Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

5.8 The Kelvin and International
Temperature Scales
The results of Secs. 5.6 and 5.7 establish theoretical upper limits on the performance
of power, refrigeration, and heat pump cycles communicating thermally with two
reservoirs. Expressions for the maximum theoretical thermal efficiency of power
cycles and the maximum theoretical coefficients of performance of refrigeration and
heat pump cycles are developed in Sec. 5.9 using the Kelvin temperature scale con-
sidered next.
5.8.1
The Kelvin Scale
From the second Carnot corollary we know that all reversible power cycles operat-
ing between the same two thermal reservoirs have the same thermal efficiency,
regardless of the nature of the substance making up the system executing the cycle
or the series of processes. Since the thermal efficiency is independent of these factors,
its value can be related only to the nature of the reservoirs themselves. Noting that
it is the difference in temperature between the two reservoirs that provides the impe-
tus for heat transfer between them, and thereby for the production of work during
the cycle, we reason that the thermal efficiency depends only on the temperatures of
the two reservoirs.
From Eq. 5.4 it also follows that for such reversible power cycles the ratio of
the heat transfers Q
C
/Q
H
depends only on the temperatures of the two reservoirs.
That is
a
Q
C
Q
H
b
rev
cycle
5 c1u
C
, u
H
2
(a)
where u
H
and u
C
denote the temperatures of the reservoirs and the function c is for
the present unspecified. Note that the words “rev cycle” are added to this expression
to emphasize that it applies only to systems undergoing reversible cycles while oper-
ating between two thermal reservoirs.
Equation (a) provides a basis for defining a thermodynamic temperature scale: a
scale independent of the properties of any substance. There are alternative choices
for the function c that lead to this end. The Kelvin scale is obtained by making a
5.8 The Kelvin and International Temperature Scales 253
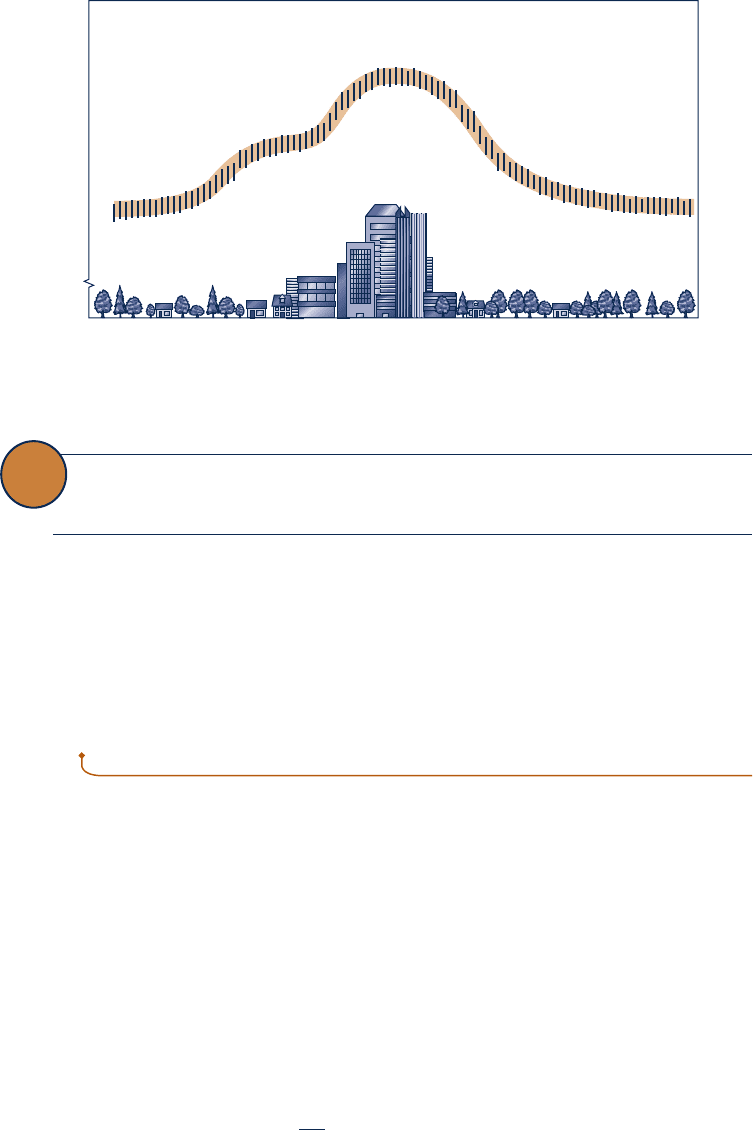
Suburban City center Suburban
Surface Temperature
Fig. 5.9 Surface temperature variation in an urban area.
Kelvin scale
c05TheSecondLawofThermodynamics.253 Page 253 5/21/10 12:27:20 PM user-s146c05TheSecondLawofThermodynamics.253 Page 253 5/21/10 12:27:20 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
254 Chapter 5
The Second Law of Thermodynamics
particularly simple choice, namely, c 5 T
C
/T
H
, where T is the symbol used by inter-
national agreement to denote temperatures on the Kelvin scale. With this, we get
a
Q
C
Q
H
b
rev
cycle
5
T
C
T
H
(5.7)
Thus, two temperatures on the Kelvin scale are in the same ratio as the values of
the heat transfers absorbed and rejected, respectively, by a system undergoing a
reversible cycle while communicating thermally with reservoirs at these tempera-
tures.
If a reversible power cycle were operated in the opposite direction as a refrig-
eration or heat pump cycle, the magnitudes of the energy transfers Q
C
and Q
H
would remain the same, but the energy transfers would be oppositely directed.
Accordingly, Eq. 5.7 applies to each type of cycle considered thus far, provided the
system undergoing the cycle operates between two thermal reservoirs and the cycle
is reversible.
More on the Kelvin Scale
Equation 5.7 gives only a ratio of temperatures. To complete the definition of the
Kelvin scale, it is necessary to proceed as in Sec. 1.7.3 by assigning the value 273.16 K
to the temperature at the triple point of water. Then, if a reversible cycle is operated
between a reservoir at 273.16 K and another reservoir at temperature T, the two
temperatures are related according to
T 5 273.16 a
Q
Q
tp
b
rev
cycle
(5.8)
where Q
tp
and Q are the heat transfers between the cycle and reservoirs at 273.16 K
and temperature T, respectively. In the present case, the heat transfer Q plays the role
of the thermometric property. However, since the performance of a reversible cycle is
independent of the makeup of the system executing the cycle, the definition of tem-
perature given by Eq. 5.8 depends in no way on the properties of any substance or
class of substances.
In Sec. 1.7 we noted that the Kelvin scale has a zero of 0 K, and lower temperatures
than this are not defined. Let us take up these points by considering a reversible
power cycle operating between reservoirs at 273.16 K and a lower temperature T.
Referring to Eq. 5.8, we know that the energy rejected from the cycle by heat trans-
fer Q would not be negative, so T must be nonnegative. Equation 5.8 also shows that
the smaller the value of Q, the lower the value of T, and conversely. Accordingly, as
Q approaches zero the temperature T approaches zero. It can be concluded that a
temperature of zero on the Kelvin scale is the lowest conceivable temperature. This
temperature is called the absolute zero, and the Kelvin scale is called an absolute
temperature scale.
When numerical values of the thermodynamic temperature are to be determined,
it is not possible to use reversible cycles, for these exist only in our imaginations.
However, temperatures evaluated using the constant-volume gas thermometer dis-
cussed in Sec. 5.8.2 to follow are identical to those of the Kelvin scale in the range of
temperatures where the gas thermometer can be used. Other empirical approaches
can be employed for temperatures above and below the range accessible to gas ther-
mometry. The Kelvin scale provides a continuous definition of temperature valid over
all ranges and provides an essential connection between the several empirical mea-
sures of temperature.
TAKE NOTE...
Some readers may prefer to
proceed directly to Sec. 5.9,
where Eq. 5.7 is applied.
c05TheSecondLawofThermodynamics.254 Page 254 5/22/10 7:39:20 PM user-s146c05TheSecondLawofThermodynamics.254 Page 254 5/22/10 7:39:20 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
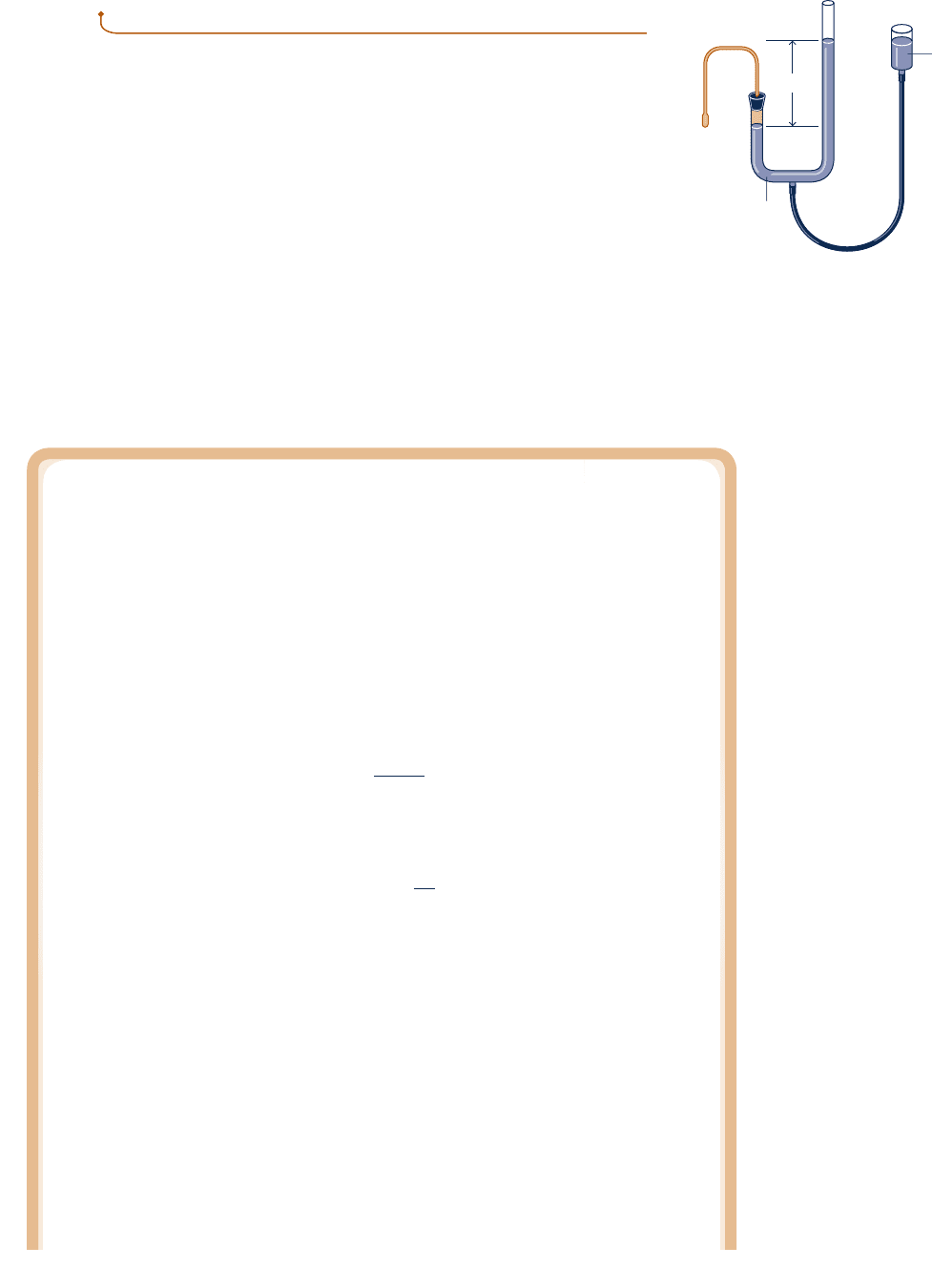
Measuring Temperature with the Gas Thermometer—The Gas Scale
It is instructive to consider how numerical values are associated with levels of tem-
perature by the gas thermometer shown in Fig. 5.10. Let p stand for the pressure in the
bulb of a constant-volume gas thermometer in thermal equilibrium with a bath. A value
can be assigned to the bath temperature by a linear relation
T = ap (a)
where a is an arbitrary constant.
The value of a is determined by inserting the thermometer into another bath main-
tained at the triple point of water and measuring the pressure, call it p
tp
, of the con-
fined gas at the triple point temperature, 273.16 K. Substituting values into Eq. (a) and
solving for a
a 5
273.1
6
p
tp
Inserting this in Eq. (a), the temperature of the original bath, at which the pressure of the
confined gas is p, is then
T 5 273.16 a
p
p
tp
b
(b)
However, since the values of both pressures, p and p
tp
, depend in part on the
amount of gas in the bulb, the value assigned by Eq. (b) to the bath temperature var-
ies with the amount of gas in the thermometer. This difficulty is overcome in precision
thermometry by repeating the measurements (in the original bath and the reference
bath) several times with less gas in the bulb in each successive attempt. For each trial
the ratio p/p
tp
is calculated from Eq. (b) and plotted versus the corresponding refer-
ence pressure p
tp
of the gas at the triple point temperature. When several such points
have been plotted, the resulting curve is extrapolated to the ordinate where p
tp
5 0.
This is illustrated in Fig. 5.11 for constant-volume thermometers with a number of dif-
ferent gases.
Inspection of Fig. 5.11 shows that at each nonzero value of the reference pressure,
the p/p
tp
values differ with the gas employed in the thermometer. However, as pressure
decreases, the p/p
tp
values from thermometers with different gases approach one
another, and in the limit as pressure tends to zero, the same value for p/p
tp
is obtained
5.8 The Kelvin and International Temperature Scales 255
5.8.2
The Gas Thermometer
The constant-volume gas thermometer shown in Fig. 5.10 is so exceptional in
terms of precision and accuracy that it has been adopted internationally as
the standard instrument for calibrating other thermometers. The thermometric
substance is the gas (normally hydrogen or helium), and the thermometric
property is the pressure exerted by the gas. As shown in the figure, the gas is
contained in a bulb, and the pressure exerted by it is measured by an open-
tube mercury manometer. As temperature increases, the gas expands, forcing
mercury up in the open tube. The gas is kept at constant volume by raising
or lowering the reservoir. The gas thermometer is used as a standard world-
wide by bureaus of standards and research laboratories. However, because
gas thermometers require elaborate apparatus and are large, slowly respond-
ing devices that demand painstaking experimental procedures, smaller, more
rapidly responding thermometers are used for most temperature measurements and
they are calibrated (directly or indirectly) against gas thermometers. For further discus-
sion of gas thermometry, see the box.
L
Manometer
Mercury
reservoi
r
Capillary
Gas bulb
Fig. 5.10 Constant-volume gas
thermometer.
c05TheSecondLawofThermodynamics.255 Page 255 5/28/10 1:15:18 PM user-s146c05TheSecondLawofThermodynamics.255 Page 255 5/28/10 1:15:18 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
256 Chapter 5
The Second Law of Thermodynamics
5.8.3
International Temperature Scale
To provide a standard for temperature measurement taking into account both theo-
retical and practical considerations, the International Temperature Scale (ITS) was
adopted in 1927. This scale has been refined and extended in several revisions, most
recently in 1990. The International Temperature Scale of 1990 (ITS-90) is defined in
such a way that the temperature measured on it conforms with the thermodynamic
temperature, the unit of which is the kelvin, to within the limits of accuracy of mea-
surement obtainable in 1990. The ITS-90 is based on the assigned values of tem-
perature of a number of reproducible fixed points (Table 5.1). Interpolation between
the fixed-point temperatures is accomplished by formulas that give the relation
between readings of standard instruments and values of the ITS. In the range from
0.65 to 5.0 K, ITS-90 is defined by equations giving the temperature as functions of
the vapor pressures of particular helium isotopes. The range from 3.0 to 24.5561 K
is based on measurements using a helium constant-volume gas thermometer. In the
range from 13.8033 to 1234.93 K, ITS-90 is defined by means of certain platinum
resistance thermometers. Above 1234.93 K the temperature is defined using Planck’s
equation for blackbody radiation and measurements of the intensity of visible-spectrum
radiation.
5.9 Maximum Performance Measures
for Cycles Operating Between
Two Reservoirs
The discussion continues in this section with the development of expressions for the
maximum thermal efficiency of power cycles and the maximum coefficients of per-
formance of refrigeration and heat pump cycles in terms of reservoir temperatures
evaluated on the Kelvin scale. These expressions can be used as standards of com-
parison for actual power, refrigeration, and heat pump cycles.
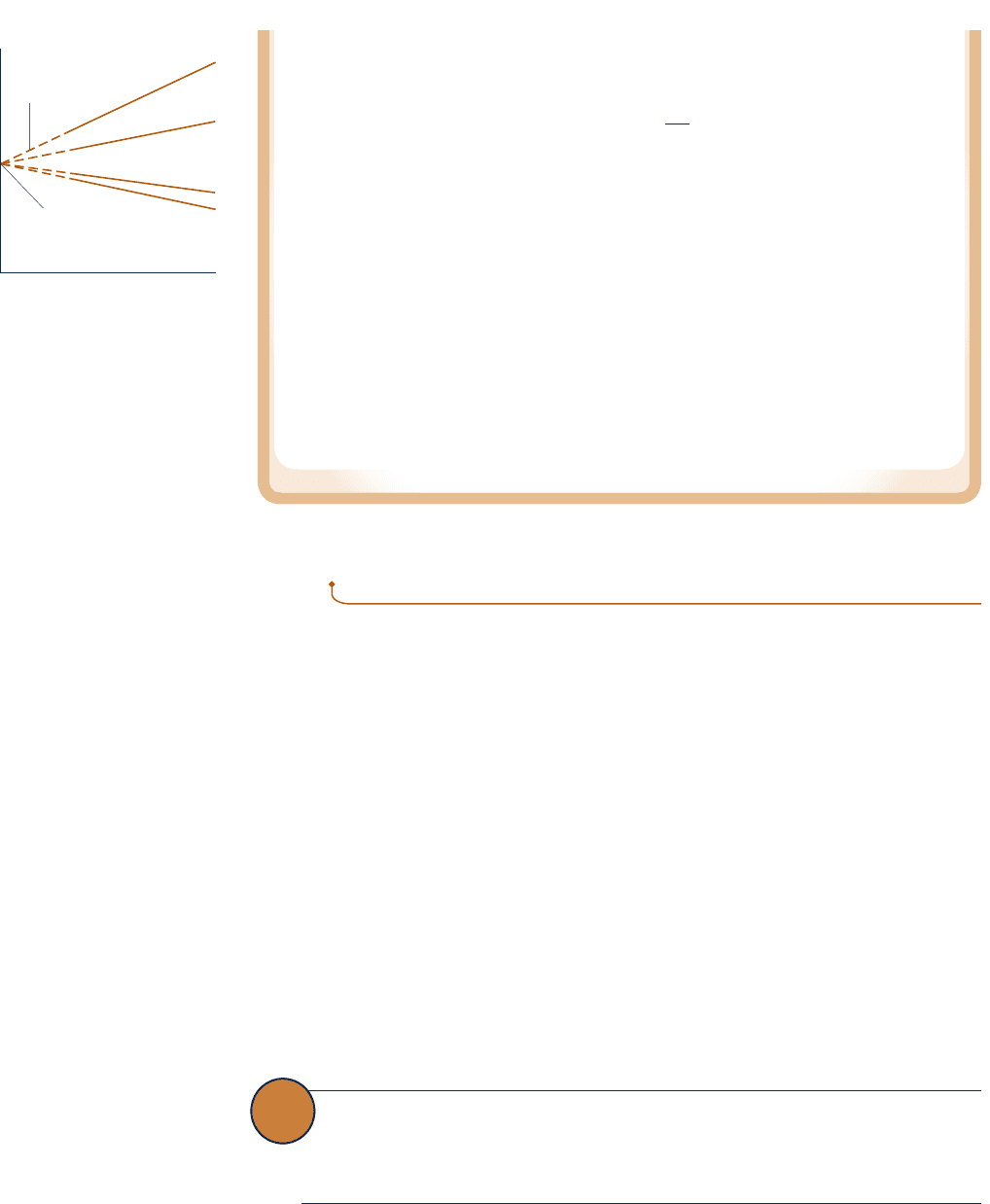
for each gas. Based on these general results, the gas temperature scale is defined by
the relationship
T 5 273.16 lim
p
p
tp
(c)
where “lim” means that both p and p
tp
tend to zero. It should be evident that the deter-
mination of temperatures by this means requires extraordinarily careful and elaborate
experimental procedures.
Although the temperature scale of Eq. (c) is independent of the properties of any
one gas, it still depends on the properties of gases in general. Accordingly, the measure-
ment of low temperatures requires a gas that does not condense at these temperatures,
and this imposes a limit on the range of temperatures that can be measured by a gas
thermometer. The lowest temperature that can be measured with such an instrument is
about 1 K, obtained with helium. At high temperatures gases dissociate, and therefore
these temperatures also cannot be determined by a gas thermometer. Other empirical
means, utilizing the properties of other substances, must be employed to measure tem-
perature in ranges where the gas thermometer is inadequate. For further discussion see
Sec. 5.8.3.
p
––
p
tp
p
––
p
tp
O
2
N
2
He
H
2
p
tp
T = 273.16 lim
Measured data for a fixed level
of temperature, extrapolated
to zero pressure
Fig. 5.11 Readings of constant-
volume gas thermometers,
when several gases are used.
c05TheSecondLawofThermodynamics.256 Page 256 5/28/10 1:15:29 PM user-s146c05TheSecondLawofThermodynamics.256 Page 256 5/28/10 1:15:29 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
5.9.1
Power Cycles
The use of Eq. 5.7 in Eq. 5.4 results in an expression for the thermal efficiency of a
system undergoing a reversible power cycle while operating between thermal reser-
voirs at temperatures T
H
and T
C
. That is
h
max
5 1 2
T
C
T
H
(5.9)
which is known as the Carnot efficiency. As temperatures on the Rankine scale differ
from Kelvin temperatures only by the factor 1.8, the T’s in Eq. 5.9 may be on either
scale of temperature.
Recalling the two Carnot corollaries, it should be evident that
the efficiency given by Eq. 5.9 is the thermal efficiency of all
reversible power cycles operating between two reservoirs at tem-
peratures T
H
and T
C
, and the maximum efficiency any power cycle
can have while operating between the two reservoirs. By inspec-
tion, the value of the Carnot efficiency increases as T
H
increases
and/or T
C
decreases.
Equation 5.9 is presented graphically in Fig. 5.12. The temperature
T
C
used in constructing the figure is 298 K in recognition that actual
power cycles ultimately discharge energy by heat transfer at about
the temperature of the local atmosphere or cooling water drawn from
a nearby river or lake. Note that the possibility of increasing the
thermal efficiency by reducing T
C
below that of the environment is
Carnot efficiency
a
b
η → 1 (100%)
0
1000 2000
Temperature, T
H
(K)
3000298
0.5
1.0
η
max
= 1 –
T
C
–––
T
H
Fig. 5.12 Carnot efficiency versus T
H
, for
T
C
5 298 K.
5.9 Maximum Performance Measures for Cycles Operating Between Two Reservoirs 257
Defining Fixed Points of the International Temperature Scale of 1990
T (K) Substance
a
State
b
3 to 5 He Vapor pressure point
13.8033 e-H
2
Triple point
< 17 e-H
2
Vapor pressure point
< 20.3 e-H
2
Vapor pressure point
24.5561 Ne Triple point
54.3584 O
2
Triple point
83.8058 Ar Triple point
234.3156 Hg Triple point
273.16 H
2
O Triple point
302.9146 Ga Melting point
429.7485 In Freezing point
505.078 Sn Freezing point
692.677 Zn Freezing point
933.473 Al Freezing point
1234.93 Ag Freezing point
1337.33 Au Freezing point
1357.77 Cu Freezing point
a
He denotes
3
He or
4
He; e-H
2
is hydrogen at the equilibrium concentration of the ortho- and
para-molecular forms.
b
Triple point: temperature at which the solid, liquid, and vapor phases are in equilibrium.
Melting point, freezing point: temperature, at a pressure of 101.325 kPa, at which the solid
and liquid phases are in equilibrium.
Source: H. Preston-Thomas, “The International Temperature Scale of 1990 (ITS-90),” Metrolo-
gia 27, 3–10 (1990). See also www.ITS-90.com.
TABLE 5.1
c05TheSecondLawofThermodynamics.257 Page 257 5/21/10 12:27:31 PM user-s146c05TheSecondLawofThermodynamics.257 Page 257 5/21/10 12:27:31 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
258 Chapter 5 The Second Law of Thermodynamics
not practical, for maintaining T
C
lower than the ambient temperature would require
a refrigerator that would have to be supplied work to operate.
Figure 5.12 shows that the thermal efficiency increases with T
H
. Referring to seg-
ment a–b of the curve, where T
H
and h are relatively low, we see that h increases
rapidly as T
H
increases, showing that in this range even a small increase in T
H
can have
a large effect on efficiency. Though these conclusions, drawn as they are from Eq. 5.9,
apply strictly only to systems undergoing reversible cycles, they are qualitatively cor-
rect for actual power cycles. The thermal efficiencies of actual cycles are observed to
increase as the average temperature at which energy is added by heat transfer increases
and/or the average temperature at which energy is discharged by heat transfer decreases.
However, maximizing the thermal efficiency of a power cycle may not be the only
objective. In practice, other considerations such as cost may be overriding.
Conventional power-producing cycles have thermal efficiencies ranging up to about
40%. This value may seem low, but the comparison should be made with an appropri-
ate limiting value and not 100%.
consider a system executing a power cycle for which the aver-
age temperature of heat addition is 745 K and the average temperature at which
heat is discharged is 298 K. For a reversible cycle receiving and discharging energy
by heat transfer at these temperatures, the thermal efficiency given by Eq. 5.9 is
60%. When compared to this value, an actual thermal efficiency of 40% does not
appear to be so low. The cycle would be operating at two-thirds of the theoretical
maximum. b b b b b
In the next example, we evaluate an inventor’s claim about the performance of a
power cycle, illustrating the use of the Carnot corollaries (Sec. 5.6.2) and the Carnot
efficiency, Eq. 5.9.
Evaluating a Power Cycle Performance Claim
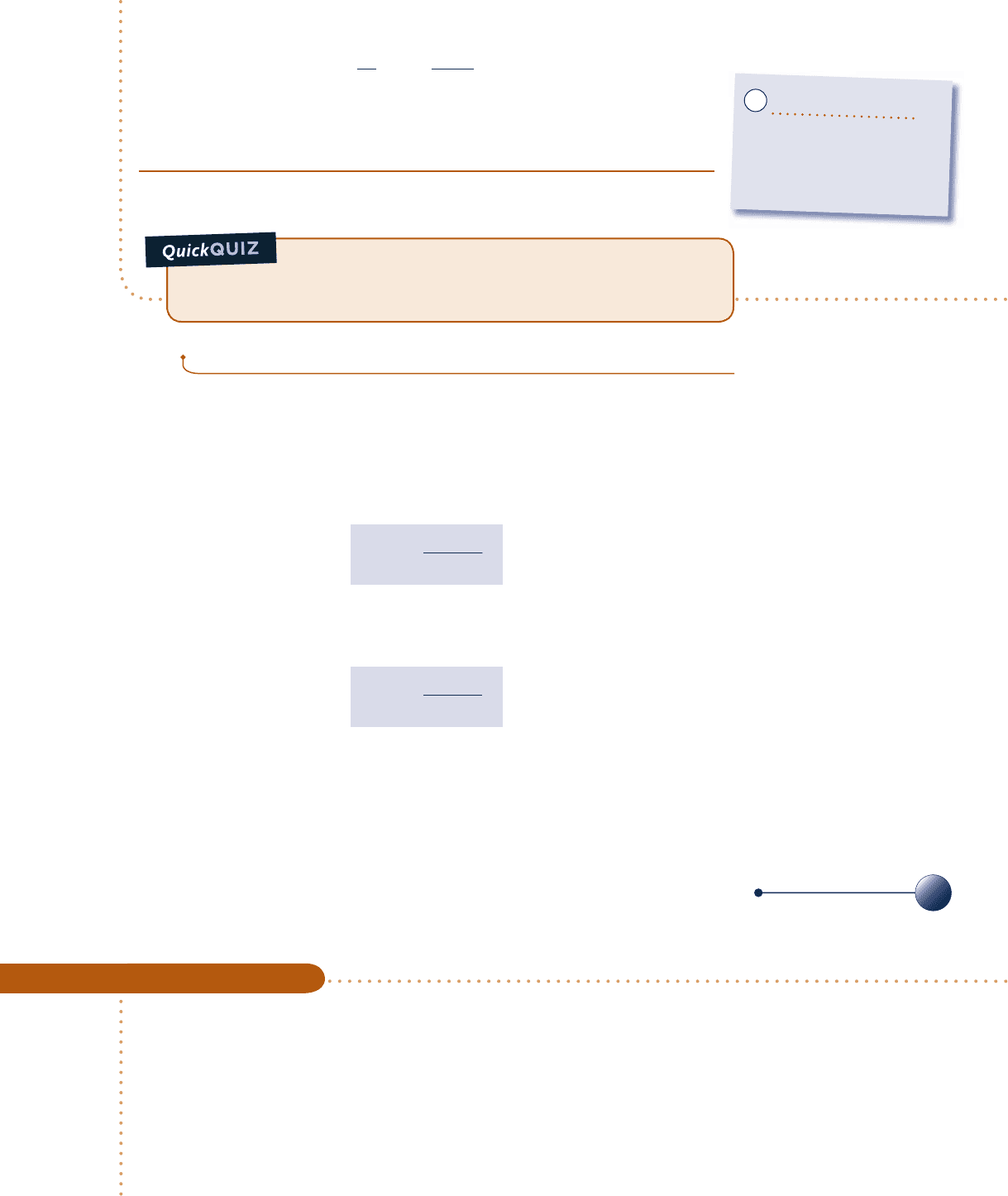
c c c c EXAMPLE 5.1 c
An inventor claims to have developed a power cycle capable of delivering a net work output of 410 kJ for an energy
input by heat transfer of 1000 kJ. The system undergoing the cycle receives the heat transfer from hot gases at a
temperature of 500 K and discharges energy by heat transfer to the atmosphere at 300 K. Evaluate this claim.
SOLUTION
Known:
A system operates in a cycle and produces a net amount of work while receiving and discharging energy
by heat transfer at fixed temperatures.
Find: Evaluate the claim that the cycle can develop 410 kJ of work for an energy input by heat of 1000 kJ.
Schematic and Given Data:
Engineering Model:
1.
The system shown on the accompanying figure executes a power cycle.
2. The hot gases and the atmosphere play the roles of hot and cold reservoirs,
respectively.
Analysis: Inserting the values supplied by the inventor into Eq. 5.4, the cycle thermal efficiency is
h 5
410 kJ
1000 kJ
5 0.41 141%2
Power
cycle
Q
in
= 1000 kJ
Q
out
W = 410 kJ
500 K
300 K
Fig. E5.1
A
A
Power_Cycle
A.9 – Tab c
c05TheSecondLawofThermodynamics.258 Page 258 6/30/10 11:08:37 AM user-s146c05TheSecondLawofThermodynamics.258 Page 258 6/30/10 11:08:37 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
The maximum thermal efficiency any power cycle can have while operating between reservoirs at T
H
5 500 K
and T
C
5 300 K is given by Eq. 5.9.
➊
h
max
5 1 2
T
C
T
H
5 1 2
300
K
500 K
5 0.40 140%2
The Carnot corollaries provide a basis for evaluating the claim: Since the ther-
mal efficiency of the actual cycle exceeds the maximum theoretical value, the
claim cannot be valid.
➊
The temperatures T
C
and T
H
used in evaluating h
max
must be in K or 8R.
Ability to…
❑
apply the Carnot corollaries,
using Eqs. 5.4 and 5.9
appropriately.
✓
Skills Developed
If the cycle receives heat transfer from a hot gas at 600 K while
all other data remain unchanged, evaluate the inventor’s claim. Ans. Claim
is in accord with the second law.
5.9.2
Refrigeration and Heat Pump Cycles
Equation 5.7 is also applicable to reversible refrigeration and heat pump cycles operat-
ing between two thermal reservoirs, but for these Q
C
represents the heat added to the
cycle from the cold reservoir at temperature T
C
on the Kelvin scale and Q
H
is the heat
discharged to the hot reservoir at temperature T
H
. Introducing Eq. 5.7 in Eq. 5.5 results
in the following expression for the coefficient of performance of any system undergo-
ing a reversible refrigeration cycle while operating between the two reservoirs
b
max
5
T
C
T
H
2 T
C
(5.10)
Similarly, substituting Eq. 5.7 into Eq. 5.6 gives the following expression for the coef-
ficient of performance of any system undergoing a reversible heat pump cycle while
operating between the two reservoirs
g
max
5
T
H
T
H
2 T
C
(5.11)
Note that the temperatures used to evaluate b
max
and g
max
must be absolute tem-
peratures on the Kelvin or Rankine scale.
From the discussion of Sec. 5.7.2, it follows that Eqs. 5.10 and 5.11 are the maximum
coefficients of performance that any refrigeration and heat pump cycles can have
while operating between reservoirs at temperatures T
H
and T
C
. As for the case of the
Carnot efficiency, these expressions can be used as standards of comparison for actual
refrigerators and heat pumps.
In the next example, we evaluate the coefficient of performance of a refrigerator
and compare it with the maximum theoretical value, illustrating the use of the second
law corollaries of Sec. 5.7.2 together with Eq. 5.10.
Evaluating Refrigerator Performance
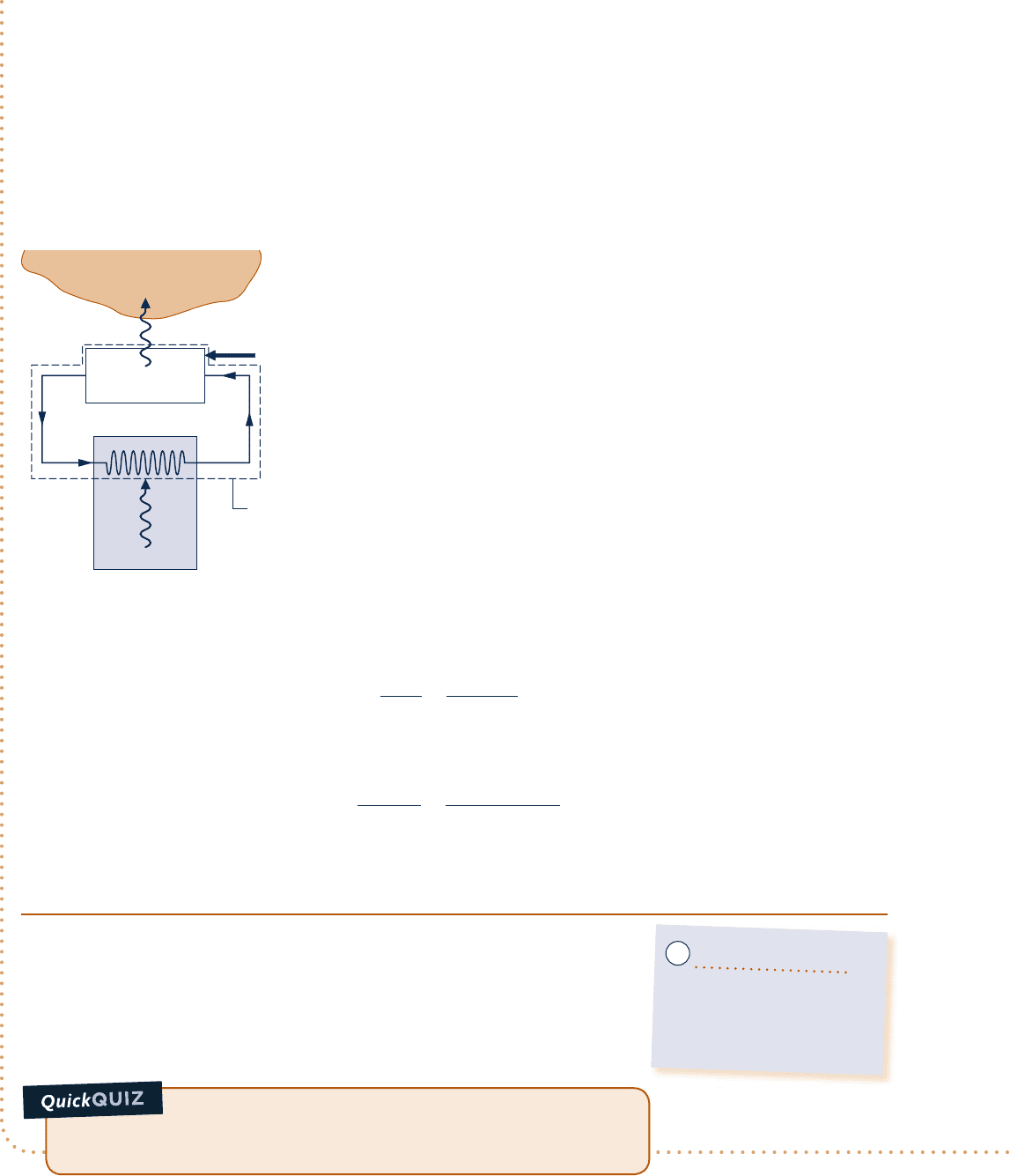
c c c c EXAMPLE 5.2 c
By steadily circulating a refrigerant at low temperature through passages in the walls of the freezer compartment,
a refrigerator maintains the freezer compartment at 258C when the air surrounding the refrigerator is at 228C.
The rate of heat transfer from the freezer compartment to the refrigerant is 8000 kJ/h and the power input
5.9 Maximum Performance Measures for Cycles Operating Between Two Reservoirs
259
A
A
Refrig_Cycle
A.10 – Tab c
Heat_Pump_Cycle
A.11 – Tab c
c05TheSecondLawofThermodynamics.259 Page 259 7/1/10 11:21:28 AM user-s146c05TheSecondLawofThermodynamics.259 Page 259 7/1/10 11:21:28 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
260 Chapter 5 The Second Law of Thermodynamics
required to operate the refrigerator is 3200 kJ/h. Determine the coefficient of performance of the refrigerator
and compare with the coefficient of performance of a reversible refrigeration cycle operating between reservoirs
at the same two temperatures.
SOLUTION
Known:
A refrigerator maintains a freezer compartment at a specified temperature. The rate of heat transfer from
the refrigerated space, the power input to operate the refrigerator, and the ambient temperature are known.
Find: Determine the coefficient of performance and compare with that of a reversible refrigerator operating
between reservoirs at the same two temperatures.
Schematic and Given Data:
Analysis: Inserting the given operating data into Eq. 5.5 expressed on a time-rate basis, the coefficient of perfor-
mance of the refrigerator is
b 5
Q
#
C
W
#
c
y
cle
5
8000 kJ
/
h
3200 kJ
/
h
5 2.5
Substituting values into Eq. 5.10 gives the coefficient of performance of a reversible refrigeration cycle operating
between reservoirs at T
C
5 268 K and T
H
5 295 K
➊
b
max
5
T
C
T
H
2 T
C
5
268 K
295 K 2 268 K
5 9.9
In accord with the corollaries of Sec. 5.7.2, the coefficient of performance of the refrigerator is less than for
a reversible refrigeration cycle operating between reservoirs at the same two temperatures. That is, irrevers-
ibilities are present within the system.
➊ The temperatures T
C
and T
H
used in evaluating b
max
must be in K or 8R.
➋ The difference between the actual and maximum coefficients of performance
suggests that there may be some potential for improving the thermodynamic
performance. This objective should be approached judiciously, however, for
improved performance may require increases in size, complexity, and cost.
Engineering Model:
1.
The system shown on the accompanying figure is at steady state.
2. The freezer compartment and the surrounding air play the roles of
cold and hot reservoirs, respectively.
Freezer compartment
at –5°C (268 K)
System
boundary
Surroundings at 22°C (295 K)
Q
·
H
Q
·
C
= 8000 kJ/h
W
·
cycle
= 3200 kJ/h
Fig. E5.2
➋
Ability to…
❑
apply the second law corollar-
ies of Sec. 5.7.2, using Eqs.
5.5 and 5.10 appropriately.
✓
Skills Developed
An inventor claims the power required to operate the refrig-
erator can be reduced to 800 kJ/h while all other data remain the same.
Evaluate this claim using the second law. Ans. b 5 10. Claim invalid.
c05TheSecondLawofThermodynamics.260 Page 260 7/1/10 11:21:37 AM user-s146c05TheSecondLawofThermodynamics.260 Page 260 7/1/10 11:21:37 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
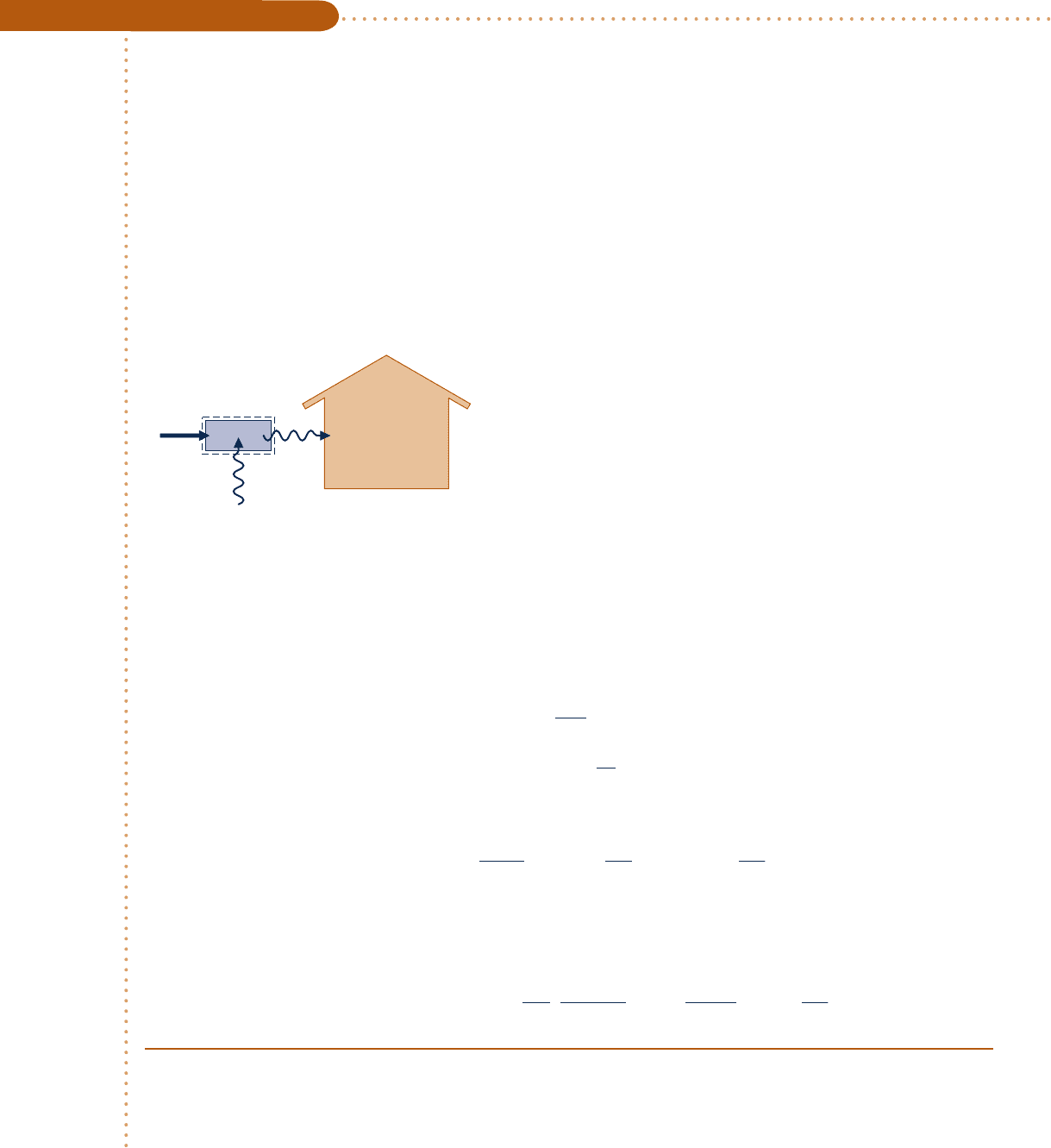
In Example 5.3, we determine the minimum theoretical work input and cost for
one day of operation of an electric heat pump, illustrating the use of the second law
corollaries of Sec. 5.7.2 together with Eq. 5.11.
5.9 Maximum Performance Measures for Cycles Operating Between Two Reservoirs 261
Evaluating Heat Pump Performance
c c c c EXAMPLE 5.3 c
A dwelling requires 6 3 10
5
Btu per day to maintain its temperature at 708F when the outside temperature is
328F. (a) If an electric heat pump is used to supply this energy, determine the minimum theoretical work input
for one day of operation, in Btu/day. (b) Evaluating electricity at 8 cents per kW ? h, determine the minimum
theoretical cost to operate the heat pump, in $/day.
SOLUTION
Known:
A heat pump maintains a dwelling at a specified temperature. The energy supplied to the dwelling, the
ambient temperature, and the unit cost of electricity are known.
Find: Determine the minimum theoretical work required by the heat pump and the corresponding electricity cost.
Schematic and Given Data:
Analysis:
(a)
Using Eq. 5.6, the work for any heat pump cycle can be expressed as W
cycle
5 Q
H
/g. The coefficient of per-
formance g of an actual heat pump is less than, or equal to, the coefficient of performance g
max
of a reversible
heat pump cycle when each operates between the same two thermal reservoirs: g # g
max
. Accordingly, for a given
value of Q
H
, and using Eq. 5.11 to evaluate g
max
, we get
W
cycle
$
Q
H
g
max
$
a
1 2
T
C
T
H
b
Q
H
Inserting values
W
cycle
$
a
1 2
4928R
5308R
ba
6 3 10
5
Btu
da
y
b
5 4.3 3 10
4
Btu
da
y
The minimum theoretical work input is 4.3 3 10
4
Btu/day.
(b) Using the result of part (a) together with the given cost data and an appropriate conversion factor
£
minimum
theoretical
cost per da
y
§5 a4.3 3 10
4
Btu
day
`
1 kW ? h
3413 Btu
`ba0.08
$
kW ? h
b5 1.01
$
day
Engineering Model:
1.
The system shown on the accompanying figure executes a
heat pump cycle.
2. The dwelling and the outside air play the roles of hot and
cold reservoirs, respectively.
3. The value of electricity is 8 cents per kW ? h.
Heat pump
Dwelling
at 70°F
(530°R)
Surroundings at 32°F (492°R)
Q
C
Q
H
W
cycle
Fig. E5.3
➊
➋
c05TheSecondLawofThermodynamics.261 Page 261 5/21/10 12:27:40 PM user-s146c05TheSecondLawofThermodynamics.261 Page 261 5/21/10 12:27:40 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
262 Chapter 5 The Second Law of Thermodynamics
➊ Note that the temperatures T
C
and T
H
must be in °R or K.
➋ Because of irreversibilities, an actual heat pump must be supplied more work
than the minimum to provide the same heating effect. The actual daily cost
could be substantially greater than the minimum theoretical cost.
Ability to…
❑
apply the second law corollar-
ies of Sec. 5.7.2, using Eqs.
5.6 and 5.11 appropriately.
❑
conduct an elementary
economic evaluation.
✓Skills Developed
If the cost of electricity is 10 cents per kW
?
h, evaluate the
minimum theoretical cost to operate the heat pump, in $/day, keeping all
other data the same. Ans. $1.26/day.
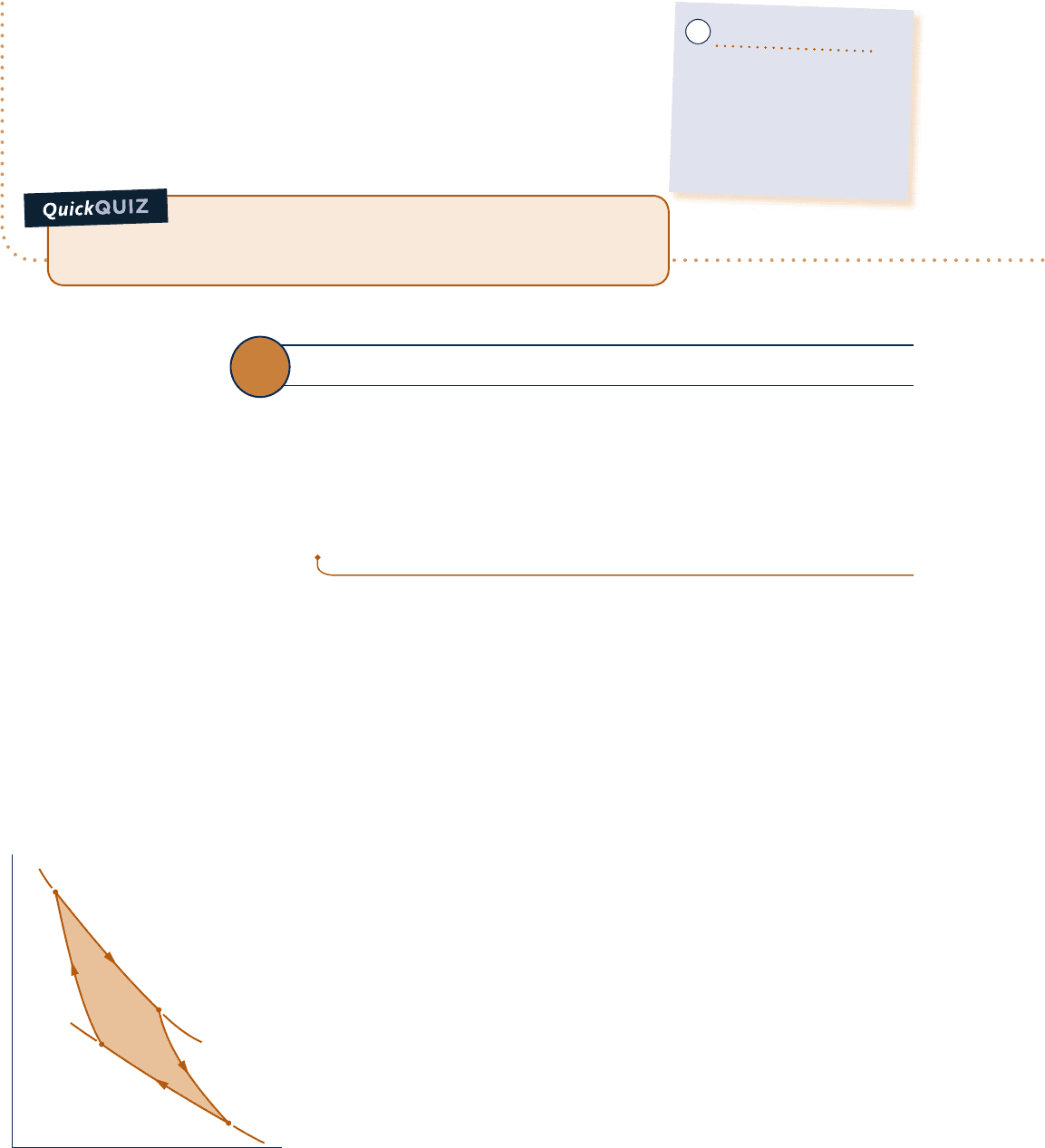
5.10 Carnot Cycle
The Carnot cycles introduced in this section provide specific examples of reversible
cycles operating between two thermal reservoirs. Other examples are provided in
Chap. 9: the Ericsson and Stirling cycles. In a Carnot cycle, the system executing the
cycle undergoes a series of four internally reversible processes: two adiabatic pro-
cesses alternated with two isothermal processes.
Carnot cycle
5.10.1
Carnot Power Cycle
Figure 5.13 shows the p–y diagram of a Carnot power cycle in which the system is a
gas in a piston–cylinder assembly. Figure 5.14 provides details of how the cycle is exe-
cuted. The piston and cylinder walls are nonconducting. The heat transfers are in the
directions of the arrows. Also note that there are two reservoirs at temperatures T
H
and T
C
, respectively, and an insulating stand. Initially, the piston–cylinder assembly is
on the insulating stand and the system is at state 1, where the temperature is T
C
. The
four processes of the cycle are
Process 1–2: The gas is compressed adiabatically to state 2, where the tempera-
ture is T
H
.
Process 2–3: The assembly is placed in contact with the reservoir at T
H
. The gas
expands isothermally while receiving energy Q
H
from the hot reservoir by heat
transfer.
Process 3–4: The assembly is again placed on the insulating stand and the gas
is allowed to continue to expand adiabatically until the temperature drops
to T
C
.
Process 4–1: The assembly is placed in contact with the reservoir at T
C
. The gas
is compressed isothermally to its initial state while it discharges energy Q
C
to
the cold reservoir by heat transfer.
For the heat transfer during Process 2–3 to be reversible, the difference
between the gas temperature and the temperature of the hot reservoir must
be vanishingly small. Since the reservoir temperature remains constant, this
implies that the temperature of the gas also remains constant during Process
2–3. The same can be concluded for the gas temperature during Process 4–1.
For each of the four internally reversible processes of the Carnot cycle, the
work can be represented as an area on Fig. 5.13. The area under the adiabatic
process line 1–2 represents the work done per unit of mass to compress the
gas in this process. The areas under process lines 2–3 and 3–4 represent the
T
H
T
C
p
v
2
3
1
4
Fig. 5.13 p–y diagram for a Carnot
gas power cycle.
c05TheSecondLawofThermodynamics262 Page 262 7/1/10 11:22:07 AM user-s146 c05TheSecondLawofThermodynamics262 Page 262 7/1/10 11:22:07 AM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
