Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

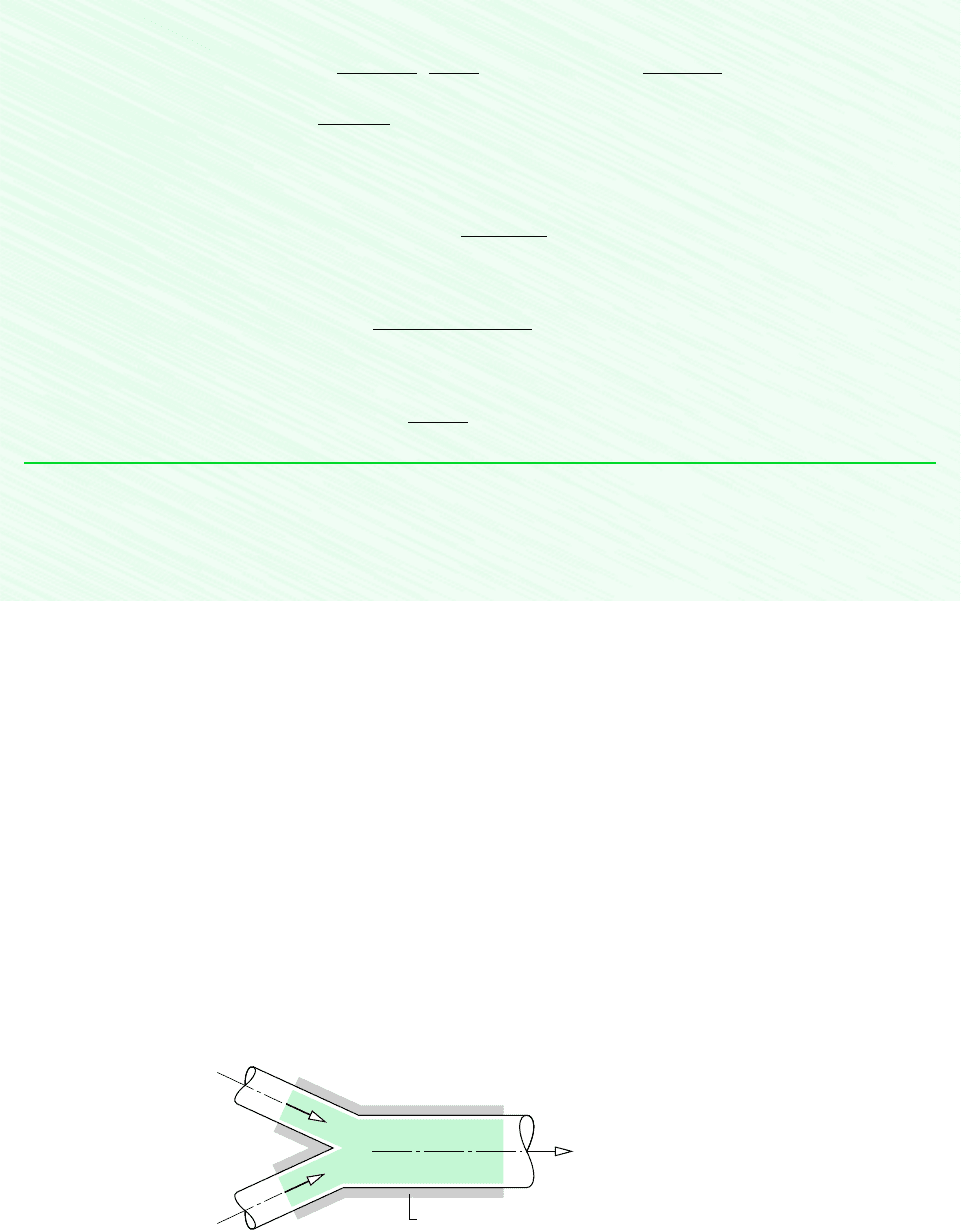
606 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
12.8.6 Adiabatic Mixing of Two Moist Air Streams
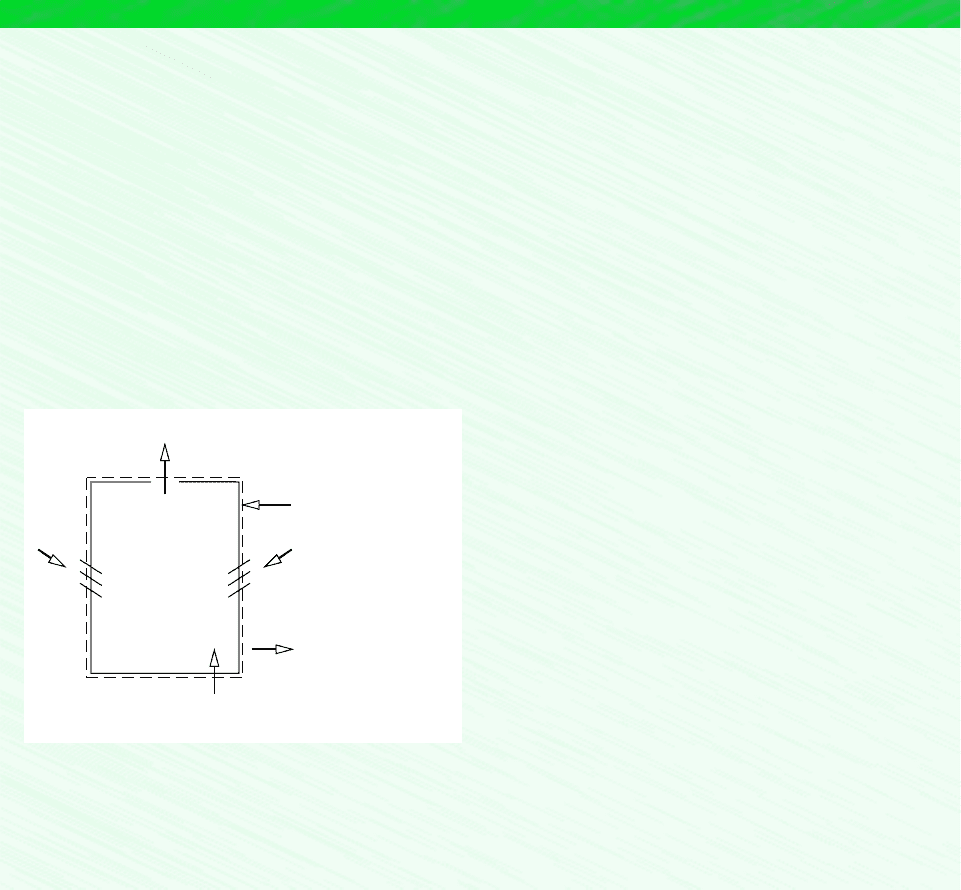
A common process in air-conditioning systems is the mixing of moist air streams, as shown
in Fig. 12.14. The objective of the thermodynamic analysis of such a process is normally
to fix the flow rate and state of the exiting stream for specified flow rates and states of
each of the two inlet streams. The case of adiabatic mixing is governed by Eqs. 12.56
to follow.
The mass rate balances for the dry air and water vapor at steady state are, respectively,
(12.56a)
With , the water vapor mass balance becomes
(12.56b)v
1
m
#
a1
v
2
m
#
a2
v
3
m
#
a3
1water vapor2
m
#
v
vm
#
a
m
#
v1
m
#
v2
m
#
v3
1water vapor2
m
#
a1
m
#
a2
m
#
a3
1dry air2
Substituting values for ,
1
, and
2
into the expression for
(b) The relative humidity of the moist air at the exit can be determined using Eq. 12.44. The partial pressure of the water va-
por required by this expression can be found by solving Eq. 12.43 to obtain
Inserting values
At 21C, the saturation pressure is 0.02487 bar. Thus, the relative humidity at the exit is
Since the underlined term in this equation is much smaller than either of the moist air enthalpies, the enthalpy of the moist
air remains nearly constant, and thus evaporative cooling takes place at nearly constant wet-bulb temperature. This can be
verified by locating the incoming and outgoing moist air states on the psychrometric chart.
A constant value of the specific heat c
pa
has been used here to evaluate the term (h
a1
h
a2
). As shown in previous exam-
ples, this term can be evaluated alternatively using the ideal gas table for air.
f
2
0.0176
0.02487
0.708
170.8% 2
p
v2
10.0112
11.01325 bars2
1.011 0.6222
0.0176 bar
p
v2
v
2
p
v
2
0.622
65.5
kg1water2
h
m
#
w
c157.8
kg1dry air2
min
`
60 min
1 h
`d 10.011 0.004082
kg1water2
kg1dry air2
m
#
w
m
#
a
❶
❷
Figure 12.14 Adiabatic mixing of two
moist air streams.
3
Insulation
1 m
·
a1
, T
1
,
1
ω
2 m
·
a2
, T
2
,
2
ω
m
·
a3
T
3
3
ω
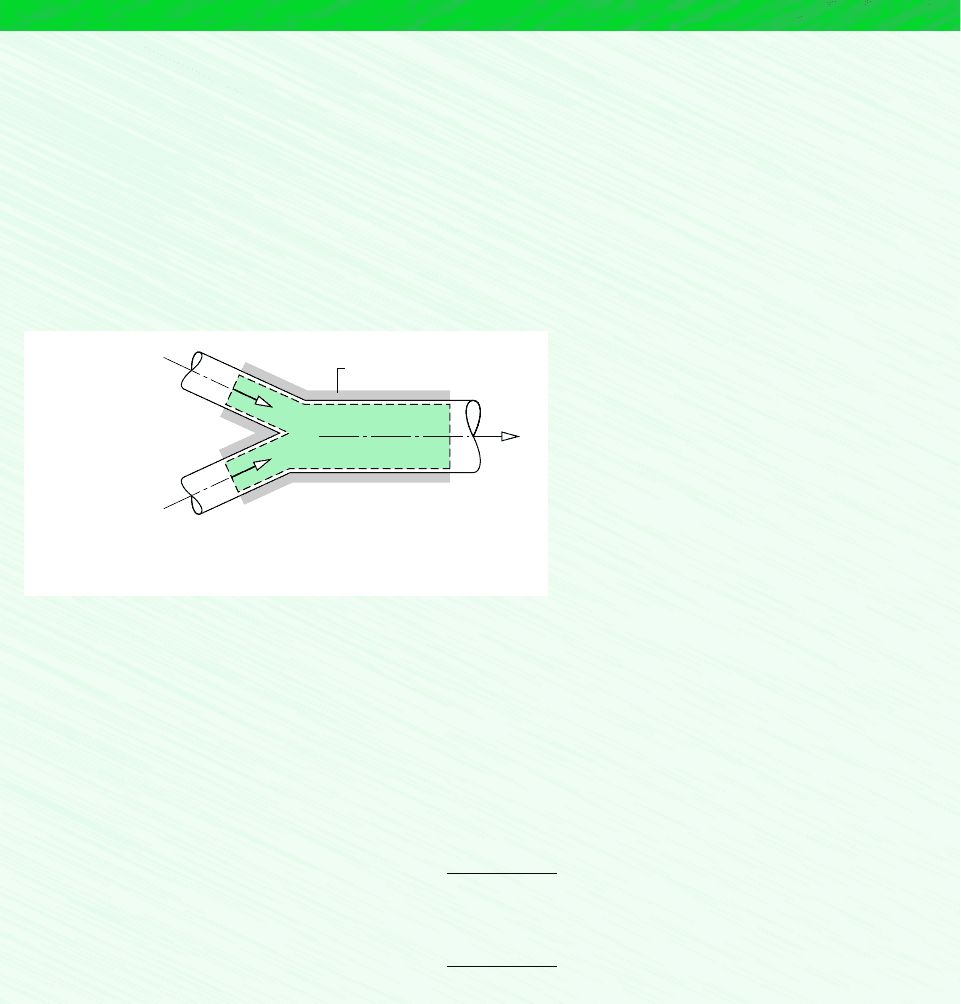
12.8 Analyzing Air-Conditioning Processes 607
Assuming and ignoring the effects of kinetic and potential energy, the
energy rate balance reduces at steady state to
(12.56c)
where the enthalpies of the entering and exiting water vapor are evaluated as the saturated
vapor values at the respective dry-bulb temperatures.
If the inlet flow rates and states are known, Eqs. 12.56 are three equations in three unknowns:
, and (h
a3
3
h
g3
). The solution of these equations is illustrated by the next example.m
#
a3
, v
3
m
#
a1
1h
a1
v
1
h
g1
2 m
#
a2
1h
a2
v
2
h
g2
2 m
#
a3
1h
a3
v
3
h
g3
2
Q
#
cv
W
#
cv
0
EXAMPLE 12.14 Adiabatic Mixing of Moist Streams
A stream consisting of 142 m
3
/min of moist air at a temperature of 5C and a humidity ratio of 0.002 kg(vapor)kg(dry air)
is mixed adiabatically with a second stream consisting of 425 m
3
/min of moist air at 24C and 50% relative humidity. The
pressure is constant throughout at 1 bar. Using the psychrometric chart, determine
(a) the humidity ratio and (b) the temper-
ature of the exiting mixed stream, in C.
SOLUTION
Known: A moist air stream at 5C, 0.002 kg(vapor)kg(dry air), and a volumetric flow rate of 142 m
3
/min is mixed
adiabatically with a stream consisting of 425 m
3
/min of moist air at 24C and 50%.
Find: Determine the humidity ratio and the temperature, in C, of the mixed stream exiting the control volume.
Schematic and Given Data:
3
1
2
kg (vapor)
__________
kg (dry air)
(AV)
1
T
1
1
= 142 m
3
/min
= 5°C
= 0.002
ω
(AV)
2
T
2
2
= 425 m
3
/min
= 24°C
= 50%
φ
T
3
= ?
3
= ?
ω
Insulation
Assumptions:
1. The control volume shown in the accompany-
ing figure operates at steady state. Changes in ki-
netic and potential energy can be neglected and
2. There is no heat transfer with the surroundings.
3. The pressure remains constant throughout at
1 bar.
W
#
cv
0.
Figure E12.14
Analysis:
(a) The humidity ratio
3
can be found by means of mass rate balances for the dry air and water vapor, respectively
With and the second of these balances becomes
Solving
Since this can be expressed as
v
3
v
1
m
#
a1
v
2
m
#
a2
m
#
a1
m
#
a2
m
#
a3
m
#
a1
m
#
a2
,
v
3
v
1
m
#
a1
v
2
m
#
a2
m
#
a3
v
1
m
#
a1
v
2
m
#
a2
v
3
m
#
a3
m
#
v3
v
3
m
#
a3
,m
#
v1
v
1
m
#
a1
, m
#
v2
v
2
m
#
a2
,
m
#
v1
m
#
v2
m
#
v3
1water vapor2
m
#
a1
m
#
a2
m
#
a3
1dry air2
608 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
To determine
3
requires values for
2
, and The mass flow rates of the dry air, and can be found as in
previous examples using the given volumetric flow rates
The values of v
a1
, and v
a2
, and
2
are readily found from the psychrometric chart, Fig. A-9. Thus, at
1
0.002 and T
1
5C, v
a1
0.79 m
3
/kg(dry air). At
2
50% and T
2
24C, v
a2
0.855 m
3
/kg(dry air) and
2
0.0094. The mass flow
rates of the dry air are then 180 kg(dry air)/min and 497 kg(dry air)/min. Inserting values into the expression
for
3
(b) The temperature T
3
of the exiting mixed stream can be found from an energy rate balance. Reduction of the energy rate
balance using assumptions 1 and 2 gives
(1)
Solving
(2)
With (h
a
h
v
)
1
10 kJ/kg(dry air) and (h
a
h
v
)
2
47.8 kJ/kg(dry air) from Fig. A-9 and other known values
This value for the enthalpy of the moist air at the exit, together with the previously determined value for
3
, fixes the state of
the exiting moist air. From inspection of Fig. A-9, T
3
19C.
Alternative Solutions:
The use of the psychrometric chart facilitates the solution for T
3
. Without the chart, an iterative solution of Eq. (2) using
table data could be used as noted in the solution to Example 12.12. Alternatively, T
3
can be determined using the following
IT program, where
2
is denoted as phi2, the volumetric flow rates at 1 and 2 are denoted as AV1 and AV2, respectively,
and so on.
// Given data
T1 = 5 // C
w1 = 0.002 // kg(vapor) / kg(dry air)
AV1 = 142 // m
3
/min
T2 = 24 // C
phi2 = 0.5
AV2 = 425 // m
3
/min
p = 1 // bar
// Mass balances for water vapor and dry air:
w1 * mdota1 + w2 * mdota2 = w3 * mdota3
mdota1 + mdota2 = mdota3
// Evaluate mass flow rates of dry air
mdota1 = AV1 / va1
va1 = va_Tw(T1, w1, p)
mdota2 = AV2 / va2
va2 = va_Tphi(T2, phi2, p)
// Determine w2
w2 = w_Tphi(T2, phi2, p)
1h
a
vh
v
2
3
1801102 497147.82
180 497
37.7
kJ
kg1dry air2
1h
a
vh
v
2
3
m
#
a1
1h
a
vh
v
2
1
m
#
a2
1h
a
vh
v
2
2
m
#
a1
m
#
a2
m
#
a1
1h
a
vh
v
2
1
m
#
a2
1h
a
vh
v
2
2
m
#
a3
1h
a
vh
v
2
3
v
3
10.0022
11802 10.00942 14972
180 497
0.0074
kg1vapor2
kg1dry air2
m
#
a2
m
#
a1
m
#
a1
1AV2
1
v
a1
,
m
#
a2
1AV2
2
v
a2
m
#
a2
,m
#
a1
m
#
a2
.m
#
a1
,
❶
12.9 Cooling Towers 609
// The energy balance, Eq. (1), reads
mdota1 * h1 + mdota2 * h2 = mdota3 * h3
h1 = ha_Tw(T1, w1)
h2 = ha_Tphi(T2, phi2, p)
h3 = ha_Tw(T3, w3)
Using the Solve button, the result is T
3
19.01C and
3
0.00745 kg (vapor)kg (dry air), which agree with the psychro-
metric chart solution.
Note the use here of special Moist Air functions listed in the Properties menu of IT.
❶
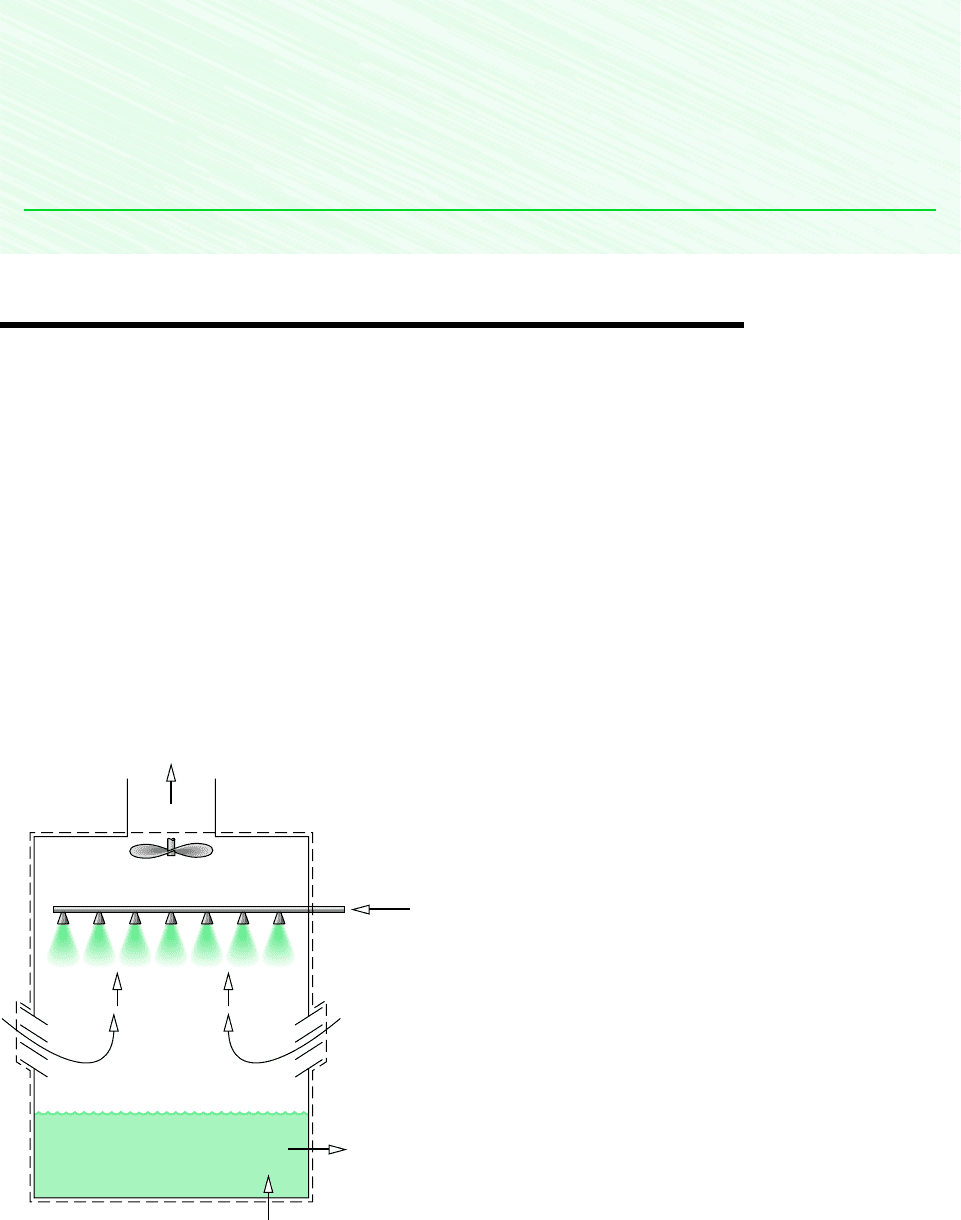
12.9 Cooling Towers
Power plants invariably discharge considerable energy to their surroundings by heat transfer
(Chap. 8). Although water drawn from a nearby river or lake can be employed to carry away
this energy, cooling towers provide an alternative in locations where sufficient cooling water
cannot be obtained from natural sources or where concerns for the environment place a limit
on the temperature at which cooling water can be returned to the surroundings. Cooling tow-
ers also are frequently employed to provide chilled water for applications other than those
involving power plants.
Cooling towers can operate by natural or forced convection. Also they may be counterflow,
cross-flow, or a combination of these. A schematic diagram of a forced-convection, coun-
terflow cooling tower is shown in Fig. 12.15. The warm water to be cooled enters at 1 and
is sprayed from the top of the tower. The falling water usually passes through a series of baf-
fles intended to keep it broken up into fine drops to promote evaporation. Atmospheric air
drawn in at 3 by the fan flows upward, counter to the direction of the falling water droplets.
Fan
Warm water inlet
T
1
, m
·
w
1
Return water
m
·
w
T
2
< T
1
2
4
5 Makeup
water
3
Liquid
Discharged moist air
m
·
a
, T
4
,
4
>
3
ωω
Atmospheric air
m
·
a
, T
3
,
3
ω
Figure 12.15 Schematic of a
cooling tower.
610 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
As the two streams interact, a small fraction of the water stream evaporates into the moist
air, which exits at 4 with a greater humidity ratio than the incoming moist air at 3. The en-
ergy required for evaporation is provided mainly by the portion of the incoming water stream
that does not evaporate, with the result that the water exiting at 2 is at a lower temperature
than the water entering at 1. Since some of the incoming water is evaporated into the moist
air stream, an equivalent amount of makeup water is added at 5 so that the return mass flow
rate of the cool water equals the mass flow rate of the warm water entering at 1.
For operation at steady state, mass balances for the dry air and water and an energy bal-
ance on the overall cooling tower provide information about cooling tower performance. In
applying the energy balance, heat transfer with the surroundings is usually neglected. The
power input to the fan of forced-convection towers also may be negligible relative to other
energy rates involved. The example to follow illustrates the analysis of a cooling tower us-
ing conservation of mass and energy together with property data for the dry air and water.
EXAMPLE 12.15 Power Plant Cooling Tower
Water exiting the condenser of a power plant at 38C enters a cooling tower with a mass flow rate of 4.5 10
7
kg/h. A stream
of cooled water is returned to the condenser from a cooling tower with a temperature of 30C and the same flow rate. Makeup
water is added in a separate stream at 20C. Atmospheric air enters the cooling tower at 25C and 35% relative humidity.
Moist air exits the tower at 35C and 90% relative humidity. Determine the mass flow rates of the dry air and the makeup
water, in kg/h. The cooling tower operates at steady state. Heat transfer with the surroundings and the fan power can each be
neglected, as can changes in kinetic and potential energy. The pressure remains constant throughout at 1 atm.
SOLUTION
Known: A liquid water stream enters a cooling tower from a condenser at 38C with a known mass flow rate. A stream of
cooled water is returned to the condenser at 30C and the same flow rate. Makeup water is added at 20C. Atmospheric air
enters the tower at 25C and 35%. Moist air exits the tower at 35C and 90%.
Find: Determine the mass flow rates of the dry air and the makeup water, in kg/h.
Schematic and Given Data:
1
3
4
2
5
Makeup water
T
5
= 20°C
Atmospheric air
T
3
= 25°C,
3
= 35%
φ
Liquid water, T
1
= 38°C
m
·
1
= 4.5 × 10
7
kg/h
Liquid water, T
2
= 30°C
m
·
2
= 4.5 × 10
7
kg/h
Moist air
φ
T
4
= 35°C
4
= 90%
Assumptions:
1. The control volume shown in the accompanying figure op-
erates at steady state. Heat transfer with the surroundings can
be neglected, as can changes in kinetic and potential energy;
also
2. To evaluate specific enthalpies, each liquid stream is
regarded as a saturated liquid at the corresponding specified
temperature.
3. The pressure is constant throughout at 1 atm.
W
#
cv
0.
Figure E12.15
Analysis: The required mass flow rates can be found from mass and energy rate balances. Mass balances for the dry air and
water individually reduce at steady state to
m
#
1
m
#
5
m
#
v3
m
#
2
m
#
v4
1water2
m
#
a3
m
#
a4
1dry air2

Chapter Summary and Study Guide 611
The common mass flow rate of the dry air is denoted as Since the second of these equations becomes
With and
Accordingly, the two required mass flow rates, and are related by this equation. Another equation relating the flow rates
is provided by the energy rate balance.
Reducing the energy rate balance with assumption 1 results in
Evaluating the enthalpies of the water vapor as the saturated vapor values at the respective temperatures and the enthalpy of
each liquid stream as the saturated liquid enthalpy at the respective temperature, the energy rate equation becomes
Introducing and and solving for
The humidity ratios
3
and
4
required by this expression can be determined from Eq. 12.43, using the partial pressure of
the water vapor obtained with the respective relative humidity. Thus,
3
0.00688 kg(vapor)kg(dry air) and
4
0.0327
kg(vapor)kg(dry air).
With enthalpies from Tables A-2 and A-22, as appropriate, and the known values for
3
,
4
, and , the expression for
becomes
Finally, inserting known values into the expression for results in
This expression for can be rearranged to read
The underlined terms and
3
and
4
can be obtained by inspection of the psychrometric chart.
m
#
a
m
#
1
1h
f1
h
f2
2
1h
a4
v
4
h
g4
2 1h
a3
v
3
h
g3
2 1v
4
v
3
2
h
f5
m
#
a
m
#
5
12.03 10
7
210.0327 0.006882 5.24 10
5
kg/h
m
#
5
2.03 10
7
kg/h
m
#
a
14.5 10
7
2
1159.21 125.792
1308.2 298.22 10.03272 12565.32 10.006882 12547.22 10.02582 183.962
m
#
a
m
#
1
m
#
a
m
#
1
1h
f1
h
f2
2
h
a4
h
a3
v
4
h
g4
v
3
h
g3
1v
4
v
3
2
h
f5
m
#
a
m
#
v4
v
4
m
#
a
m
#
1
m
#
2
, m
#
5
m
#
a
1v
4
v
3
2, m
#
v3
v
3
m
#
a
,
0 m
#
1
h
f1
1m
#
a
h
a3
m
#
v3
h
g3
2 m
#
5
h
f5
m
#
2
h
f2
1m
#
a
h
a4
m
#
v4
h
g4
2
0 m
#
1
h
w1
1m
#
a
h
a3
m
#
v3
h
v3
2 m
#
5
h
w5
m
#
2
h
w2
1m
#
a
h
a4
m
#
v4
h
v4
2
m
#
5
,m
#
a
m
#
5
m
#
a
1v
4
v
3
2
m
#
v4
v
4
m
#
a
m
#
v3
v
3
m
#
a
m
#
5
m
#
v4
m
#
v3
m
#
1
m
#
2
,m
#
a
.
❶
❶
Chapter Summary and Study Guide
In this chapter we have applied the principles of thermody-
namics to systems involving ideal gas mixtures, including the
special case of psychrometric applications involving air–water
vapor mixtures, possibly in the presence of liquid water. Both
closed system and control volume applications are presented.
The first part of the chapter deals with general ideal gas
mixture considerations and begins by describing mixture
composition in terms of the mass fractions or mole fractions.
Two models are then introduced for the p–v–T relation of ideal
gas mixtures: the Dalton model, which includes the partial
pressure concept, and the Amagat model. Means are also
introduced for evaluating the enthalpy, internal energy, and
entropy of a mixture by adding the contribution of each
component at its condition in the mixture. Applications are
considered where ideal gas mixtures undergo processes at
constant composition as well as where ideal gas mixtures are
formed from their component gases.
In the second part of the chapter, we study psychrometrics.
Special terms commonly used in psychrometrics are
introduced, including moist air, humidity ratio, relative
humidity, mixture enthalpy, and the dew point, dry-bulb, and
wet-bulb temperatures. The psychrometric chart, which gives
612 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
Key Engineering Concepts
mass fraction p. 559
mole fraction p. 559
apparent molecular
weight p. 559
Dalton model p. 563
partial pressure p. 563
psychrometrics p. 579
moist air p. 579
humidity ratio p. 581
relative humidity p. 581
mixture enthalpy p. 582
dew point temperature
p. 583
dry-bulb temperature
p. 590
wet-bulb temperature
p. 590
psychrometric chart
p. 592
Exercises: Things Engineers Think About
1. In an equimolar mixture of O
2
and N
2
, are the mass fractions
equal?
2. Is it possible to have a two-component mixture in which the
mass and mole fractions are equal?
3. How would you calculate the specific heat ratio, k, at 300 K
of a mixture of N
2
,O
2
, and CO
2
if the molar analysis were known?
4. How would you evaluate the entropy change of a component
in an ideal gas mixture undergoing a process between states where
the temperature and pressure are T
1
, p
1
and T
2
, p
2
, respectively?
5. If two samples of the same gas at the same temperature and
pressure were mixed adiabatically, would entropy be produced?
6. Which component of the fuel–air mixture in a cylinder of an
automobile engine would have the greater mass fraction?
7. Is it possible for a control volume operating at steady state
with to take in a binary ideal gas mixture at tem-
perature T and pressure p and separate the mixture into compo-
nents, each at T and p?
W
#
cv
Q
#
cv
0
8. Which do you think is most closely related to human comfort,
the humidity ratio or the relative humidity?
9. How do you explain the different rates of evaporation from a
dish of water in winter and summer?
10. Why does your bathroom mirror often fog up when you
shower?
11. Although water vapor in air is typically a superheated vapor,
why can we use the saturated vapor value, h
g
(T ), to represent its
enthalpy?
12. Can the dry-bulb and wet-bulb temperatures be equal?
13. How do you explain the water dripping from the tailpipe of
an automobile on a cold morning?
14. How does an automobile’s windshield defroster achieve its
purpose?
15. Would you recommend an evaporative cooling system for
use in Florida? In Arizona?
a graphical representation of important moist air properties,
is introduced. The principles of conservation of mass and en-
ergy are formulated in terms of psychrometric quantities, and
typical air-conditioning applications are considered, includ-
ing dehumidification and humidification, evaporative cooling,
and mixing of moist air streams. A discussion of cooling tow-
ers is also provided.
The following list provides a study guide for this chapter.
When your study of the text and end-of-chapter exercises has
been completed, you should be able to
write out the meanings of the terms listed in the margin
throughout the chapter and understand each of the
related concepts. The subset of key concepts listed below
is particularly important.
describe mixture composition in terms of mass fractions
or mole fractions.
relate pressure, volume, and temperature of ideal gas
mixtures using the Dalton model, and evaluate U, H, c
v
,
c
p
, and S of ideal gas mixtures in terms of the mixture
composition and the respective contribution of each
component.
apply the conservation of mass and energy principles and
the second law of thermodynamics to systems involving
ideal gas mixtures.
For psychrometric applications, you should be able to
evaluate the humidity ratio, relative humidity, mixture
enthalpy, and dew point temperature.
use the psychrometric chart.
apply the conservation of mass and energy principles
and the second law of thermodynamics to analyze air-
conditioning processes and cooling towers.

Problems: Developing Engineering Skills 613
Problems: Developing Engineering Skills
Determining Mixture Composition
12.1 Answer the following questions involving a mixture of two
gases:
(a) When would the analysis of the mixture in terms of mass
fractions be identical to the analysis in terms of mole
fractions?
(b) When would the apparent molecular weight of the mix-
ture equal the average of the molecular weights of the
two gases?
12.2 The molar analysis of a gas mixture at 30C, 2 bar is 40%
N
2
, 50% CO
2
, 10% CH
4
. Determine
(a) the analysis in terms of mass fractions.
(b) the partial pressure of each component, in bar.
(c) the volume occupied by 10 kg of mixture, in m
3
.
12.3 The molar analysis of a gas mixture at 25C, 0.1 MPa is
60% N
2
, 30% CO
2
, 10% O
2
. Determine
(a) the analysis in terms of mass fractions.
(b) the partial pressure of each component, in MPa.
(c) the volume occupied by 50 kg of the mixture, in m
3
.
12.4 Natural gas at 23C, 1 bar enters a furnace with the
following molar analysis: 40% propane (C
3
H
8
), 40% ethane
(C
2
H
6
), 20% methane (CH
4
). Determine
(a) the analysis in terms of mass fractions.
(b) the partial pressure of each component, in bar.
(c) the mass flow rate, in kg/s, for a volumetric flow rate of
20 m
3
/s.
12.5 A rigid vessel having a volume of 3 m
3
initially contains
a mixture at 21C, 1 bar consisting of 79% N
2
and 21% O
2
on
a molar basis. Helium is allowed to flow into the vessel until
the pressure is 2 bar. If the final temperature of the mixture
within the vessel is 27C, determine the mass, in kg, of each
component present.
12.6 A vessel having a volume of 0.3 m
3
contains a mixture at
40C, 6.9 bar with a molar analysis of 75% O
2
, 25% CH
4
. De-
termine the mass of methane that would have to be added and
the mass of oxygen that would have to be removed, each in
kg, to obtain a mixture having a molar analysis of 30% O
2
,
70% CH
4
at the same temperature and pressure.
12.7 A control volume operating at steady state has two en-
tering streams and a single exiting stream. A mixture with a
mass flow rate of 11.67 kg/min and a molar analysis 9% CH
4
,
91% air enters at one location and is diluted by a separate
stream of air entering at another location. The molar analysis
of the air is 21% O
2
, 79% N
2
. If the mole fraction of CH
4
in
the exiting stream is required to be 5%, determine
(a) the molar flow rate of the entering air, in kmol/min.
(b) the mass flow rate of oxygen in the exiting stream, in
kg/min.
Considering Constant-Composition Processes
12.8 A gas mixture consists of 2 kg of N
2
and 3 kg of He
Determine
(a) the composition in terms of mass fractions.
(b) the composition in terms of mole fractions.
(c) the heat transfer, in kJ, required to increase the mixture
temperature from 21 to 66C, while keeping the pressure
constant.
(d) the change in entropy of the mixture for the process of part
(c), in kJ/K.
For parts (c) and (d), use the ideal gas model with constant
specific heats.
12.9 Two kg of a mixture having an analysis on a mass basis
of 30% N
2
, 40% CO
2
, 30% O
2
is compressed adiabatically
from 1 bar, 300 K to 4 bar, 500 K. Determine
(a) the work, in kJ.
(b) the amount of entropy produced, in kJ/K.
12.10 A mixture having a molar analysis of 50% CO
2
, 33.3%
CO, and 16.7% O
2
enters a compressor operating at steady
state at 37C, 1 bar, 40 m/s with a mass flow rate of 1 kg/s
and exits at 237C, 30 m/s. The rate of heat transfer from the
compressor to its surroundings is 5% of the power input.
(a) Neglecting potential energy effects, determine the power
input to the compressor, in kW.
(b) If the compression is polytropic, evaluate the polytropic
exponent n and the exit pressure, in bar.
12.11 A mixture of 2 kg of H
2
and 4 kg of N
2
is compressed
in a piston–cylinder assembly in a polytropic process for which
n 1.2. The temperature increases from 22 to 150C. Using
constant values for the specific heats, determine
(a) the heat transfer, in kJ.
(b) the entropy change, in kJ/K.
12.12 A gas turbine receives a mixture having the following
molar analysis: 10% CO
2
, 19% H
2
O, 71% N
2
at 720 K,
0.35 MPa and a volumetric flow rate of 3.2 m
3
/s. Products exit
the turbine at 380 K, 0.11 MPa. For adiabatic operation with
negligible kinetic and potential energy effects, determine the
power developed at steady state, in kW.
12.13 A gas mixture at 1500 K with the molar analysis 10%
CO
2
, 20% H
2
O, 70% N
2
enters a waste-heat boiler operating
at steady state, and exits the boiler at 600 K. A separate stream
of saturated liquid water enters at 25 bar and exits as saturated
vapor with a negligible pressure drop. Ignoring stray heat trans-
fer and kinetic and potential energy changes, determine the
mass flow rate of the exiting saturated vapor, in kg per kmol
of gas mixture.
12.14 A gas mixture having a molar analysis of 60% O
2
and
40% N
2
enters an insulated compressor operating at steady
state at 1 bar, 20C with a mass flow rate of 0.5 kg/s and is
614 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
compressed to 5.4 bar. Kinetic and potential energy effects are
negligible. For an isentropic compressor efficiency of 78%,
determine
(a) the temperature at the exit, in C.
(b) the power required, in kW.
(c) the rate of entropy production, in kW/K.
12.15 A mixture having an analysis on a mass basis of 80% N
2
,
20% CO
2
enters a nozzle operating at steady state at 1000 K
with a velocity of 5 m/s and expands adiabatically through a
7.5 :1 pressure ratio, exiting with a velocity of 900 m/s. De-
termine the isentropic nozzle efficiency.
12.16 A mixture having a molar analysis of 60% N
2
and 40%
CO
2
enters an insulated compressor operating at steady state
at 1 bar, 30C with a mass flow rate of 1 kg/s and is compressed
to 3 bar, 147C. Neglecting kinetic and potential energy effects,
determine
(a) the power required, in kW.
(b) the isentropic compressor efficiency.
(c) the rate of exergy destruction, in kW, for T
0
300 K.
12.17 Natural gas having a molar analysis of 60% methane
(CH
4
) and 40% ethane (C
2
H
6
) enters a compressor at 340 K,
6 bar and is compressed isothermally without internal irre-
versibilities to 20 bar. The compressor operates at steady state,
and kinetic and potential energy effects are negligible.
(a) Assuming ideal gas behavior, determine for the compres-
sor the work and heat transfer, each in kJ per kmol of mix-
ture flowing.
(b) Compare with the values for work and heat transfer, re-
spectively, determined assuming ideal solution behavior
(Sec. 11.9.5). For the pure components at 340 K:
h (kJ/kg) s (kJ/kg K)
6 bar 20 bar 6 bar 20 bar
Methane 715.33 704.40 10.9763 10.3275
Ethane 462.39 439.13 7.3493 6.9680
Forming Mixtures
12.18 One kilogram of argon at 27C, 1 bar is contained in a
rigid tank connected by a valve to another rigid tank contain-
ing 0.8 kg of O
2
at 127C, 5 bar. The valve is opened, and the
gases are allowed to mix, achieving an equilibrium state at
87C. Determine
(a) the volume of each tank, in m
3
.
(b) the final pressure, in bar.
(c) the heat transfer to or from the gases during the process,
in kJ.
(d) the entropy change of each gas, in kJ/K.
12.19 Using the ideal gas model with constant specific heats,
determine the mixture temperature, in K, for each of two cases:
(a) Initially, 0.6 kmol of O
2
at 500 K is separated by a parti-
tion from 0.4 kmol of H
2
at 300 K in a rigid insulated ves-
#
sel. The partition is removed and the gases mix to obtain
a final equilibrium state.
(b) Oxygen (O
2
) at 500 K and a molar flow rate of 0.6 kmol/s
enters an insulated control volume operating at steady state
and mixes with H
2
entering as a separate stream at 300 K
and a molar flow rate of 0.4 kmol/s. A single mixed stream
exits. Kinetic and potential energy effects can be ignored.
12.20 A system consists initially of n
A
moles of gas A at pres-
sure p and temperature T and n
B
moles of gas B separate from
gas A but at the same pressure and temperature. The gases are
allowed to mix with no heat or work interactions with the sur-
roundings. The final equilibrium pressure and temperature are
p and T, respectively, and the mixing occurs with no change
in total volume.
(a) Assuming ideal gas behavior, obtain an expression for the
entropy produced in terms of , n
A
, and n
B
.
(b) Using the result of part (a), demonstrate that the entropy
produced has a positive value.
(c) Would entropy be produced when samples of the same gas
at the same temperature and pressure mix? Explain.
12.21 An insulated tank has two compartments connected by a
valve. Initially, one compartment contains 0.7 kg of CO
2
at
500 K, 6.0 bar and the other contains 0.3 kg of N
2
at 300 K,
6.0 bar. The valve is opened and the gases are allowed to mix
until equilibrium is achieved. Determine
(a) the final temperature, in K.
(b) the final pressure, in bar.
(c) the amount of entropy produced, in kJ/K.
12.22 A rigid insulated tank has two compartments. Initially
one contains 0.5 kmol of carbon dioxide (CO
2
) at 27C, 2 bar
and the other contains 1 kmol of oxygen (O
2
) at 152C, 5 bar.
The gases are allowed to mix while 500 kJ of energy are added
by electrical work. Determine
(a) the final temperature, in C.
(b) the final pressure, in bar.
(c) the change in exergy, in kJ, for T
0
20C.
(d) the exergy destruction, in kJ.
12.23 Air at 40C, 1 atm and a volumetric flow rate of
50 m
3
/min enters an insulated control volume operating at
steady state and mixes with helium entering as a separate
stream at 100C, 1 atm and a volumetric flow rate of 20 m
3
/min.
A single mixed stream exits at 1 atm. Ignoring kinetic and po-
tential energy effects, determine for the control volume
(a) the temperature of the exiting mixture, in C.
(b) the rate of entropy production, in kW/K.
12.24 Air at 77C, 1 bar, and a molar flow rate of 0.1 kmol/s
enters an insulated mixing chamber operating at steady state
and mixes with water vapor entering at 277C, 1 bar, and a
molar flow rate of 0.3 kmol/s. The mixture exits at 1 bar.
Kinetic and potential energy effects can be ignored. For the
chamber, determine
(a) the temperature of the exiting mixture, in C.
(b) the rate of entropy production, in kW/K.
R
Problems: Developing Engineering Skills 615
12.25 Helium at 400 K, 1 bar enters an insulated mixing cham-
ber operating at steady state, where it mixes with argon enter-
ing at 300 K, 1 bar. The mixture exits at a pressure of 1 bar.
If the argon mass flow rate is x times that of helium, plot
versus x
(a) the exit temperature, in K.
(b) the rate of exergy destruction within the chamber, in kJ
per kg of helium entering.
Kinetic and potential energy effects can be ignored. Let T
0
300 K.
12.26 An insulated, rigid tank initially contains 1 kmol of argon
(Ar) at 300 K, 1 bar. The tank is connected by a valve to a
large vessel containing nitrogen (N
2
) at 500 K, 4 bar. A quan-
tity of nitrogen flows into the tank, forming an argon-nitrogen
mixture at temperature T and pressure p. Plot T, in K, and p,
in bar, versus the amount of N
2
within the tank, in kmol.
12.27 A stream of air (21% O
2
and 79% N
2
on a molar basis)
at 300 K and 0.1 MPa is to be separated into pure oxygen and
nitrogen streams, each at 300 K and 0.1 MPa. A device to
achieve the separation is claimed to require a work input at
steady state of 1200 kJ per kmol of air. Heat transfer between
the device and its surroundings occurs at 300 K. Ignoring ki-
netic and potential energy effects, evaluate whether the work
value can be as claimed.
12.28 A device is being designed to separate into components
a natural gas consisting of CH
4
and C
2
H
6
in which the mole
fraction of C
2
H
6
, denoted by y, may vary from 0.05 to 0.50.
The device will receive natural gas at 20C, 1 atm with a vol-
umetric flow rate of 100 m
3
/s. Separate streams of CH
4
and
C
2
H
6
will exit, each at 20C, 1 atm. Heat transfer between the
device and its surroundings occurs at 20C. Ignoring kinetic
and potential energy effects, plot versus y the minimum theo-
retical work input required at steady state, in kW.
Exploring Psychrometric Principles
12.29 A water pipe at 5C runs above ground between two
buildings. The surrounding air is at 35C. What is the maxi-
mum relative humidity the air can have before condensation
occurs on the pipe?
12.30 On entering a dwelling maintained at 20C from the out-
doors where the temperature is 10C, a person’s eye-glasses
are observed not to become fogged. A humidity gauge indi-
cates that the relative humidity in the dwelling is 55%. Can
this reading be correct? Provide supporting calculations.
12.31 A fixed amount of moist air initially at 1 bar and a relative
humidity of 60% is compressed isothermally until condensa-
tion of water begins. Determine the pressure of the mixture at
the onset of condensation, in bar. Repeat if the initial relative
humidity is 90%.
12.32 A vessel whose volume is 0.5 m
3
initially contains dry
air at 0.2 MPa and 20C. Water is added to the vessel until the
air is saturated at 20C. Determine the
(a) mass of water added, in kg.
(b) final pressure in the vessel, in bar.
12.33 Wet grain at 20C containing 40% moisture by mass en-
ters a dryer operating at steady state. Dry air enters the dryer
at 90C, 1 atm at a rate of 15 kg per kg of wet grain entering.
Moist air exits the dryer at 38C, 1 atm, and 52% relative hu-
midity. For the grain exiting the dryer, determine the percent
moisture by mass.
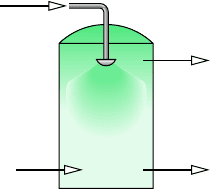
12.34 Figure P12.34 shows a spray dryer operating at steady
state. The mixture of liquid water and suspended solid parti-
cles entering through the spray head contains 30% solid matter
by mass. Dry air enters at 177C, 1 atm, and moist air exits
at 85C, 1 atm, and 21% relative humidity with a volumetric
flow rate of 310 m
3
/min. The dried particles exit separately.
Determine
(a) the volumetric flow rate of the entering dry air, in m
3
/min.
(b) the rate that dried particles exit, in kg/min.
Solid-liquid
mixture
70% liquid
30% solid
Dry air,
177°C,
1 atm
Dried
particles
φ
Moist air
310 m
3
/min,
85°C,
1 atm,
= 21%
Figure P12.34
12.35 A system consisting initially of 0.5 m
3
of air at 35C,
1 bar, and 70% relative humidity is cooled at constant pres-
sure to 29C. Determine the work and heat transfer for the
process, each in kJ.
12.36 A closed, rigid tank having a volume of 3 m
3
initially
contains air at 100C, 4.4 bar, and 40% relative humidity. De-
termine the heat transfer, in kJ, if the tank contents are cooled
to (a) 80C, (b) 20C.
12.37 A closed, rigid tank initially contains 0.5 m
3
of moist air
in equilibrium with 0.1 m
3
of liquid water at 80C and 0.1 MPa.
If the tank contents are heated to 200C, determine
(a) the final pressure, in MPa.
(b) the heat transfer, in kJ.
12.38 Combustion products with the molar analysis of 10%
CO
2
, 20% H
2
O, 70% N
2
enter an engine’s exhaust pipe at
1000F, 1 atm and are cooled as they pass through the pipe,
exiting at 100F, 1 atm. Determine the heat transfer at steady
state, in Btu per lb of entering mixture.
12.39 Air at 35C, 3 bar, 30% relative humidity, and a velocity
of 50 m/s expands isentropically through a nozzle. Determine
the lowest exit pressure, in bar, that can be attained without
condensation. For this exit pressure, determine the exit veloc-
ity, in m/s. The nozzle operates at steady state and without sig-
nificant potential energy effects.
