Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

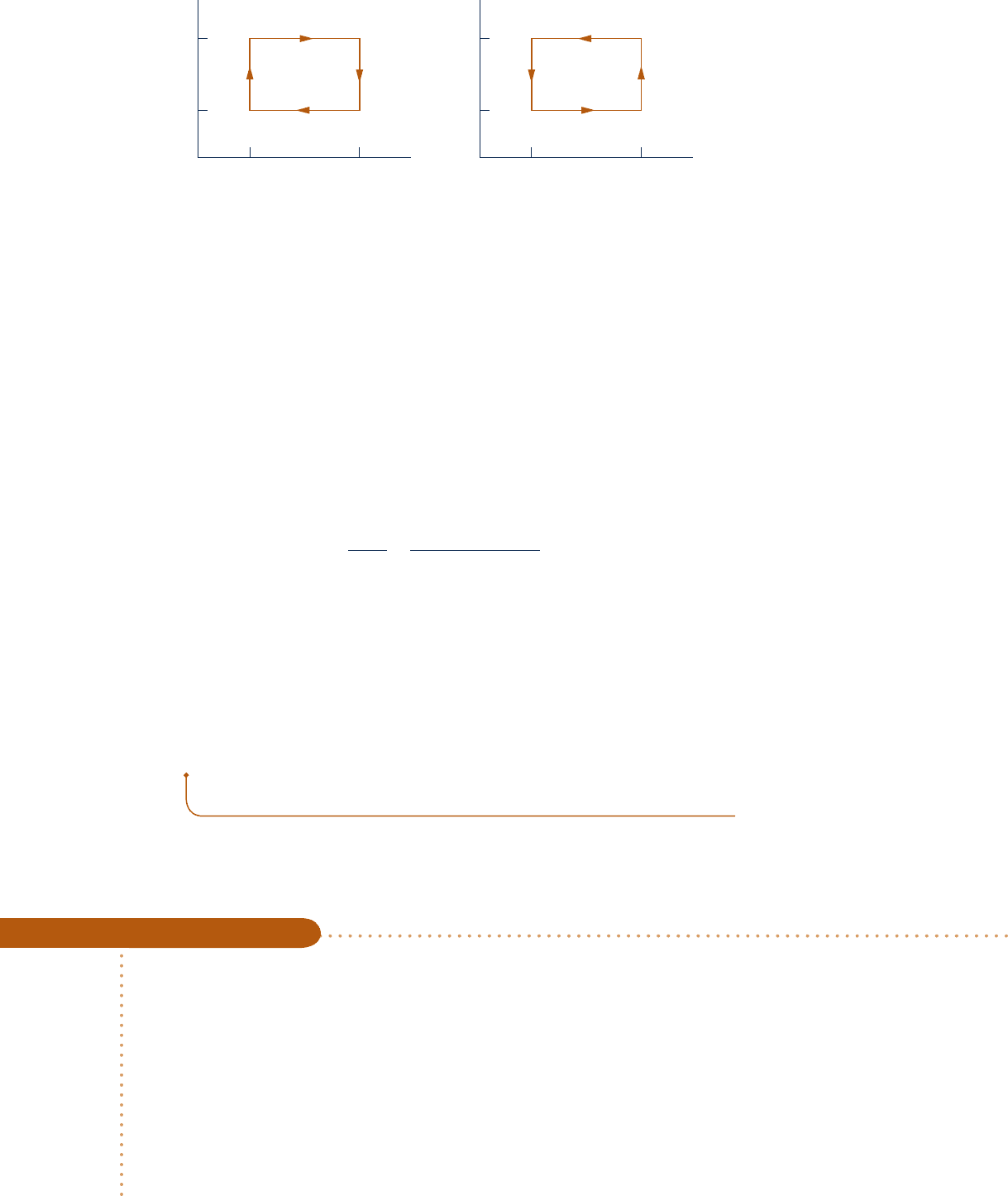
at T
H
. The system entropy increases due to the accompanying entropy transfer. For
this process, Eq. 6.23 gives Q
23
5 T
H
(S
3
2 S
2
), so area 2–3–a–b–2 on Fig. 6.5a repre-
sents the heat transfer during the process. Process 3–4 is an adiabatic and internally
reversible process and thus is an isentropic (constant-entropy) process. Process 4–1 is an
isothermal process at T
C
during which heat is transferred from the system. Since
entropy transfer accompanies the heat transfer, system entropy decreases. For this
process, Eq. 6.23 gives Q
41
5 T
C
(S
1
2 S
4
), which is negative in value. Area 4–1–b–a–4
on Fig. 6.5a represents the magnitude of the heat transfer Q
41
. Process 1–2, which
completes the cycle, is adiabatic and internally reversible (isentropic).
The net work of any cycle is equal to the net heat transfer, so enclosed area
1–2–3–4–1 represents the net work of the cycle. The thermal efficiency of the cycle
also can be expressed in terms of areas:
h 5
W
cycle
Q
23
5
area 122232421
area 2232a2b22
The numerator of this expression is (T
H
2 T
C
)(S
3
2 S
2
) and the denominator is
T
H
(S
3
2 S
2
), so the thermal efficiency can be given in terms of temperatures only as
h
5 1 2 T
C
/
T
H
. This of course agrees with Eq. 5.9.
If the cycle were executed as shown in Fig. 6.5b, the result would be a Carnot
refrigeration or heat pump cycle. In such a cycle, heat is transferred to the system
while its temperature remains at T
C
, so entropy increases during Process 1–2. In Pro-
cess 3–4 heat is transferred from the system while the temperature remains constant
at T
H
and entropy decreases.
6.6.3
Work and Heat Transfer in an Internally
Reversible Process of Water
To further illustrate concepts introduced in this section, Example 6.1 considers water
undergoing an internally reversible process while contained in a piston–cylinder
assembly.
Evaluating Work and Heat Transfer for an Internally
Reversible Process of Water
c c c c EXAMPLE 6.1 c
Water, initially a saturated liquid at 1508C (423.15 K), is contained in a piston–cylinder assembly. The water under-
goes a process to the corresponding saturated vapor state, during which the piston moves freely in the cylinder.
If the change of state is brought about by heating the water as it undergoes an internally reversible process at
constant pressure and temperature, determine the work and heat transfer per unit of mass, each in kJ/kg.
6.6 Entropy Change in Internally Reversible Processes of Closed Systems 293
Fig. 6.5
Carnot cycles on the temperature–entropy diagram. (a) Power cycle. (b) Refrigeration
or heat pump cycle.
23
14
T
H
T
C
T
ba
S
(a)
43
12
T
H
T
C
T
ba
S
(b)
c06UsingEntropy.indd Page 293 5/26/10 2:40:31 PM user-s146c06UsingEntropy.indd Page 293 5/26/10 2:40:31 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
294 Chapter 6 Using Entropy
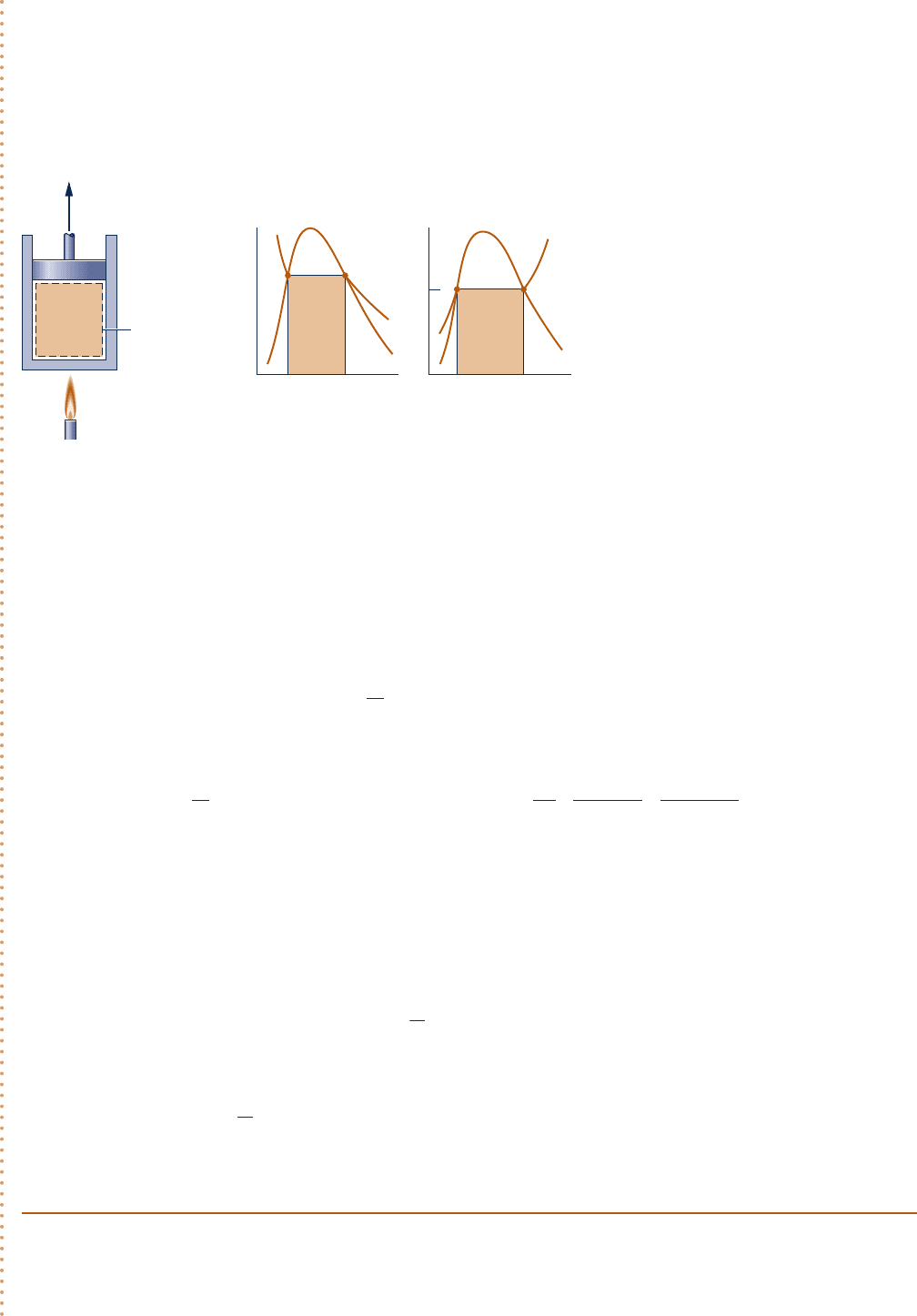
SOLUTION
Known:
Water contained in a piston–cylinder assembly undergoes an internally reversible process at 1508C from
saturated liquid to saturated vapor.
Find: Determine the work and heat transfer per unit mass.
Schematic and Given Data:
Engineering Model:
1.
The water in the piston–cylinder assembly is a closed system.
2. The process is internally reversible.
3. Temperature and pressure are constant during the process.
4. There is no change in kinetic or potential energy between the two end states.
Analysis: At constant pressure the work is
W
m
5
#
2
1
p dy 5 p1y
2
2 y
1
2
With values from Table A-2 at 1508C
W
m
5 14.758 bar210.3928 2 1.0905 3 10
23
2a
m
3
kg
b`
10
5
N
/
m
2
1 bar
``
1 kJ
10
3
N ? m
`
5 186.38 kJ
/
kg
Since the process is internally reversible and at constant temperature, Eq. 6.23 gives
Q 5
#
2
1
T dS 5 m
#
2
1
T ds
or
Q
m
5 T1s
2
2 s
1
2
With values from Table A-2
➊
Q
m
5 1423.15 K216.8379 2 1.84182 kJ
/
kg ? K 5 2114.1 kJ
/
kg
As shown in the accompanying figure, the work and heat transfer can be represented as areas on p–y and T–s
diagrams, respectively.
Fig. E6.1
p
v
150°C
150°C
W
––
m
T
s
Q
––
m
System boundary
Water
1
1
2
2
c06UsingEntropy.indd Page 294 5/26/10 2:40:33 PM user-s146c06UsingEntropy.indd Page 294 5/26/10 2:40:33 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
➊ The heat transfer can be evaluated alternatively from an energy balance written on a unit mass basis as
u
2
2 u
1
5
Q
m
2
W
m
Introducing W/m 5 p(y
2
2 y
1
) and solving
Q
m
5 1u
2
2 u
1
21 p1y
2
2 y
1
2
5
1
u
2
1 py
2
2
2
1
u
1
1 py
1
2
5 h
2
2 h
1
From Table A-2 at 1508C, h
2
2 h
1
5 2114.3 kJ/kg, which agrees with the value
for Q/m obtained in the solution.
If the initial and final states were saturation states at 1008C
(373.15 K), determine the work and heat transfer per unit of mass, each
in kJ/kg. Ans. 170 kJ/kg, 2257 kJ/kg.
6.7 Entropy Balance for Closed Systems
In this section, we begin our study of the entropy balance. The entropy balance is an
expression of the second law that is particularly effective for thermodynamic analysis.
The current presentation is limited to closed systems. The entropy balance is extended
to control volumes in Sec. 6.9.
Just as mass and energy are accounted for by mass and energy balances, respec-
tively, entropy is accounted for by an entropy balance. In Eq. 5.2, the entropy balance
is introduced in words as
≥
change in the amount of
entropy contained within
the system during some
time interval
¥
5
E
net amount of
entropy transferred in
across the system
boundary during the
time interval
U
1
C
amount of entropy produced
within the system
during the time interval
S
In symbols, the closed system entropy balance takes the form
S
2
2 S
1
5
#
2
1
a
@
Q
T
b
b
1
s
(6.24)
entropy entropy entropy
change transfer production
where subscript b signals that the integrand is evaluated at the system boundary. For
the development of Eq. 6.24, see the box.
It is sometimes convenient to use the entropy balance expressed in differential form
dS 5
a
dQ
T
b
b
1 ds
(6.25)
Note that the differentials of the nonproperties Q and s are shown, respectively, as
dQ and ds. When there are no internal irreversibilities, ds vanishes and Eq. 6.25
reduces to Eq. 6.2b.
In each of its alternative forms the entropy balance can be regarded as a statement
of the second law of thermodynamics. For the analysis of engineering systems, the
entropy balance is a more effective means for applying the second law than the
Clausius and Kelvin–Planck statements given in Chap. 5.
entropy balance
closed system entropy
balance
6.7 Entropy Balance for Closed Systems 295
Ability to…
❑
evaluate work and heat
transfer for an internally
reversible process, and
represent them as areas
on p–y and T–s diagrams,
respectively.
❑
retrieve entropy data for
water.
✓Skills Developed
c06UsingEntropy.indd Page 295 5/26/10 5:20:20 PM user-s146c06UsingEntropy.indd Page 295 5/26/10 5:20:20 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
296 Chapter 6
Using Entropy
6.7.1
Interpreting the Closed System Entropy Balance
If the end states are fixed, the entropy change on the left side of Eq. 6.24 can be
evaluated independently of the details of the process. However, the two terms on the
right side depend explicitly on the nature of the process and cannot be determined
solely from knowledge of the end states. The first term on the right side of Eq. 6.24
is associated with heat transfer to or from the system during the process. This term
can be interpreted as the entropy transfer accompanying heat transfer. The direction of
entropy transfer is the same as the direction of the heat transfer, and the same sign
convention applies as for heat transfer: A positive value means that entropy is trans-
ferred into the system, and a negative value means that entropy is transferred out.
When there is no heat transfer, there is no entropy transfer.
The entropy change of a system is not accounted for solely by the entropy transfer,
but is due in part to the second term on the right side of Eq. 6.24 denoted by s. The
term s is positive when internal irreversibilities are present during the process and van-
ishes when no internal irreversibilities are present. This can be described by saying that
entropy is produced (or generated) within the system by the action of irreversibilities.
The second law of thermodynamics can be interpreted as requiring that entropy
is produced by irreversibilities and conserved only in the limit as irreversibilities are
reduced to zero. Since s measures the effect of irreversibilities present within the
system during a process, its value depends on the nature of the process and not solely
on the end states. Entropy production is not a property.
Developing the Closed System Entropy Balance
The entropy balance for closed systems can be developed using the Clausius inequality
expressed by Eq. 5.13 (Sec. 5.11) and the defining equation for entropy change, Eq. 6.2a,
as follows:
Shown in Fig. 6.6 is a cycle executed by a closed system. The cycle consists of process I,
during which internal irreversibilities are present, followed by internally reversible process R.
For this cycle, Eq. 5.13 takes the form
#
2
1
a
dQ
T
b
b
1
#
1
2
a
dQ
T
b
rev
int
52s
(a)
where the first integral is for process I and the second is for process R. The subscript
b in the first integral serves as a reminder that the integrand is evaluated at the system
boundary. The subscript is not required in the second integral because temperature is
uniform throughout the system at each intermediate state of an internally reversible
process. Since no irreversibilities are associated with process R, the term s
cycle
of
Eq. 5.13, which accounts for the effect of irreversibilities during the cycle, refers only
to process I and is shown in Eq. (a) simply as s.
Applying the definition of entropy change, Eq. 6.2a, we can express the second integral
of Eq. (a) as
#
1
2
a
dQ
T
b
rev
int
5 S
1
2 S
2
(b)
With this, Eq. (a) becomes
#
2
1
a
d
Q
T
b
b
1 1S
1
2 S
2
252s
(c)
On rearrangement, Eq. (c) gives Eq. 6.24, the closed system entropy balance.
entropy transfer
accompanying heat
transfer
entropy production
Fig. 6.6 Cycle used to
develop the entropy balance.
1
R
I
2
c06UsingEntropy.indd Page 296 5/26/10 2:40:38 PM user-s146c06UsingEntropy.indd Page 296 5/26/10 2:40:38 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
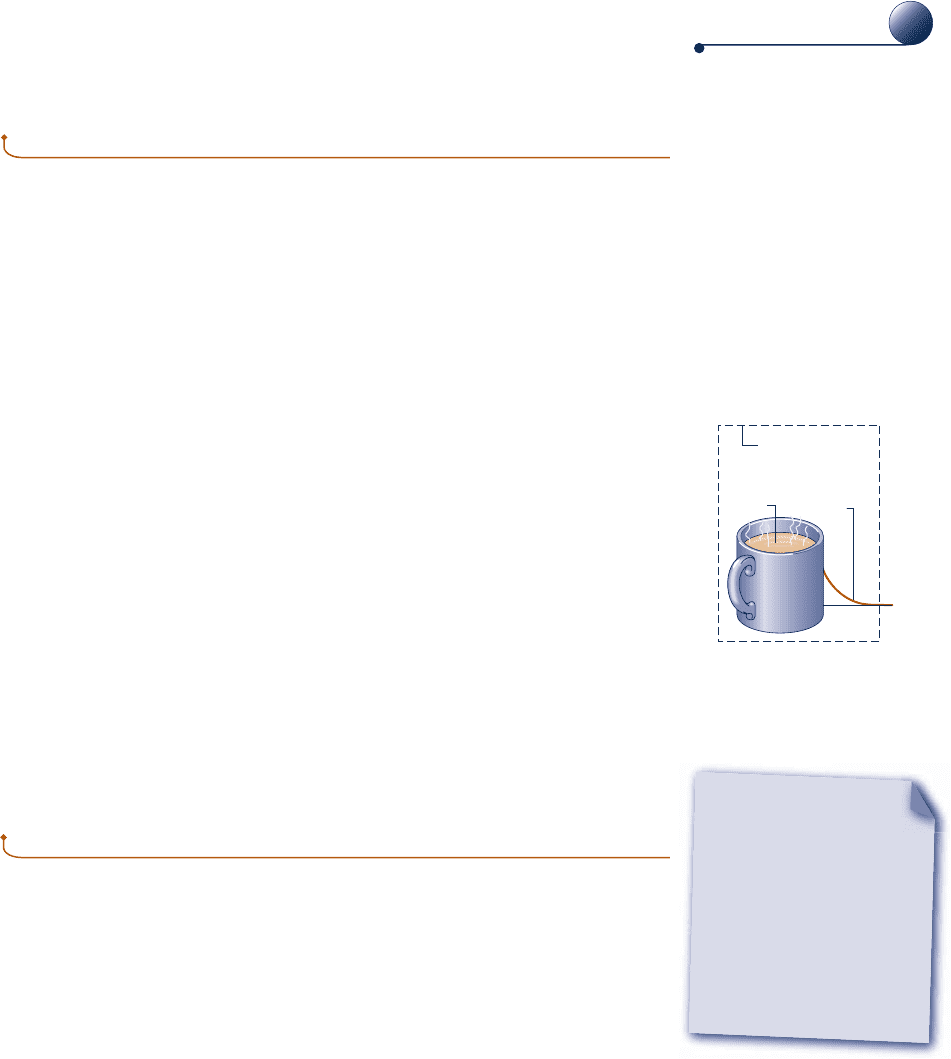
When applying the entropy balance to a closed system, it is essential to remember
the requirements imposed by the second law on entropy production: The second law
requires that entropy production be positive, or zero, in value
s: e
. 0 irreversibilities present within the system
5 0 no irreversibilities present within the s
y
ste
m
(6.26)
The value of the entropy production cannot be negative. In contrast, the change in
entropy of the system may be positive, negative, or zero:
S
2
2 S
1
:
c
. 0
5 0
, 0
(6.27)
Like other properties, entropy change for a process between two specified states can
be determined without knowledge of the details of the process.
6.7.2
Evaluating Entropy Production and Transfer
The objective in many applications of the entropy balance is to evaluate the entropy
production term. However, the value of the entropy production for a given process
of a system often does not have much significance by itself. The significance is
normally determined through comparison. For example, the entropy production
within a given component might be compared to the entropy production values of
the other components included in an overall system formed by these components.
By comparing entropy production values, the components where appreciable irre-
versibilities occur can be identified and rank ordered. This allows attention to be
focused on the components that contribute most to inefficient operation of the overall
system.
To evaluate the entropy transfer term of the entropy balance requires informa-
tion regarding both the heat transfer and the temperature on the boundary where
the heat transfer occurs. The entropy transfer term is not always subject to direct
evaluation, however, because the required information is either unknown or not
defined, such as when the system passes through states sufficiently far from equi-
librium. In such applications, it may be convenient, therefore, to enlarge the system
to include enough of the immediate surroundings that the temperature on the
boundary of the enlarged system corresponds to the temperature of the surround-
ings away from the immediate vicinity of the system, T
f
. The entropy transfer term
is then simply Q/T
f
. However, as the irreversibilities present would not be just for
the system of interest but for the enlarged system, the entropy production term
would account for the effects of internal irreversibilities within the original system
and external irreversibilities present within that portion of the surroundings included
within the enlarged system.
6.7.3
Applications of the Closed System Entropy Balance
The following examples illustrate the use of the energy and entropy balances for the
analysis of closed systems. Property relations and property diagrams also contribute
significantly in developing solutions. Example 6.2 reconsiders the system and end
states of Example 6.1 to demonstrate that entropy is produced when internal irrevers-
ibilities are present and that the amount of entropy production is not a property. In
Example 6.3, the entropy balance is used to determine the minimum theoretical com-
pression work.
6.7 Entropy Balance for Closed Systems 297
T > T
f
Temperature
variation
T
f
Boundary of
enlarged system
TAKE NOTE...
On property diagrams,
solid lines are used for inter-
nally reversible processes.
A dashed line signals only
that a process has
occurred between initial
and final equilibrium states
and does not define a path
for the process.
A
A
Entropy_Bal_Closed
_Sys A.24 – All Tabs
c06UsingEntropy.indd Page 297 6/30/10 2:25:59 PM user-s146c06UsingEntropy.indd Page 297 6/30/10 2:25:59 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
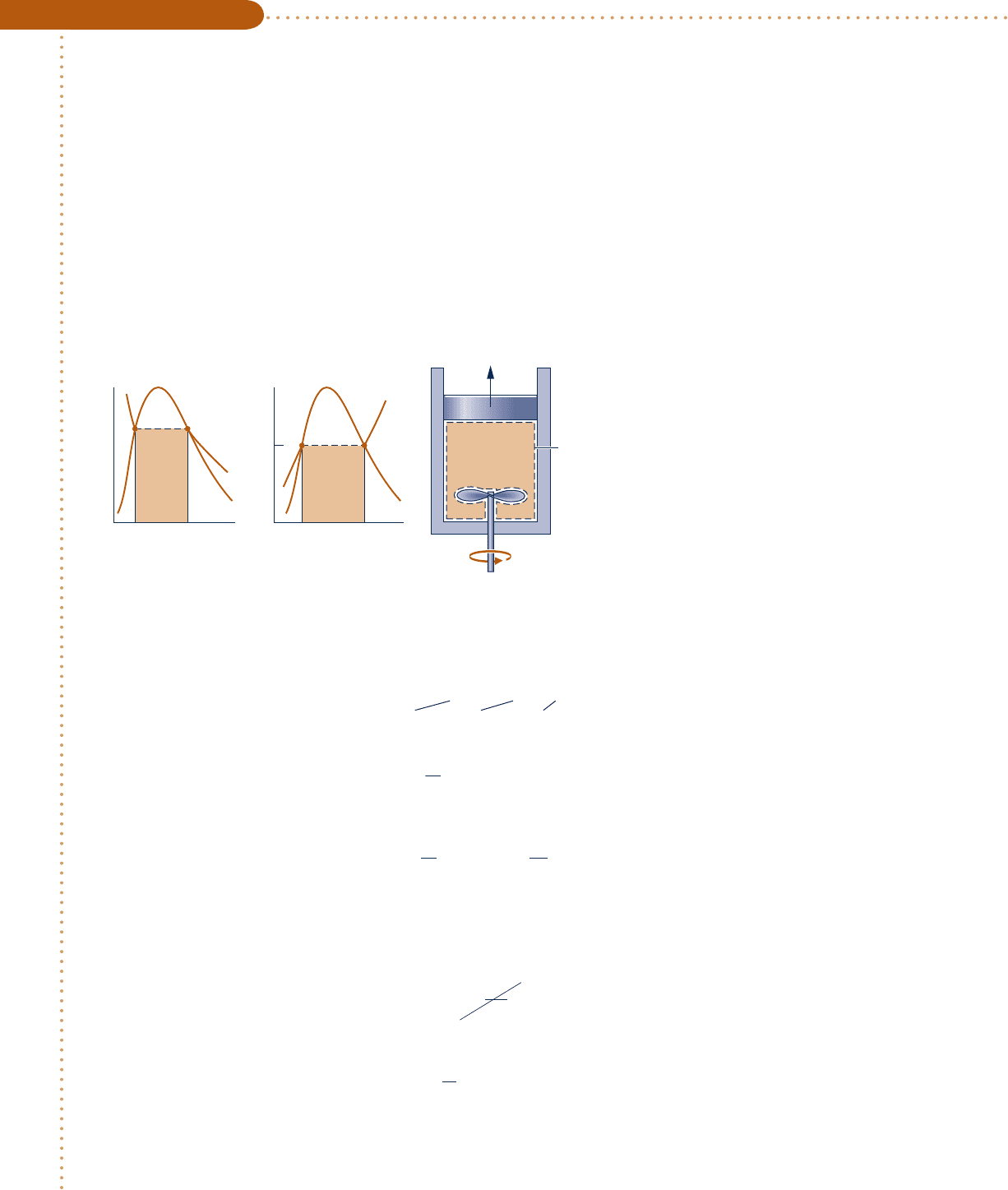
298 Chapter 6 Using Entropy
Determining Work and Entropy Production for an Irreversible Process of Water
c c c c EXAMPLE 6.2 c
Water initially a saturated liquid at 1508C is contained within a piston–cylinder assembly. The water undergoes a
process to the corresponding saturated vapor state, during which the piston moves freely in the cylinder. There is
no heat transfer with the surroundings. If the change of state is brought about by the action of a paddle wheel,
determine the net work per unit mass, in kJ/kg, and the amount of entropy produced per unit mass, in kJ/kg
?
K.
SOLUTION
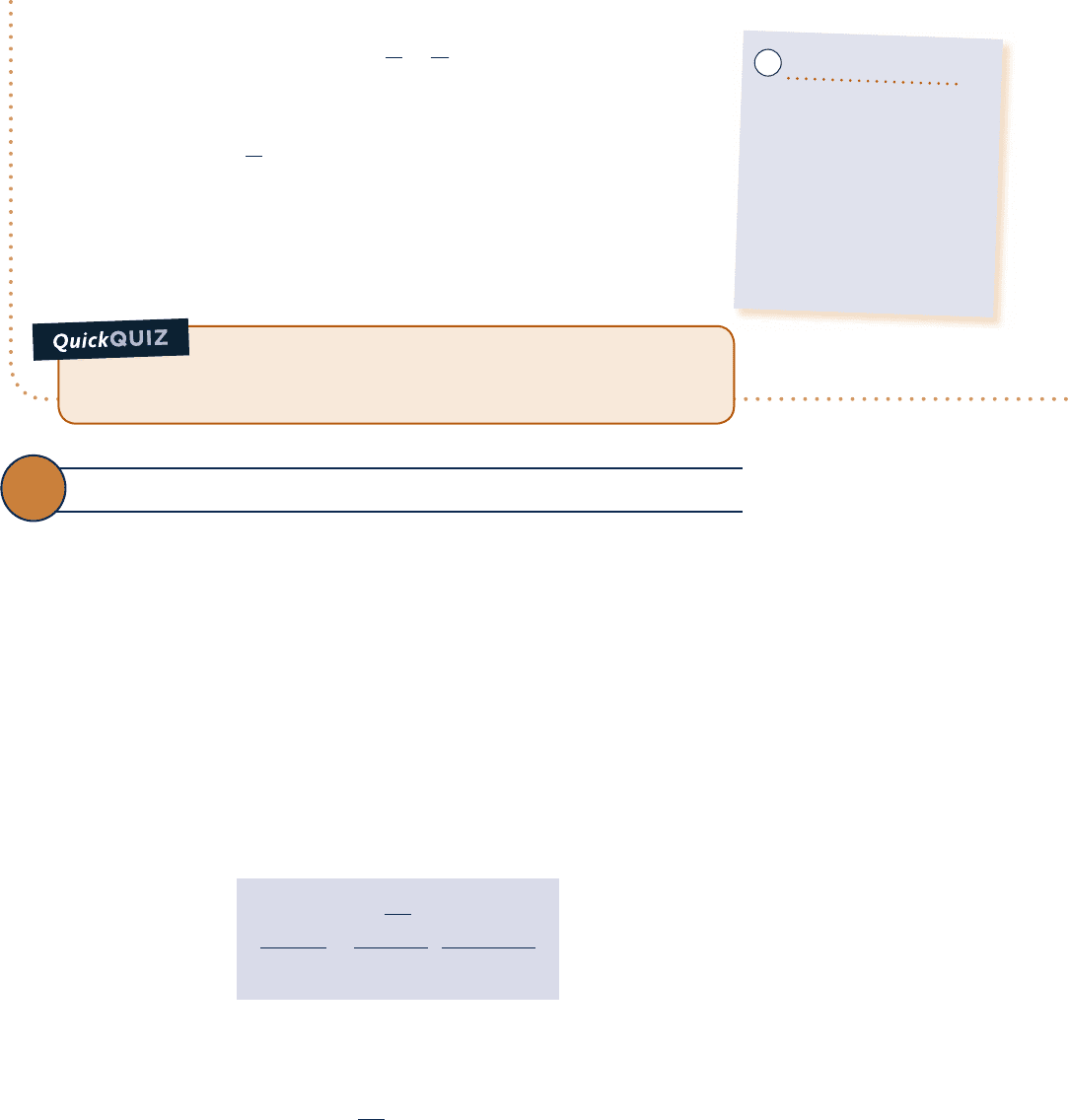
Known:
Water contained in a piston–cylinder assembly undergoes an adiabatic process from saturated liquid to
saturated vapor at 1508C. During the process, the piston moves freely, and the water is rapidly stirred by a
paddle wheel.
Find: Determine the net work per unit mass and the entropy produced per unit mass.
Schematic and Given Data:
Analysis:
As the volume of the system increases during the process, there is an energy transfer by work from
the system during the expansion, as well as an energy transfer by work to the system via the paddle wheel. The
net work can be evaluated from an energy balance, which reduces with assumptions 2 and 3 to
¢U 1 ¢KE
0
1 ¢PE
0
5 Q
0
2
W
On a unit mass basis, the energy balance is then
W
m
521u
2
2 u
1
2
With specific internal energy values from Table A-2 at 1508C, u
1
5 631.68 kJ/kg, u
2
5 2559.5 kJ/kg, we get
W
m
521927.82
kJ
k
g
The minus sign indicates that the work input by stirring is greater in magnitude than the work done by the water
as it expands.
The amount of entropy produced is evaluated by applying the entropy balance Eq. 6.24. Since there is no heat
transfer, the term accounting for entropy transfer vanishes
¢S 5
#
2
1
a
d
Q
T
b
b
0
1 s
On a unit mass basis, this becomes on rearrangement
s
m
5 s
2
2 s
1
Engineering Model:
1.
The water in the piston–
cylinder assembly is a closed
system.
2. There is no heat transfer with
the surroundings.
3. The system is at an equilibrium
state initially and finally. There is
no change in kinetic or potential
energy between these two states.
Fig. E6.2
p
v
150°C
150°C
Area is
not work
T
s
Area is
not heat
Water
System
boundary
1
2
1
2
➊
c06UsingEntropy.indd Page 298 5/26/10 2:40:44 PM user-s146c06UsingEntropy.indd Page 298 5/26/10 2:40:44 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
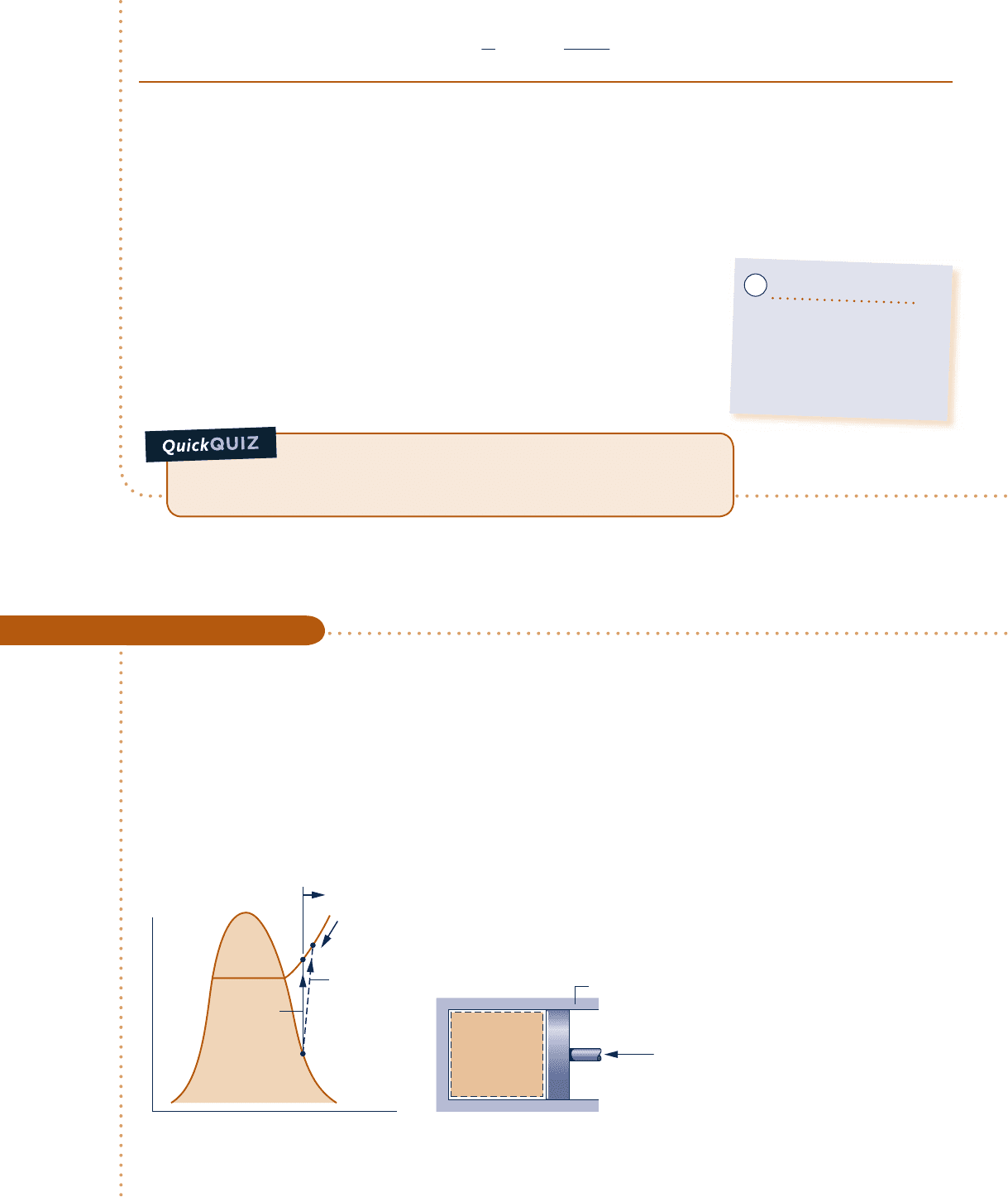
As an illustration of second law reasoning, minimum theoretical compression work
is evaluated in Example 6.3 using the fact that the entropy production term of the
entropy balance cannot be negative.
With specific entropy values from Table A-2 at 1508C, s
1
5 1.8418 kJ/kg
?
K, s
2
5 6.8379 kJ/kg
?
K, we get
➋
s
m
5 4.9961
k
J
k
g
? K
➊
Although each end state is an equilibrium state at the same pressure and temperature, the pressure and
temperature are not necessarily uniform throughout the system at intervening states, nor are they necessarily
constant in value during the process. Accordingly, there is no well-defined “path” for the process. This is
emphasized by the use of dashed lines to represent the process on these p–y and T–s diagrams. The dashed
lines indicate only that a process has taken place, and no “area” should be associated with them. In particu-
lar, note that the process is adiabatic, so the “area” below the dashed line on the T–s diagram can have no
significance as heat transfer. Similarly, the work cannot be associated with an area on the p–y diagram.
➋ The change of state is the same in the present example as in Example 6.1.
However, in Example 6.1 the change of state is brought about by heat trans-
fer while the system undergoes an internally reversible process. Accordingly,
the value of entropy production for the process of Example 6.1 is zero. Here,
fluid friction is present during the process and the entropy production is
positive in value. Accordingly, different values of entropy production are
obtained for two processes between the same end states. This demonstrates
that entropy production is not a property.
Ability to…
❑
apply the closed system
energy and entropy balances.
❑
retrieve property data for
water.
✓
Skills Developed
If the initial and final states were saturation states at 1008C,
determine the net work, in kJ/kg, and the amount of entropy produced, in
kJ/kg
?
K. Ans. 22087.56 kJ/kg, 6.048 kJ/kg
?
K.
6.7 Entropy Balance for Closed Systems 299
Evaluating Minimum Theoretical Compression Work
c c c c EXAMPLE 6.3 c
Refrigerant 134a is compressed adiabatically in a piston–cylinder assembly from saturated vapor at 108F to a final
pressure of 120 lbf/in.
2
Determine the minimum theoretical work input required per unit mass of refrigerant, in Btu/lb.
SOLUTION
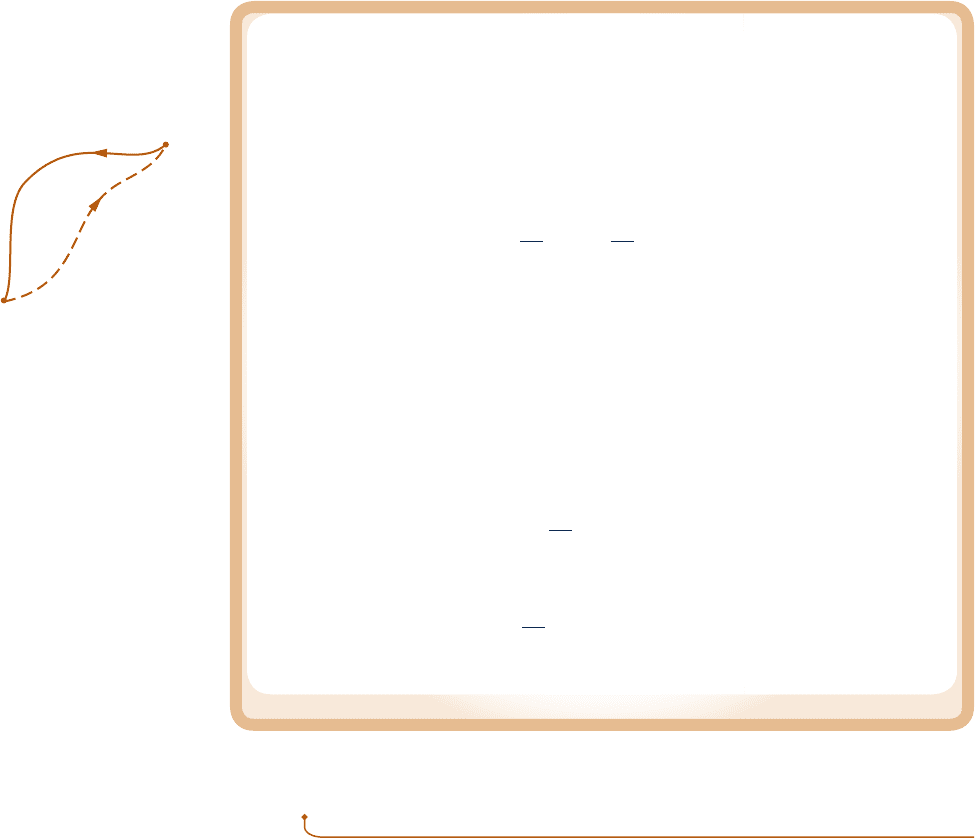
Known:
Refrigerant 134a is compressed without heat transfer from a specified initial state to a specified final pressure.
Find: Determine the minimum theoretical work input required per unit of mass.
Schematic and Given Data:
Engineering Model:
1.
The Refrigerant 134a is a closed
system.
2. There is no heat transfer with the
surroundings.
3. The initial and final states are
equilibrium states. There is no
change in kinetic or potential
energy between these states.
Fig. E6.3
T
Internally
reversible
compression
Actual
compression
s
1
2
2s
Internal
energy
decreases
R-134a
Insulation
Accessible
states
c06UsingEntropy.indd Page 299 5/26/10 2:40:46 PM user-s146c06UsingEntropy.indd Page 299 5/26/10 2:40:46 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
300 Chapter 6 Using Entropy
6.7.4
Closed System Entropy Rate Balance
If the temperature T
b
is constant, Eq. 6.24 reads
S
2
2 S
1
5
Q
T
b
1 s
where Q/T
b
represents the amount of entropy transferred through the portion of the
boundary at temperature T
b
. Similarly, the quantity Q
#
/
T
j
represents the time rate of
Analysis: An expression for the work can be obtained from an energy balance. By applying assumptions 2 and 3,
we get
¢U 1 ¢KE
0
1 ¢PE
0
5 Q
0
2
W
When written on a unit mass basis, the work input is then
a
2
W
m
b
5 u
2
2 u
1
The specific internal energy u
1
can be obtained from Table A-10E as u
1
5 94.68 Btu/lb. Since u
1
is known, the
value for the work input depends on the specific internal energy u
2.
The minimum work input corresponds to
the smallest allowed value for u
2,
determined using the second law as follows.
Applying the entropy balance, Eq. 6.24, we get
¢S 5
#
2
1
a
d
Q
T
b
b
0
1 s
where the entropy transfer term is set equal to zero because the process is adiabatic. Thus, the allowed final
states must satisfy
s
2
2 s
1
5
s
m
$ 0
The restriction indicated by the foregoing equation can be interpreted using the accompanying T–s diagram.
Since s cannot be negative, states with s
2
, s
1
are not accessible adiabatically. When irreversibilities are present
during the compression, entropy is produced, so s
2
. s
1.
The state labeled 2s on the diagram would be attained
in the limit as irreversibilities are reduced to zero. This state corresponds to an isentropic compression.
By inspection of Table A-12E, we see that when pressure is fixed, the specific internal energy decreases as
specific entropy decreases. Thus, the smallest allowed value for u
2
corresponds to state 2s. Interpolating in Table
A-12E at 120 lb/in.
2
, with s
2s
5 s
1
5 0.2214 Btu/lb
?
8R, we find that u
2s
5 107.46 Btu/lb, which corresponds to a
temperature at state 2s of about 1018F. Finally, the minimum work input is
➊
a2
W
m
b
min
5 u
2s
2 u
1
5 107.46 2 94.68 5 12.78 Btu
/
lb
➊ The effect of irreversibilities exacts a penalty on the work input required: A
greater work input is needed for the actual adiabatic compression process
than for an internally reversible adiabatic process between the same initial
state and the same final pressure. See the Quick Quiz to follow.
Ability to…
❑
apply the closed system
energy and entropy balances.
❑
retrieve property data for
Refrigerant 134a.
✓
Skills Developed
If the refrigerant were compressed adiabatically to a final state
where p
2
5 120 lbf/in.
2
, T
2
5 1208F, determine the work input, in Btu/lb,
and the amount of entropy produced, in Btu/lb
?
8R. Ans. 17.16 Btu/lb,
0.0087 Btu/lb
?
8R.
c06UsingEntropy.indd Page 300 5/26/10 5:20:25 PM user-s146c06UsingEntropy.indd Page 300 5/26/10 5:20:25 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
entropy transfer through the portion of the boundary whose instantaneous temperature
is T
j
. This quantity appears in the closed system entropy rate balance considered next.
On a time rate basis, the closed system entropy rate balance is
dS
dt
5
a
j
Q
#
j
T
j
1 s
#
(6.28)
where dS/dt is the time rate of change of entropy of the system. The term Q
#
j
/
T
j
rep-
resents the time rate of entropy transfer through the portion of the boundary whose
instantaneous temperature is T
j
. The term
s
#
accounts for the time rate of entropy
production due to irreversibilities within the system.
To pinpoint the relative significance of the internal and external irreversibilities,
Example 6.4 illustrates the application of the entropy rate balance to a system and
to an enlarged system consisting of the system and a portion of its immediate sur-
roundings.
closed system entropy
rate balance
6.7 Entropy Balance for Closed Systems 301
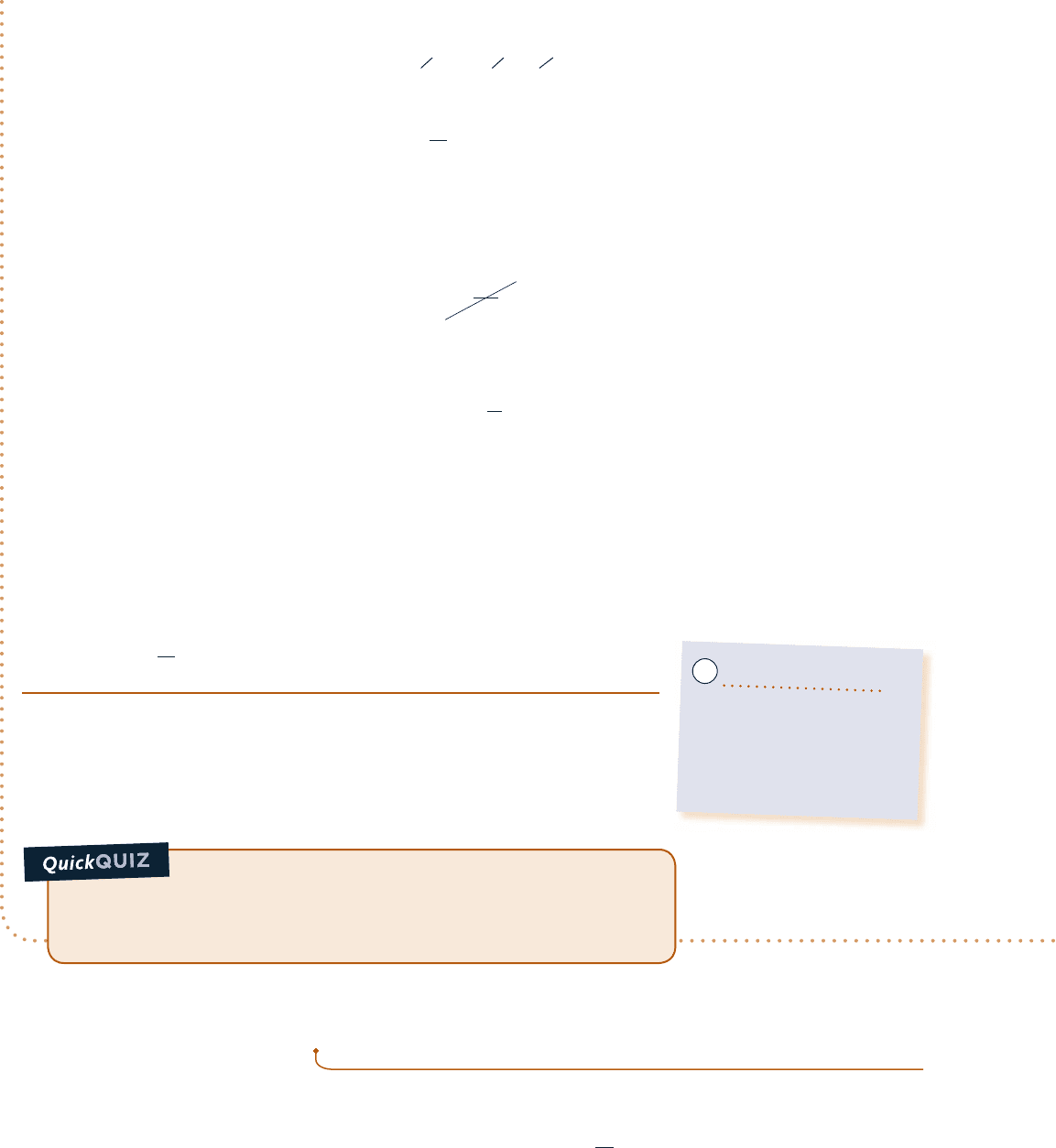
Pinpointing Irreversibilities
c c c c EXAMPLE 6.4 c
Referring to Example 2.4, evaluate the rate of entropy production s
#
, in kW/K, for (a) the gearbox as the system
and (b) an enlarged system consisting of the gearbox and enough of its surroundings that heat transfer occurs
at the temperature of the surroundings away from the immediate vicinity of the gearbox, T
f
5 293 K (208C).
SOLUTION
Known:
A gearbox operates at steady state with known values for the power input through the high-speed shaft,
power output through the low-speed shaft, and heat transfer rate. The temperature on the outer surface of the
gearbox and the temperature of the surroundings away from the gearbox are also known.
Find: Evaluate the entropy production rate s
#
for each of the two specified systems shown in the schematic.
Schematic and Given Data:
Engineering Model:
1.
In part (a), the gearbox is taken as a closed system operating at steady state, as shown on the
accompanying sketch labeled with data from Example 2.4.
2. In part (b) the gearbox and a portion of its surroundings are taken as a closed system, as shown on the
accompanying sketch labeled with data from Example 2.4.
3. The temperature of the outer surface of the gearbox and the temperature of the surroundings do not vary.
Fig. E6.4
System
boundary
Q
= –1.2 kW
58.8 kW
60 kW
Gearbox
T
b
= 300 K
58.8 kW
60 kW
T
b
T
f
At this
boundary the
temperature is
T
f
= 293 K
Temperature
variation
(a)(b)
c06UsingEntropy.indd Page 301 5/26/10 2:40:50 PM user-s146c06UsingEntropy.indd Page 301 5/26/10 2:40:50 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
302 Chapter 6 Using Entropy
If the power delivered were 59.32 kW, evaluate the outer sur-
face temperature, in K, and the rate of entropy production, in kW/K, for
the gearbox as the system, keeping input power, h, and A from Example 2.4
the same. Ans. 297 K, 2.3 3 10
23
kW/K.
Analysis:
(a)
To obtain an expression for the entropy production rate, begin with the entropy balance for a closed system
on a time rate basis: Eq. 6.28. Since heat transfer takes place only at temperature T
b
, the entropy rate balance
reduces at steady state to
dS
0
dt
5
Q
#
T
b
1 s
#
Solving
s
#
52
Q
#
T
b
Introducing the known values for the heat transfer rate
Q
#
and the surface temperature T
b
s
#
52
1
21.2 kW
2
1
300 K
2
5 4 3 10
23
kW
/
K
(b) Since heat transfer takes place at temperature T
f
for the enlarged system, the entropy rate balance reduces
at steady state to
dS
0
dt
5
Q
#
T
f
1 s
#
Solving
s
#
52
Q
#
T
f
Introducing the known values for the heat transfer rate
Q
#
and the temperature T
f
➊ s
#
52
1
21.2 kW
2
1
293 K
2
5 4.1 3 10
23
kW
/
K
➊ The value of the entropy production rate calculated in part (a) gauges the
significance of irreversibilities associated with friction and heat transfer
within the gearbox. In part (b), an additional source of irreversibility is
included in the enlarged system, namely the irreversibility associated with
the heat transfer from the outer surface of the gearbox at T
b
to the sur-
roundings at T
f
. In this case, the irreversibilities within the gearbox are dom-
inant, accounting for about 98% of the total rate of entropy production.
Ability to…
❑
apply the closed system
entropy rate balance.
❑
develop an engineering model.
✓
Skills Developed
6.8 Directionality of Processes
Our study of the second law of thermodynamics began in Sec. 5.1 with a discussion of
the directionality of processes. In this section we consider two related aspects for which
there are significant applications: the increase in entropy principle and a statistical
interpretation of entropy.
6.8.1
Increase of Entropy Principle
In the present discussion, we use the closed system energy and entropy balances to
introduce the increase of entropy principle. Discussion centers on an enlarged system
c06UsingEntropy.indd Page 302 5/26/10 2:40:53 PM user-s146c06UsingEntropy.indd Page 302 5/26/10 2:40:53 PM user-s146 /Users/user-s146/Desktop/Merry_X-Mas/New/Users/user-s146/Desktop/Merry_X-Mas/New
