Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

The SI unit for entropy is J/K. However, in this book it is convenient to work in
terms of kJ/K. A commonly employed English unit for entropy is Btu/8R. Units in SI
for specific entropy are kJ/kg
?
K for s and kJ/kmol
?
K for s. Commonly used English
units for specific entropy are Btu/lb
?
8R and Btu/lbmol
?
8R.
It should be clear that entropy is defined and evaluated in terms of a particular
expression (Eq. 6.2a) for which no accompanying physical picture is given. We encoun-
tered this previously with the property enthalpy. Enthalpy is introduced without
physical motivation in Sec. 3.6.1. Then, in Chap. 4, we learned how enthalpy is used
for thermodynamic analysis of control volumes. As for the case of enthalpy, to gain
an appreciation for entropy you need to understand how it is used and what it is used
for. This is the aim of the rest of this chapter.
6.1.2
Evaluating Entropy
Since entropy is a property, the change in entropy of a system in going from one state
to another is the same for all processes, both internally reversible and irreversible,
between these two states. Thus, Eq. 6.2a allows the determination of the change in
entropy, and once it has been evaluated, this is the magnitude of the entropy change
for all processes of the system between the two states.
The defining equation for entropy change, Eq. 6.2a, serves as the basis for evaluat-
ing entropy relative to a reference value at a reference state. Both the reference value
and the reference state can be selected arbitrarily. The value of entropy at any state
y relative to the value at the reference state x is obtained in principle from
S
y
5 S
x
1
a
#
y
x
dQ
T
b
int
rev
(6.3)
where S
x
is the reference value for entropy at the specified reference state.
The use of entropy values determined relative to an arbitrary reference state is satis-
factory as long as they are used in calculations involving entropy differences, for then the
reference value cancels. This approach suffices for applications where composition remains
constant. When chemical reactions occur, it is necessary to work in terms of absolute
values of entropy determined using the third law of thermodynamics (Chap. 13).
6.1.3
Entropy and Probability
The presentation of engineering thermodynamics provided in this book takes a mac-
roscopic view as it deals mainly with the gross, or overall, behavior of matter. The
macroscopic concepts of engineering thermodynamics introduced thus far, including
energy and entropy, rest on operational definitions whose validity is shown directly
or indirectly through experimentation. Still, insights concerning energy and entropy
can result from considering the microstructure of matter. This brings in the use of
probability and the notion of disorder. Further discussion of entropy, probability, and
disorder is provided in Sec. 6.8.2.
units for entropy
6.2 Retrieving Entropy Data
In Chap. 3, we introduced means for retrieving property data, including tables, graphs,
equations, and the software available with this text. The emphasis there is on evaluat-
ing the properties p, y, T, u, and h required for application of the conservation of
mass and energy principles. For application of the second law, entropy values are
usually required. In this section, means for retrieving entropy data for water and
several refrigerants are considered.
6.2 Retrieving Entropy Data 283
c06UsingEntropy.indd Page 283 6/30/10 9:45:28 AM user-s146c06UsingEntropy.indd Page 283 6/30/10 9:45:28 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
284 Chapter 6
Using Entropy
Tables of thermodynamic data are introduced in Secs. 3.5 and 3.6 (Tables A-2
through A-18). Specific entropy is tabulated in the same way as considered there for
the properties y, u, and h, and entropy values are retrieved similarly. The specific
entropy values given in Tables A-2 through A-18 are relative to the following reference
states and values. For water, the entropy of saturated liquid at 0.018C (32.028F) is set
to zero. For the refrigerants, the entropy of the saturated liquid at 2408C (2408F) is
assigned a value of zero.
6.2.1
Vapor Data
In the superheat regions of the tables for water and the refrigerants, specific entropy
is tabulated along with y, u, and h versus temperature and pressure.
consider two states of water. At state 1 the pressure is 3 MPa
and the temperature is 5008C. At state 2, the pressure is 0.3 MPa and the specific
entropy is the same as at state 1, s
2
5 s
1
. The object is to determine the temperature
at state 2. Using T
1
and p
1
, we find the specific entropy at state 1 from Table A-4 as
s
1
5 7.2338 kJ/kg
?
K. State 2 is fixed by the pressure, p
2
5 0.3 MPa, and the specific
entropy, s
2
5 7.2338 kJ/kg
?
K. Returing to Table A-4 at 0.3 MPa and interpolating
with s
2
between 160 and 2008C results in T
2
5 1838C. b b b b b
6.2.2
Saturation Data
For saturation states, the values of s
f
and s
g
are tabulated as a function of either
saturation pressure or saturation temperature. The specific entropy of a two-phase
liquid–vapor mixture is calculated using the quality
s 5
1
1 2 x
2
s
f
1 xs
g
(6.4)
5 s
f
1 x
1
s
g
2 s
f
2
These relations are identical in form to those for y, u, and h (Secs. 3.5 and 3.6).
let us determine the specific entropy of Refrigerant 134a at a
state where the temperature is 08C and the specific internal energy is 138.43 kJ/kg.
Referring to Table A-10, we see that the given value for u falls between u
f
and u
g
at
08C, so the system is a two-phase liquid–vapor mixture. The quality of the mixture
can be determined from the known specific internal energy
x
5
u 2 u
f
u
g
2 u
f
5
138
.
43
2
49
.7
9
227.06 2 49.79
5 0.5
Then with values from Table A-10, Eq. 6.4 gives
s 5
1
1 2 x
2
s
f
1 xs
g
5
1
0.5
21
0.1970
2
1
1
0.5
21
0.9190
2
5 0.5580 kJ
/
kg ? K b b b b b
6.2.3
Liquid Data
Compressed liquid data are presented for water in Tables A-5. In these tables s, y, u, and
h are tabulated versus temperature and pressure as in the superheat tables, and the tables
are used similarly. In the absence of compressed liquid data, the value of the specific
entropy can be estimated in the same way as estimates for y and u are obtained for
liquid states (Sec. 3.10.1), by using the saturated liquid value at the given temperature
s1T, p2< s
f
1T2 (6.5)
c06UsingEntropy.indd Page 284 5/26/10 2:40:17 PM user-s146c06UsingEntropy.indd Page 284 5/26/10 2:40:17 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
suppose the value of specific entropy is required for water at
25 bar, 2008C. The specific entropy is obtained directly from Table A-5 as s 5 2.3294 kJ/
kg
?
K. Using the saturated liquid value for specific entropy at 2008C from Table A-2,
the specific entropy is approximated with Eq. 6.5 as s 5 2.3309 kJ/kg
?
K, which
agrees closely with the previous value. b b b b b
6.2.4
Computer Retrieval
The software available with this text, Interactive Thermodynamics: IT, provides data
for the substances considered in this section. Entropy data are retrieved by simple
call statements placed in the workspace of the program.
consider a two-phase liquid–vapor mixture of H
2
O at p 5 1 bar,
y 5 0.8475 m
3
/kg. The following illustrates how specific entropy and quality x are
obtained using IT
p 5 1 // bar
v 5 0.8475 // m
3
/kg
v 5 vsat_Px(“Water/Steam”,p,x)
s 5 ssat_Px(“Water/Steam”,p,x)
The software returns values of x 5 0.5 and s 5 4.331 kJ/kg
?
K, which can be checked
using data from Table A-3. Note that quality x is implicit in the expression for specific
volume, and it is not necessary to solve explicitly for x. As another example, consider
superheated ammonia vapor at p 5 1.5 bar, T 5 88C. Specific entropy is obtained
from IT as follows:
p 5 1.5 // bar
T 5 8 // 8C
s 5 s_PT (“Ammonia”, p,T)
The software returns s 5 5.981 kJ/kg
?
K, which agrees closely with the value obtained
by interpolation in Table A-15. b b b b b
6.2.5
Using Graphical Entropy Data
The use of property diagrams as an adjunct to problem solving is emphasized through-
out this book. When applying the second law, it is frequently helpful to locate states
and plot processes on diagrams having entropy as a coordinate. Two commonly used
figures having entropy as one of the coordinates are the temperature–entropy dia-
gram and the enthalpy–entropy diagram.
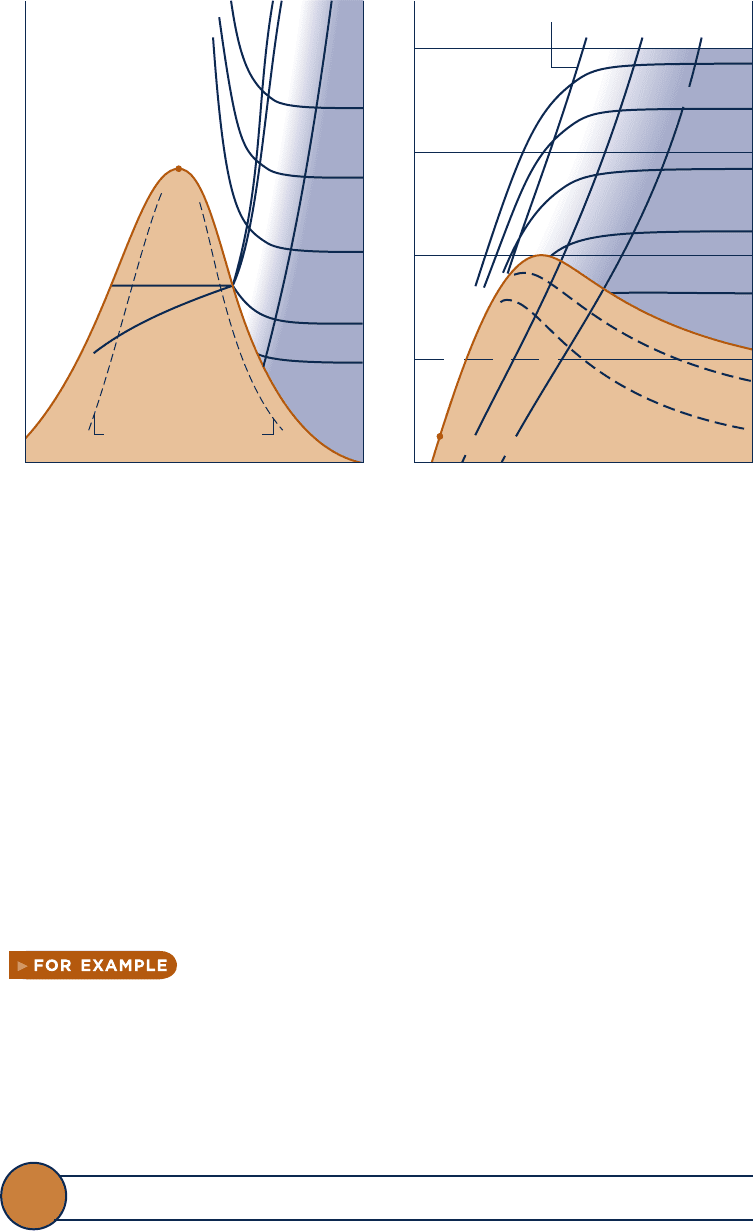
Temperature–Entropy Diagram
The main features of a temperature–entropy diagram are shown in Fig. 6.2. For detailed
figures for water in SI and English units, see Figs. A-7. Observe that lines of constant
enthalpy are shown on these figures. Also note that in the superheated vapor region
constant specific volume lines have a steeper slope than constant-pressure lines. Lines
of constant quality are shown in the two-phase liquid–vapor region. On some figures,
lines of constant quality are marked as percent moisture lines. The percent moisture
is defined as the ratio of the mass of liquid to the total mass.
In the superheated vapor region of the T–s diagram, constant specific enthalpy
lines become nearly horizontal as pressure is reduced. These superheated vapor states
are shown as the shaded area on Fig. 6.2. For states in this region of the diagram, the
enthalpy is determined primarily by the temperature: h(T, p)
<
h(T). This is the
T–s diagram
TAKE NOTE...
Note that IT does not
provide compressed liquid
data for any substance. IT
returns liquid entropy data
using the approximation of
Eq. 6.5. Similarly, Eqs. 3.11,
3.12, and 3.14 are used
to return liquid values for
y, u, and h, respectively.
6.2 Retrieving Entropy Data 285
c06UsingEntropy.indd Page 285 5/26/10 2:40:18 PM user-s146c06UsingEntropy.indd Page 285 5/26/10 2:40:18 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
286 Chapter 6
Using Entropy
region of the diagram where the ideal gas model provides a reasonable approxima-
tion. For superheated vapor states outside the shaded area, both temperature and
pressure are required to evaluate enthalpy, and the ideal gas model is not suitable.
Enthalpy–Entropy Diagram
The essential features of an enthalpy–entropy diagram, commonly known as a Mollier
diagram
, are shown in Fig. 6.3. For detailed figures for water in SI and English units,
see Figs. A-8. Note the location of the critical point and the appearance of lines of
constant temperature and constant pressure. Lines of constant quality are shown in
the two-phase liquid–vapor region (some figures give lines of constant percent mois-
ture). The figure is intended for evaluating properties at superheated vapor states and
for two-phase liquid–vapor mixtures. Liquid data are seldom shown. In the super-
heated vapor region, constant-temperature lines become nearly horizontal as pressure
is reduced. These superheated vapor states are shown, approximately, as the shaded
area on Fig. 6.3. This area corresponds to the shaded area on the temperature–entropy
diagram of Fig. 6.2, where the ideal gas model provides a reasonable approximation.
to illustrate the use of the Mollier diagram in SI units, consider
two states of water. At state 1, T
1
5 240°C, p
1
5 0.10 MPa. The specific enthalpy and
quality are required at state 2, where p
2
= 0.01 MPa and s
2
5 s
1
. Turning to Fig. A-8,
state 1 is located in the superheated vapor region. Dropping a vertical line into the
two-phase liquid–vapor region, state 2 is located. The quality and specific enthalpy at
state 2 read from the figure agree closely with values obtained using Tables A-3 and
A-4: x
2
5 0.98 and h
2
5 2537 kJ/kg. b b b b b
Mollier diagram
6.3 Introducing the T dS Equations
Although the change in entropy between two states can be determined in principle
by using Eq. 6.2a, such evaluations are generally conducted using the T dS equations
developed in this section. The T dS equations allow entropy changes to be evaluated
from other more readily determined property data. The use of the T dS equations to
Fig. 6.2
Temperature–entropy diagram.
S
a
t
u
r
a
t
e
d
v
a
p
o
r
S
a
t
u
r
a
t
e
d
l
i
q
u
i
d
Critical point
p = constant
v = constant
x = 0.2 x
= 0.9
h = constant
v = constant
p = constant
p = constant
T
s
Fig. 6.3 Enthalpy–entropy diagram.
x
=
0
.
9
0
x
=
0
.
9
6
S
a
t
u
r
a
t
e
d
v
a
p
o
r
p = constant
T = constant
T = constant
p = constant
p = constant
s
h
Critical point
c06UsingEntropy.indd Page 286 5/26/10 5:20:14 PM user-s146c06UsingEntropy.indd Page 286 5/26/10 5:20:14 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

evaluate entropy changes for an incompressible substance is illustrated in Sec. 6.4
and for ideal gases in Sec. 6.5. The importance of the T dS equations is greater than
their role in assigning entropy values, however. In Chap. 11 they are used as a point
of departure for deriving many important property relations for pure, simple com-
pressible systems, including means for constructing the property tables giving u, h,
and s.
The T dS equations are developed by considering a pure, simple compressible
system undergoing an internally reversible process. In the absence of overall system
motion and the effects of gravity, an energy balance in differential form is
1
dQ
2
int
r
ev
5 dU 1
1
dW
2
int
r
ev
(6.6)
By definition of simple compressible system (Sec. 3.1.2), the work is
1dW2
int
rev
5 p dV (6.7a)
On rearrangement of Eq. 6.2b, the heat transfer is
1dQ2
int
rev
5 T dS (6.7b)
Substituting Eqs. 6.7 into Eq. 6.6, the first T dS equation results
T ds 5 dU 1 p dV (6.8)
The second T dS equation is obtained from Eq. 6.8 using H 5 U 1 pV. Forming
the differential
dH 5 dU 1 d1pV25 dU 1 p dV 1 V dp
On rearrangement
dU 1 p dV 5 dH 2 V dp
Substituting this into Eq. 6.8 gives the second T dS equation
T dS 5 dH 2 V dp (6.9)
The T dS equations can be written on a unit mass basis as
T ds 5 du 1 p dy (6.10a)
T ds 5 dh 2 y dp (6.10b)
or on a per mole basis as
T ds 5 du 1 p
dy (6.11a)
T ds 5 dh 2
ydp
(6.11b)
Although the T dS equations are derived by considering an internally reversible
process, an entropy change obtained by integrating these equations is the change for
any process, reversible or irreversible, between two equilibrium states of a system.
Because entropy is a property, the change in entropy between two states is indepen-
dent of the details of the process linking the states.
To show the use of the T dS equations, consider a change in phase from saturated
liquid to saturated vapor at constant temperature and pressure. Since pressure is
constant, Eq. 6.10b reduces to give
ds 5
dh
T
6.3 Introducing the T dS Equations 287
first T dS equation
second T dS equation
c06UsingEntropy.indd Page 287 6/30/10 9:45:30 AM user-s146c06UsingEntropy.indd Page 287 6/30/10 9:45:30 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
288 Chapter 6
Using Entropy
Then, because temperature is also constant during the phase change
s
g
2 s
f
5
h
g
2 h
f
T
(6.12)
This relationship shows how s
g
2 s
f
is calculated for tabulation in property tables.
consider Refrigerant 134a at 08C. From Table A-10, h
g
2 h
f
=
197.21 kJ/kg, so with Eq. 6.12
s
g
2 s
f
5
197.21
kJ
/
kg
273.15 K
5 0.7220
k
J
kg ? K
which is the value calculated using s
f
and s
g
from the table. To give a similar exam-
ple in English units, consider Refrigerant 134a at 08F. From Table A-10E, h
g
2 h
f
5
90.12 Btu/lb, so
s
g
2 s
f
5
90.12 Btu
/
lb
459.678R
5 0.1961
Btu
lb ? 8R
which agrees with the value calculated using s
f
and s
g
from the table. b b b b b
6.4 Entropy Change of an Incompressible
Substance
In this section, Eq. 6.10a of Sec. 6.3 is used to evaluate the entropy change between
two states of an incompressible substance. The incompressible substance model intro-
duced in Sec. 3.10.2 assumes that the specific volume (density) is constant and the
specific internal energy depends solely on temperature. Thus, du 5 c(T)dT, where c
denotes the specific heat of the substance, and Eq. 6.10a reduces to give
ds 5
c1T2d
T
T
1
pd
y
T
0
5
c1T
2d
T
T
On integration, the change in specific entropy is
s
2
2 s
1
5
#
T
2
T
1
c1T2
T
dT
When the specific heat is constant, this becomes
s
2
2 s
1
5 c ln
T
2
T
1
1incompressible, constant c2
(6.13)
Equation 6.13, along with Eqs. 3.20 giving Du and Dh, respectively, are applicable to
liquids and solids modeled as incompressible. Specific heats of some common liquids
and solids are given in Table A-19.
consider a system consisting of liquid water initially at T
1
5 300 K,
p
1
5 2 bar undergoing a process to a final state at T
2
5 323 K, p
2
5 1 bar. There are
two ways to evaluate the change in specific entropy in this case. The first approach is to
use Eq. 6.5 together with saturated liquid data from Table A-2. That is, s
1
<
s
f
(T
1
) 5
0.3954 KJ/kg ? K and s
2
<
s
f
(T
2
) 5 0.7038 KJ/kg ? K, giving s
2
2 s
1
5 0.308 KJ/kg
?
K.
The second approach is to use the incompressible model. That is, with Eq. 6.13 and
c 5 4.18 KJ/kg ? K from Table A-19, we get
c06UsingEntropy.indd Page 288 5/26/10 2:40:24 PM user-s146c06UsingEntropy.indd Page 288 5/26/10 2:40:24 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
s
2
2 s
1
5 c ln
T
2
T
1
5 a4.18
kJ
kg ? K
b lna
323 K
300 K
b5 0.309 KJ
/
kg ? K
Comparing the values obtained for the change in specific entropy using the two
approaches considered here, we see they are in agreement. b b b b b
6.5 Entropy Change of an Ideal Gas
In this section, the T dS equations of Sec. 6.3, Eqs. 6.10, are used to evaluate the
entropy change between two states of an ideal gas. For a quick review of ideal gas
model relations, see Table 6.1.
It is convenient to begin with Eqs. 6.10 expressed as
ds 5
d
u
T
1
p
T
dy
(6.14)
ds
5
dh
T
2
y
T
dp
(6.15)
For an ideal gas, du 5 c
y
(T) dT, dh 5 c
p
(T) dT, and py 5 RT. With these relations,
Eqs. 6.14 and 6.15 become, respectively
ds 5 c
y
1T2
dT
T
1 R
dy
y
and
ds 5 c
p
1T2
dT
T
2 R
dp
p
(6.16)
On integration, Eqs. 6.16 give, respectively
s1T
2
, y
2
22 s1T
1
, y
1
25
#
T
2
T
1
c
y
1T2
dT
T
1 R ln
y
2
y
1
(6.17)
s1T
2
, p
2
22 s1T
1
, p
1
25
#
T
2
T
1
c
p
1T2
dT
T
2 R ln
p
2
p
1
(6.18)
Since R is a constant, the last terms of Eqs. 6.16 can be integrated directly. However,
because c
y
and c
p
are functions of temperature for ideal gases, it is necessary to have
information about the functional relationships before the integration of the first term
in these equations can be performed. Since the two specific heats are related by
c
p
1T25 c
y
1T21 R (3.44)
where R is the gas constant, knowledge of either specific function suffices.
6.5.1
Using Ideal Gas Tables
As for internal energy and enthalpy changes of ideal gases, the evaluation of entropy
changes for ideal gases can be reduced to a convenient tabular approach. We begin
by introducing a new variable s8(T) as
s81T25
#
T
T¿
c
p
1T2
T
dT
(6.19)
where T9 is an arbitrary reference temperature.
6.5 Entropy Change of an Ideal Gas 289
c06UsingEntropy.indd Page 289 7/1/10 7:28:07 AM user-s146c06UsingEntropy.indd Page 289 7/1/10 7:28:07 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
290 Chapter 6
Using Entropy
The integral of Eq. 6.18 can be expressed in terms of s° as follows
#
T
2
T
1
c
p
dT
T
5
#
T
2
T ¿
c
p
dT
T
2
#
T
1
T ¿
c
p
d
T
T
5 s81T
2
22 s81T
1
2
Thus, Eq. 6.18 can be written as
s1T
2
, p
2
22 s1T
1
, p
1
25 s81T
2
22 s81T
1
22 R ln
p
2
p
1
(6.20a)
or on a per mole basis as
s1T
2
, p
2
22 s1T
1
, p
1
25 s81T
2
22 s81T
1
22 R ln
p
2
p
1
(6.20b)
Because s8 depends only on temperature, it can be tabulated versus temperature,
like h and u. For air as an ideal gas, s8 with units of kJ/kg ? K or Btu/lb ? 8R is given
in Table A-22 and A-22E, respectively. Values of s8 for several other common gases are
given in Tables A-23 with units of kJ/kmol ? K or Btu/lbmol ? 8R. In passing, we note the
arbitrary reference temperature T9 of Eq. 6.19 is specified differently in Tables A-22 than
in Tables A-23. As discussed in Sec. 13.5.1, Tables A-23 give absolute entropy values.
Using Eqs. 6.20 and the tabulated values for s8 or s8, as appropriate, entropy
changes can be determined that account explicitly for the variation of specific heat
with temperature.
let us evaluate the change in specific entropy, in kJ/kg ? K, of air
modeled as an ideal gas from a state where T
1
5 300 K and p
1
= 1 bar to a state
where T
2
5 1000 K and p
2
5 3 bar. Using Eq. 6.20a and data from Table A-22
s
2
2 s
1
5 s81T
2
22 s81T
1
22 R ln
p
2
p
1
5 12.96770 2 1.702032
kJ
kg ? K
2
8.314
28.97
kJ
kg ? K
ln
3 bar
1 bar
5 0.9504 kJ
/
kg ? K b b b b b
Ideal Gas Model Review
Equations of state:
py 5 RT (3.32)
pV 5 mRT (3.33)
Changes in u and h:
u(T
2
) 2 u(T
1
)
5
#
T
2
T
1
c
y
1T2dT
(3.40)
h(T
2
) 2 h(T
1
)
5
#
T
2
T
1
c
p
1T2dT
(3.43)
Constant Specific Heats Variable Specific Heats
TABLE 6.1
u(T ) and h(T ) are evaluated from Tables A-22
for air (mass basis) and Tables A-23 for several
other gases (molar basis).
u(T
2
) 2 u(T
1
) 5 c
y
(T
2
2 T
1
) (3.50)
h(T
2
) 2 h(T
1
) 5 c
p
(T
2
2 T
1
) (3.51)
See Tables A-20, 21 for c
y
and c
p
data.
c06UsingEntropy.indd Page 290 5/26/10 5:20:19 PM user-s146c06UsingEntropy.indd Page 290 5/26/10 5:20:19 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New

If a table giving s8 (or s8) is not available for a particular gas of interest, the inte-
grals of Eqs. 6.17 and 6.18 can be performed analytically or numerically using specific
heat data such as provided in Tables A-20 and A-21.
6.5.2
Assuming Constant Specific Heats
When the specific heats c
y
and c
p
are taken as constants, Eqs. 6.17 and 6.18 reduce,
respectively, to
s1T
2
, y
2
22 s1T
1
, y
1
2
.
5 c
y
ln
T
2
T
1
1 R ln
y
2
y
1
(6.21)
s1T
2
, p
2
22 s1T
1
, p
1
25 c
p
ln
T
2
T
1
2 R ln
p
2
p
1
(6.22)
These equations, along with Eqs. 3.50 and 3.51 giving ¢u and ¢h, respectively, are
applicable when assuming the ideal gas model with constant specific heats.
let us determine the change in specific entropy, in KJ/kg
?
K, of
air as an ideal gas undergoing a process from T
1
5 300 K, p
1
5 1 bar to T
2
5 400 K,
p
2
5 5 bar. Because of the relatively small temperature range, we assume a constant value
of c
p
evaluated at 350 K. Using Eq. 6.22 and c
p
5 1.008 KJ/kg
?
K from Table A-20
¢s 5 c
p
ln
T
2
T
1
2 R ln
p
2
p
1
5 a1.008
kJ
kg ? K
b lna
400 K
300 K
b2 a
8.314
28.97
kJ
kg ? K
b lna
5 bar
1 bar
b
520.1719 kJ
/
kg ? K b b b b b
6.5.3
Computer Retrieval
For gases modeled as ideal gases, IT directly returns s(T, p) using the following special
form of Eq. 6.18:
s1T, p22 s1T
ref
, p
ref
25
#
T
T
ref
c
p
1T2
T
dT 2 R ln
p
p
ref
and the following choice of reference state and reference value: T
ref
5 0 K (08R),
p
ref
5 1 atm, and s(T
ref
, p
ref
) 5 0, giving
s1T, p25
#
T
0
c
p
1T2
T
dT 2 R ln
p
p
ref
(a)
Such reference state and reference value choices equip IT for use in combustion
applications. See the discussion of absolute entropy in Sec. 13.5.1.
Changes in specific entropy evaluated using IT agree with entropy changes evalu-
ated using ideal gas tables.
consider a process of air as an ideal gas from T
1
5 300 K, p
1
5
1 bar to T
2
5 1000 K, p
2
5 3 bar. The change in specific entropy, denoted as dels, is
determined in SI units using IT as follows:
p1 5 1//bar
T1 5 300//K
p2 5 3
T2 5 1000
s1 5 s_TP(“Air”,T1,p1)
s2 5 s_TP(“Air”,T2,p2)
dels 5 s2 2 s1
6.5 Entropy Change of an Ideal Gas 291
c06UsingEntropy.indd Page 291 6/30/10 9:45:31 AM user-s146c06UsingEntropy.indd Page 291 6/30/10 9:45:31 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
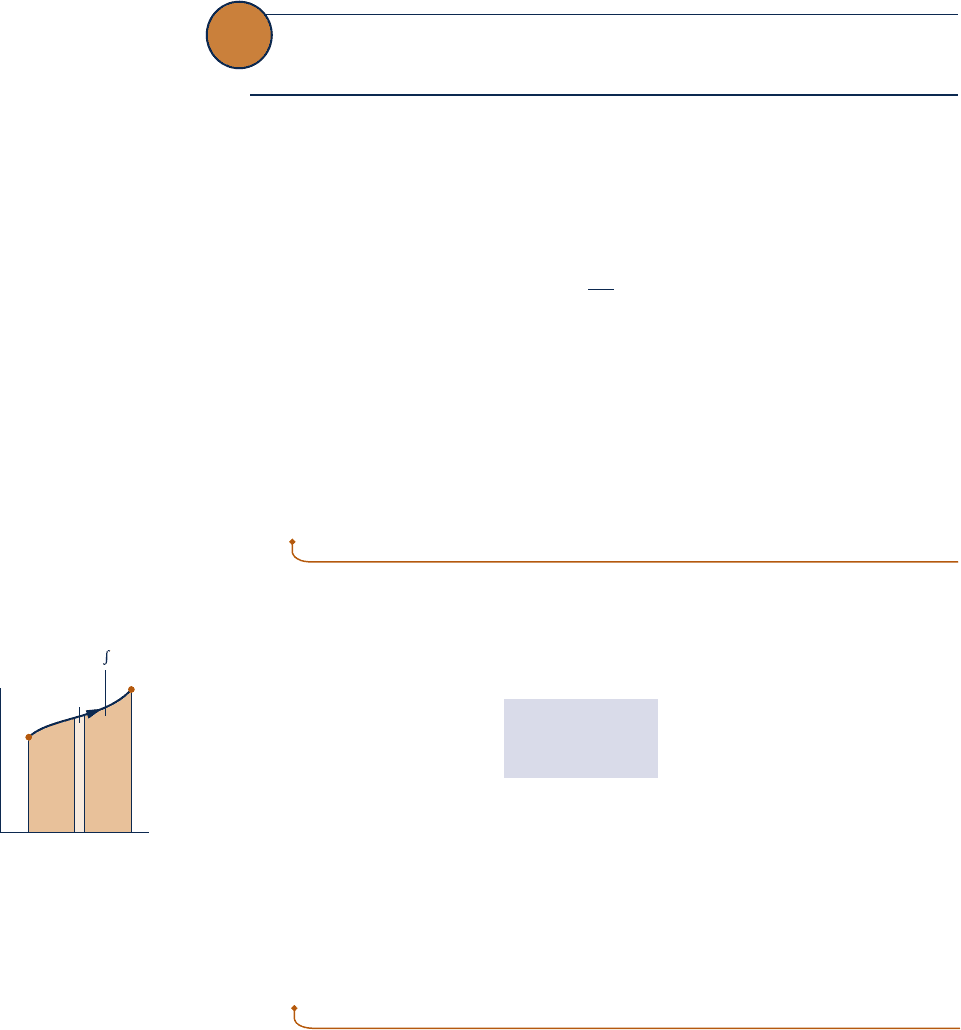
292 Chapter 6 Using Entropy
The software returns values of s
1
5 1.706, s
2
5 2.656, and dels 5 0.9501, all in units
of kJ/kg ? K. This value for ¢s agrees with the value obtained using Table A-22: 0.9504
kJ/kg ? K, as shown in the concluding example of Sec. 6.5.1. b b b b b
Note again that IT returns specific entropy directly using Eq. (a) above. IT does
not use the special function s8.
6.6 Entropy Change in Internally Reversible
Processes of Closed Systems
In this section the relationship between entropy change and heat transfer for internally
reversible processes is considered. The concepts introduced have important applications
in subsequent sections of the book. The present discussion is limited to the case of
closed systems. Similar considerations for control volumes are presented in Sec. 6.13.
As a closed system undergoes an internally reversible process, its entropy can
increase, decrease, or remain constant. This can be brought out using
dS 5 a
dQ
T
b
int
rev
(6.2b)
which indicates that when a closed system undergoing an internally reversible process
receives energy by heat transfer, the system experiences an increase in entropy. Con-
versely, when energy is removed from the system by heat transfer, the entropy of the
system decreases. This can be interpreted to mean that an entropy transfer accompa-
nies heat transfer. The direction of the entropy transfer is the same as that of the heat
transfer. In an adiabatic internally reversible process, entropy remains constant. A
constant-entropy process is called an isentropic process.
6.6.1
Area Representation of Heat Transfer
On rearrangement, Eq. 6.2b gives
1dQ2
int
rev
5 T dS
Integrating from an initial state 1 to a final state 2
Q
int
rev
5
#
2
1
T dS
(6.23)
From Eq. 6.23 it can be concluded that an energy transfer by heat to a closed system
during an internally reversible process can be represented as an area on a temperature–
entropy diagram. Figure 6.4 illustrates the area representation of heat transfer for an
arbitrary internally reversible process in which temperature varies. Carefully note that
temperature must be in kelvins or degrees Rankine, and the area is the entire area
under the curve (shown shaded). Also note that the area representation of heat trans-
fer is not valid for irreversible processes, as will be demonstrated later.
6.6.2
Carnot Cycle Application
To provide an example illustrating both the entropy change that accompanies heat
transfer and the area representation of heat transfer, consider Fig. 6.5a, which shows a
Carnot power cycle (Sec. 5.10.1). The cycle consists of four internally reversible processes
in series: two isothermal processes alternated with two adiabatic processes. In Process 2–3,
heat transfer to the system occurs while the temperature of the system remains constant
entropy transfer
Carnot cycle
isentropic process
Fig. 6.4 Area representation
of heat transfer for an
internally reversible process
of a closed system.
1
2
S
T
(δQ) = T dS
Q =
2
1
T dS
int
rev
int
rev
c06UsingEntropy.indd Page 292 5/26/10 2:40:28 PM user-s146c06UsingEntropy.indd Page 292 5/26/10 2:40:28 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
