Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.


636 Chapter 13 Reacting Mixtures and Combustion
Consider first the reactants. With the enthalpy of formation for methane from Table A-25, the given value for methane,
and enthalpy values for nitrogen and oxygen from Table A-23
Next consider the products. With enthalpy of formation values for CO
2
, CO, and H
2
O(g) from Table A-25 and enthalpy
values from Table A-23
Inserting values into the expression for the rate of heat transfer
Q
#
cv
n
#
F
h
P
h
R
228,079 168442221,235 kJ/kmol 1CH
4
2
228,079 kJ/kmol 1CH
4
2
2 3241,820 172,513 990424
0.289160,371 86822 8.515157,651 86692
0.049 3110,530 158,191 866924
h
P
0.9513393,520 188,806 936424
6844
kJ/kmol 1CH
4
2
374,850 381400 29824 2.265314,770 86824 8.515314,581 86694
h
R
1h°
f
c
p
¢T 2
CH
4
2.2651¢h2
O
2
8.5151¢h2
N
2
c
p
CONDENSATION OF COMBUSTION PRODUCTS. When the combustion products of a hy-
drocarbon fuel are cooled sufficiently, condensation of a portion of the water formed will
occur. The energy balance must then be written to account for the presence of water in the
products as both a liquid and a vapor. For cooling of combustion products at constant pressure,
the dew point temperature method of Example 13.2 is used to determine the temperature at
the onset of condensation.
CLOSED SYSTEMS
Let us consider next a closed system involving a combustion process. In the absence of ki-
netic and potential energy effects, the appropriate form of the energy balance is
where U
R
denotes the internal energy of the reactants and U
P
denotes the internal energy of
the products. If the reactants and products form ideal gas mixtures, the energy balance can
be expressed as
(13.16)
where the coefficients n on the left side are the coefficients of the reaction equation giving
the moles of each reactant or product.
Since each component of the reactants and products behaves as an ideal gas, the respec-
tive specific internal energies of Eq. 13.16 can be evaluated as so the equation
becomes
(13.17a)
Q W
a
P
n1h RT
P
2
a
R
n1h RT
R
2
u h RT,
a
P
nu
a
R
nu Q W
U
P
U
R
Q W
13.2 Conservation of Energy—Reacting Systems 637
where T
P
and T
R
denote the temperature of the products and reactants, respectively. With ex-
pressions of the form of Eq. 13.13 for each of the reactants and products, Eq. 13.17a can be
written alternatively as
(13.17b)
The enthalpy of formation terms are obtained from Table A-25. The terms are evaluated
as discussed above.
The foregoing concepts are illustrated in Example 13.6, where a gaseous mixture burns
in a closed, rigid container.
¢h
a
P
n1h°
f
¢h2
a
R
n1h°
f
¢h2 RT
P
a
P
n RT
R
a
R
n
Q W
a
P
n1h°
f
¢h RT
P
2
a
R
n1h°
f
¢h RT
R
2
EXAMPLE 13.6 Analyzing Combustion at Constant Volume
A mixture of 1 kmol of gaseous methane and 2 kmol of oxygen initially at 25C and 1 atm burns completely in a closed, rigid
container. Heat transfer occurs until the products are cooled to 900 K. If the reactants and products each form ideal gas mix-
tures, determine (a) the amount of heat transfer, in kJ, and (b) the final pressure, in atm.
SOLUTION
Known: A mixture of gaseous methane and oxygen, initially at 25C and 1 atm, burns completely within a closed rigid con-
tainer. The products are cooled to 900 K.
Find: Determine the amount of heat transfer, in kJ, and the final pressure of the combustion products, in atm.
Schematic and Given Data:
1kmol CH
4
(g)
2 kmol O
2
T
1
p
1
= 25°C
= 1 atm
State 1
Products of
combustion
T
2
p
2
State 2
Figure E13.6
Assumptions:
1. The contents of the closed, rigid container are taken as the system.
2. Kinetic and potential energy effects are absent, and W 0.
3. Combustion is complete.
4. The initial mixture and the products of combustion each form ideal gas mixtures.
5. The initial and final states are equilibrium states.
Analysis: The chemical reaction equation for the complete combustion of methane with oxygen is
CH
4
2O
2
S CO
2
2H
2
O

638 Chapter 13 Reacting Mixtures and Combustion
(a) With assumptions 2 and 3, the closed system energy balance takes the form
or
Each coefficient in this equation is the same as the corresponding term of the balanced chemical equation.
Since each reactant and product behaves as an ideal gas, the respective specific internal energies can be evaluated as
. The energy balance then becomes
where T
1
and T
2
denote, respectively, the initial and final temperatures. Collecting like terms
The specific enthalpies are evaluated in terms of the respective enthalpies of formation to give
Since the methane and oxygen are initially at 25C, for each of these reactants. Also, for oxygen.
With enthalpy of formation values for CO
2
,H
2
O(g), and CH
4
(g) from Table A-25 and enthalpy values for H
2
O and CO
2
from Table A-23
(b) By assumption 3, the initial mixture and the products of combustion each form ideal gas mixtures. Thus, for the
reactants
where n
R
is the total number of moles of reactants and p
1
is the initial pressure. Similarly, for the products
where n
P
is the total number of moles of products and p
2
is the final pressure.
Since n
R
n
P
3 and volume is constant, these equations combine to give
p
2
T
2
T
1
p
1
a
900 K
298 K
b 11 atm2 3.02 atm
p
2
V n
P
RT
2
p
1
V n
R
RT
1
745,436 kJ
174,8502 318.31421298 9002
Q 3393,520 137,405 936424 23241,820 131,828 990424
h°
f
0¢h 0
1h
°
f
¢h
0
2
CH
4
1 g2
21h°
f
0
¢h
0
2
O
2
4 3R1T
1
T
2
2
Q 31h°
f
¢h2
CO
2
21h°
f
¢h2
H
2
O 1 g2
Q 1h
CO
2
2h
H
2
O 1 g2
h
CH
4
1 g2
2h
O
2
2 3R1T
1
T
2
2
Q 311h
CO
2
RT
2
2 21h
H
2
O 1g2
RT
2
24 311h
CH
4
1g2
RT
1
2 21h
O
2
RT
1
24
u h RT
Q U
P
U
R
11u
CO
2
2u
H
2
O 1 g2
2 11u
CH
4
1 g2
2u
O
2
2
U
P
U
R
Q W
0
13.2.3 Enthalpy of Combustion and Heating Values
Although the enthalpy of formation concept underlies the formulations of the energy bal-
ances for reactive systems presented thus far, the enthalpy of formation of fuels is not always
tabulated. for example. . . fuel oil and coal are normally composed of several indi-
vidual chemical substances, the relative amounts of which may vary considerably, depend-
ing on the source. Owing to the wide variation in composition that these fuels can exhibit,
we do not find their enthalpies of formation listed in Tables A-25 or similar compilations of
thermophysical data. In many cases of practical interest, however, the enthalpy of com-
bustion, which is accessible experimentally, can be used to conduct an energy analysis when
enthalpy of formation data are lacking.

13.2 Conservation of Energy—Reacting Systems 639
The enthalpy of combustion is defined as the difference between the enthalpy of the
products and the enthalpy of the reactants when complete combustion occurs at a given tem-
perature and pressure. That is
(13.18)
where the n’s correspond to the respective coefficients of the reaction equation giving the
moles of reactants and products per mole of fuel. When the enthalpy of combustion is ex-
pressed on a unit mass of fuel basis, it is designated h
RP
. Tabulated values are usually given
at the standard temperature T
ref
and pressure p
ref
introduced in Sec. 13.2.1. The symbol
or is used for data at this temperature and pressure.
The heating value of a fuel is a positive number equal to the magnitude of the enthalpy
of combustion. Two heating values are recognized by name: the higher heating value (HHV)
and the lower heating value (LHV). The higher heating value is obtained when all the wa-
ter formed by combustion is a liquid; the lower heating value is obtained when all the water
formed by combustion is a vapor. The higher heating value exceeds the lower heating value
by the energy that would be required to vaporize the liquid formed. Values for the HHV and
LHV also depend on whether the fuel is a liquid or a gas. Heating value data for several
hydrocarbons are provided in Tables A-25.
The calculation of the enthalpy of combustion, and the associated heating value, using
table data is illustrated in the next example.
h°
RP
h°
RP
h
RP
a
P
n
e
h
e
a
R
n
i
h
i
h
RP
enthalpy of combustion
EXAMPLE 13.7 Calculating Enthalpy of Combustion
Calculate the enthalpy of combustion of gaseous methane, in kJ per kg of fuel, (a) at 25C, 1 atm with liquid water in the
products, (b) at 25C, 1 atm with water vapor in the products. (c) Repeat part (b) at 1000 K, 1 atm.
SOLUTION
Known: The fuel is gaseous methane.
Find: Determine the enthalpy of combustion, in kJ per kg of fuel, (a) at 25C, 1 atm with liquid water in the products,
(b) at 25C, 1 atm with water vapor in the products, (c) at 1000 K, 1 atm with water vapor in the products.
Assumptions:
1. Each mole of oxygen in the combustion air is accompanied by 3.76 moles of nitrogen, which is inert.
2. Combustion is complete, and both reactants and products are at the same temperature and pressure.
3. The ideal gas model applies for methane, the combustion air, and the gaseous products of combustion.
Analysis: The combustion equation is
The enthalpy of combustion is, from Eq. 13.18
Introducing the coefficients of the combustion equation and evaluating the specific enthalpies in terms of the respective
enthalpies of formation
1h
°
f
¢h2
CO
2
21h°
f
¢h2
H
2
O
1h°
f
¢h2
CH
4
1g2
21h°
f
0
¢h2
O
2
h
RP
h
CO
2
2h
H
2
O
h
CH
4
1 g2
2h
O
2
h
RP
a
P
n
e
1h°
f
¢h2
e
a
R
n
i
1h°
f
¢h2
i
CH
4
2O
2
7.52N
2
S CO
2
2H
2
O 7.52N
2
higher and lower
heating values
640 Chapter 13 Reacting Mixtures and Combustion
For nitrogen, the enthalpy terms of the reactants and products cancel. Also, the enthalpy of formation of oxygen is zero by
definition. On rearrangement, the enthalpy of combustion expression becomes
The values for and ( ) depend on whether the water in the products is a liquid or a vapor.
(a) Since the reactants and products are at 25C, 1 atm in this case, the terms drop out of the above expression for .
Thus, for liquid water in the products, the enthalpy of combustion is
With enthalpy of formation values from Table A-25
Dividing by the molecular weight of methane places this result on a unit mass of fuel basis
which agrees with the higher heating value of methane given in Table A-25.
(b) As in part (a), the terms drop out of the above expression for which for water vapor in the products reduces to
, where
With enthalpy of formation values from Table A-25
On a unit of mass of fuel basis, the enthalpy of combustion for this case is
which agrees with the lower heating value of methane given in Table A-25.
(c) For the case where the reactants and products are at 1000 K, 1 atm, the term in the above expression for has the
value determined in part (b): (fuel), and the terms for O
2
,H
2
O(g), and CO
2
can be evaluated
using specific enthalpies at 298 and 1000 K from Table A-23. The results are
For methane, the expression of Table A-21 can be used to obtain
Substituting values into the expression for the enthalpy of combustion
800,522 kJ/kmol 1fuel2
h
RP
802,310 333,405 2125,9782 38,189 2122,70724
38,189 kJ/kmol 1fuel2
R
a3.826 T
3.979
10
3
T
2
2
24.558
10
6
T
3
3
22.733
10
9
T
4
4
6.963
10
12
T
5
5
b
1000
298
1¢h2
CH
4
1 g2
1000
298
c
p
dT
c
p
1¢h2
CO
2
42,769 9364 33,405 kJ/kmol
1¢h
2
H
2
O 1 g2
35,882 9904 25,978 kJ/kmol
1¢h
2
O
2
31,389 8682 22,707 kJ/kmol
¢h
h°
RP
802,310 kJ/kmol
h
RP
h°
RP
h°
RP
802,310
16.04
50,019 kJ/kg
fuel
h
°
RP
393,520 21241,8202 174,8502802,310 kJ/kmol 1fuel2
h
°
RP
1h°
f
2
CO
2
21h°
f
2
H
2
O1 g2
1h°
f
2
CH
4
1 g2
h°
RP
h
RP
,¢h
h°
RP
890,330 kJ/kmol 1fuel2
16.04 kg 1fuel2
kmol 1fuel2
55,507 kJ/kg 1fuel2
h
°
RP
393,520 21285,8302 174,8502890,330 kJ/kmol 1fuel2
h
°
RP
1h°
f
2
CO
2
21h°
f
2
H
2
O1l2
1h°
f
2
CH
4
1g2
h
RP
¢h
H
2
O
¢hh°
RP
h°
RP
31¢h2
CO
2
21¢h2
H
2
O
1¢h2
CH
4
1g2
21¢h2
O
2
4
h
RP
1h°
f
2
CO
2
21h°
f
2
H
2
O
1h°
f
2
CH
4
1g2
31¢h2
CO
2
21¢h2
H
2
O
1¢h2
CH
4
1g2
21¢h2
O
2
4
❶

13.3 Determining the Adiabatic Flame Temperature 641
13.3 Determining the Adiabatic
Flame Temperature
Let us reconsider the reactor at steady state pictured in Fig. 13.2. In the absence of work
and appreciable kinetic and potential energy effects, the energy liberated on combustion is trans-
ferred from the reactor in two ways only: by energy accompanying the exiting combustion
products and by heat transfer to the surroundings. The smaller the heat transfer, the greater the
W
#
cv
When enthalpy of formation data are available for all the reactants and products, the enthalpy
of combustion can be calculated directly from Eq. 13.18, as illustrated in Example 13.7. Other-
wise, it must be obtained experimentally using devices known as calorimeters. Both constant-
volume (bomb calorimeters) and flow-through devices are employed for this purpose. Consider
as an illustration a reactor operating at steady state in which the fuel is burned completely with
air. For the products to be returned to the same temperature as the reactants, a heat transfer from
the reactor would be required. From an energy rate balance, the required heat transfer is
(13.19)
where the symbols have the same significance as in previous discussions. The heat transfer
per mole of fuel, would be determined from measured data. Comparing Eq. 13.19
with the defining equation, Eq. 13.18, we have In accord with the usual sign
convention for heat transfer, the enthalpy of combustion would be negative.
As noted previously, the enthalpy of combustion can be used for energy analyses of re-
acting systems. for example. . . consider a control volume at steady state for which
the energy rate balance takes the form
All symbols have the same significance as in previous discussions. This equation can be re-
arranged to read
For a complete reaction, the underlined term is just the enthalpy of combustion , at T
ref
and p
ref
. Thus, the equation becomes
(13.20)
The right side of Eq. 13.20 can be evaluated with an experimentally determined value for
and values for the reactants and products determined as discussed previously. ¢hh°
RP
Q
#
cv
n
#
F
W
#
cv
n
#
F
h°
RP
a
P
n
e
1¢h2
e
a
R
n
i
1¢h2
i
h°
RP
Q
#
cv
n
#
F
W
#
cv
n
#
F
a
P
n
e
1h°
f
2
e
a
R
n
i
1h°
f
2
i
a
P
n
e
1¢h2
e
a
R
n
i
1¢h2
i
Q
#
cv
n
#
F
W
#
cv
n
#
F
a
P
n
e
1h°
f
¢h2
e
a
R
n
i
1h°
f
¢h2
i
h
RP
Q
#
cv
n
#
F
.
Q
#
cv
n
#
F
,
Q
#
cv
n
#
F
a
P
n
e
h
e
a
R
n
i
h
i
On a unit mass basis
Using Interactive Thermodynamics: IT, we get 38,180 kJ/kmol (fuel).
Comparing the values of parts (b) and (c), the enthalpy of combustion of methane is seen to vary little with temperature.
The same is true for many hydrocarbon fuels. This fact is sometimes used to simplify combustion calculations.
h
RP
800,552
16.04
49,910 kJ/kg 1fuel2
❶
❷
❷
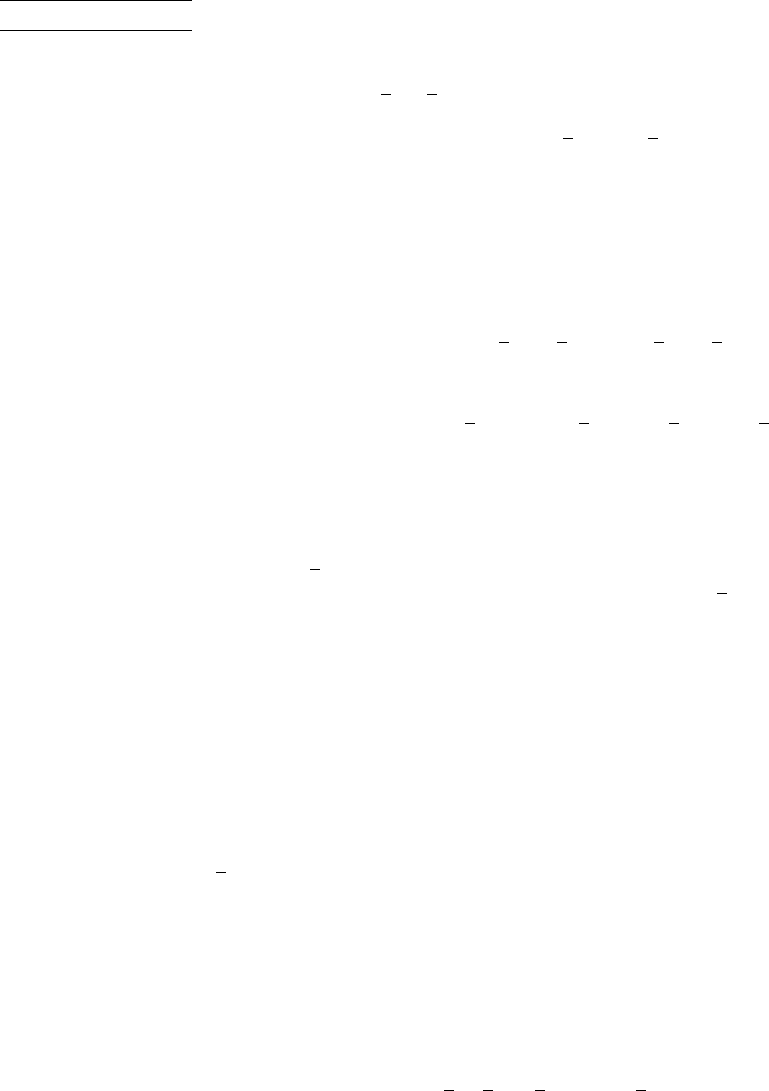
642 Chapter 13 Reacting Mixtures and Combustion
energy carried out with the combustion products and thus the greater the temperature of the prod-
ucts. The temperature that would be achieved by the products in the limit of adiabatic operation
of the reactor is called the adiabatic flame temperature or adiabatic combustion temperature.
The adiabatic flame temperature can be determined by use of the conservation of mass
and conservation of energy principles. To illustrate the procedure, let us suppose that the
combustion air and the combustion products each form ideal gas mixtures. Then, with the
other assumptions stated above, the energy rate balance on a per mole of fuel basis, Eq. 13.12b,
reduces to the form —that is
(13.21a)
where i denotes the incoming fuel and air streams and e the exiting combustion products.
With this expression, the adiabatic flame temperature can be determined using table data or
computer software, as follows.
USING TABLE DATA. When using Eq. 13.9 with table data to evaluate enthalpy terms,
Eq. 13.21a takes the form
or
(13.21b)
The n’s are obtained on a per mole of fuel basis from the balanced chemical reaction equation.
The enthalpies of formation of the reactants and products are obtained from Table A-25 or
A-25E. Enthalpy of combustion data might be employed in situations where the enthalpy of
formation for the fuel is not available. Knowing the states of the reactants as they enter the re-
actor, the terms for the reactants can be evaluated as discussed previously. Thus, all terms
on the right side of Eq. 13.21b can be evaluated. The terms on the left side account for
the changes in enthalpy of the products from T
ref
to the unknown adiabatic flame temperature.
Since the unknown temperature appears in each term of the sum on the left side of the equa-
tion, determination of the adiabatic flame temperature requires iteration: A temperature for the
products is assumed and used to evaluate the left side of Eq. 13.21b. The value obtained is
compared with the previously determined value for the right side of the equation. The proce-
dure continues until satisfactory agreement is attained. Example 13.8 gives an illustration.
USING COMPUTER SOFTWARE. Thus far we have emphasized the use of Eq. 13.9 together
with table data when evaluating the specific enthalpies required by energy balances for re-
acting systems. Such enthalpy values also can be retrieved using Interactive Thermodynam-
ics: IT. With IT, the quantities on the right side of Eq. 13.9 are evaluated by software, and
data are returned directly. for example. . . consider CO
2
at 500 K modeled as an
ideal gas. The specific enthalpy is obtained from IT as follows:
T = 500 // K
h = h_T(“CO
2
”, T)
Choosing K for the temperature unit and moles for the amount under the Units menu, IT re-
turns h 3.852 10
5
kJ/kmol.
This value agrees with the value calculated from Eq. 13.9 using enthalpy data for CO
2
from Table A-23, as follows
3.852 10
5
kJ/kmol
393,520 317,678 9364 4
h h°
f
3h1500 K2 h1298 K24
h
(¢h)
e
¢h
a
P
n
e
1¢h2
e
a
R
n
i
1¢h2
i
a
R
n
i
h°
fi
a
P
n
e
h°
fe
a
P
n
e
1h°
f
¢h2
e
a
R
n
i
1h°
f
¢h2
i
a
P
n
e
h
e
a
R
n
i
h
i
h
P
h
R
adiabatic flame
temperature
13.3 Determining the Adiabatic Flame Temperature 643
As suggested by this discussion, IT is also useful for analyzing reacting systems. In par-
ticular, the equation solver and property retrieval features of IT allow the adiabatic flame
temperature to be determined without the iteration required when using table data. This is
illustrated in Example 13.8.
EXAMPLE 13.8 Determining the Adiabatic Flame Temperature
Liquid octane at 25C, 1 atm enters a well-insulated reactor and reacts with air entering at the same temperature and pressure.
For steady-state operation and negligible effects of kinetic and potential energy, determine the temperature of the combustion
products for complete combustion with (a) the theoretical amount of air, (b) 400% theoretical air.
SOLUTION
Known: Liquid octane and air, each at 25C and 1 atm, burn completely within a well-insulated reactor operating at steady
state.
Find: Determine the temperature of the combustion products for (a) the theoretical amount of air and (b) 400% theoretical
air.
Schematic and Given Data:
Air
25°C, 1 atm
Combustion products,
T
P
C
8
H
18
(l)
25°C, 1 atm
Insulation
Figure E13.8
Assumptions:
1. The control volume indicated on the accompanying figure by a dashed line operates at steady state.
2. For the control volume, , and kinetic and potential effects are negligible.
3. The combustion air and the products of combustion each form ideal gas mixtures.
4. Combustion is complete.
5. Each mole of oxygen in the combustion air is accompanied by 3.76 moles of nitrogen, which is inert.
Analysis: At steady state, the control volume energy rate balance Eq. 13.12b reduces with assumptions 2 and 3 to give
Eq. 13.21a
(1)
When Eq. 13.9 and table data are used to evaluate the enthalpy terms, Eq. (1) is written as
On rearrangement, this becomes
a
P
n
e
1¢h2
e
a
R
n
i
1¢h2
i
a
R
n
i
h°
fi
a
P
n
e
h°
fe
a
P
n
e
1h°
f
¢h2
e
a
R
n
i
1h°
f
¢h2
i
a
P
n
e
h
e
a
R
n
i
h
i
Q
#
cv
0, W
#
cv
0

644 Chapter 13 Reacting Mixtures and Combustion
which corresponds to Eq. 13.21b. Since the reactants enter at 25C, the ( )
i
terms on the right side vanish, and the energy
rate equation becomes
(2)
(a) For combustion of liquid octane with the theoretical amount of air, the chemical equation is
Introducing the coefficients of this equation, Eq. (2) takes the form
The right side of the above equation can be evaluated with enthalpy of formation data from Table A-25, giving
Each term on the left side of this equation depends on the temperature of the products, T
P
. This temperature can be de-
termined by an iterative procedure.
The following table gives a summary of the iterative procedure for three trial values of T
P
. Since the summation of the en-
thalpies of the products equals 5,074,630 kJ/kmol, the actual value of T
P
is in the interval from 2350 to 2400 K. Interpolation
between these temperatures gives T
P
2395 K.
2500 K 2400 K 2350 K
975,408 926,304 901,816
890,676 842,436 818,478
3,492,664 3,320,597 3,234,869
5,358,748 5,089,337 4,955,163
Alternative Solution:
The following IT code can be used as an alternative to iteration with table data, where hN2_R and hN2_P denote the enthalpy
of N
2
in the reactants and products, respectively, and so on. In the Units menu, select temperature in K and amount of substance
in moles.
TR = 25 + 273.15 // K
// Evaluate reactant and product enthalpies, hR and hP, respectively
hR = hC8H18 + 12.5 * hO2_R + 47 * hN2_R
hP = 8 * hCO2_P + 9 * hH2O_P + 47 * hN2_P
hC8H18 = –249910 // kJ/kmol (Value from Table A-25)
hO2_R = h_T(“O2’’,TR)
hN2_R = h_T(“N2’’,TR)
hCO2_P = h_T(“CO2’’,TP)
hH2O_P = h_T(“H2O’’,TP)
hN2_P = h_T(“N2’’,TP)
// Energy balance
hP = hR
Using the Solve button, the result is TP = 2394 K, which agrees closely with the result obtained above.
(b) For complete combustion of liquid octane with 400% theoretical air, the chemical equation is
C
8
H
18
1l2 50O
2
188N
2
S 8CO
2
9H
2
O 37.5O
2
188N
2
a
P
n
e
1¢h2
e
471¢h2
N
2
91¢h2
H
2
O1g2
81¢h2
CO
2
¢h
81¢h2
CO
2
91¢h2
H
2
O1g2
471¢h2
N
2
5,074,630 kJ/kmol 1fuel2
381h°
f
2
CO
2
91h°
f
2
H
2
O1g2
471h°
f
2
N
2
4
0
31h°
f
2
C
8
H
18
1l2
12.51h°
f
2
O
2
0
471h°
f
2
N
2
4
0
81¢h2
CO
2
91¢h2
H
2
O1g2
471¢h2
N
2
C
8
H
18
1l2 12.5O
2
47N
2
S 8CO
2
9H
2
O1g2 47N
2
a
P
n
e
1¢h2
e
a
R
n
i
h°
fi
a
P
n
e
h°
fe
¢h

13.4 Fuel Cells 645
Equation (2), the energy rate balance, reduces for this case to
Observe that the right side has the same value as in part (a). Proceeding iteratively as above, the temperature of the products
is T
P
962 K. The use of IT to solve part (b) is left as an exercise.
The temperature determined in part (b) is considerably lower than the value found in part (a). This shows that once enough
oxygen has been provided for complete combustion, bringing in more air dilutes the combustion products, lowering their
temperature.
81¢h
2
CO
2
91¢h2
H
2
O1g2
37.51¢h2
O
2
1881¢h2
N
2
5,074,630 kJ/kmol 1fuel2
❶
❶
CLOSING COMMENTS. For a specified fuel and specified temperature and pressure of the
reactants, the maximum adiabatic flame temperature is for complete combustion with the the-
oretical amount of air. The measured value of the temperature of the combustion products
may be several hundred degrees below the calculated maximum adiabatic flame temperature,
however, for several reasons:
Once adequate oxygen has been provided to permit complete combustion, bringing in
more air dilutes the combustion products, lowering their temperature.
Incomplete combustion also tends to reduce the temperature of the products, and com-
bustion is seldom complete (see Sec. 14.4).
Heat losses can be reduced but not altogether eliminated.
As a result of the high temperatures achieved, some of the combustion products may
dissociate. Endothermic dissociation reactions lower the product temperature. The effect
of dissociation on the adiabatic flame temperature is considered in Sec. 14.4.
13.4 Fuel Cells
A fuel cell is an electrochemical device in which fuel and an oxidizer (normally oxygen from
air) undergo a chemical reaction, providing electrical current to an external circuit and pro-
ducing products. The fuel and oxidizer react catalytically in stages on separate electrodes:
the anode and the cathode. An electrolyte separating the two electrodes allows passage of
ions formed by reaction. Depending on the type of fuel cell, the ions may be positively or
negatively charged. The reaction is not a combustion process.
Rates of reaction in fuel cells are limited by the time it takes for diffusion of chemical
species through the electrodes and the electrolyte and by the speed of the chemical reactions
themselves. These features, together with other aspects of fuel cell operation, result in in-
ternal irreversibilities that are inherently less significant than encountered in power producing
devices relying on combustion.
By avoiding highly irreversible combustion, fuel cells have the potential of providing more
power from a given supply of fuel and oxidizer, while forming fewer undesirable products,
than internal combustion engines and gas turbines. In contrast to power plants studied in pre-
vious chapters, fuel cells can produce electric power without moving parts or utilizing
intermediate heat exchangers. Fuel cells do not operate as thermodynamic power cycles, and
thus the notion of a limiting thermal efficiency imposed by the second law is not applicable.
Despite these thermodynamic advantages, widespread use of fuel cells has not occurred thus
far owing primarily to cost.
For scale, Fig. 13.3a shows a solid oxide fuel cell module. Figure 13.3b gives the
schematic of a proton exchange membrane fuel cell, which is discussed next as a repre-
sentative case.
fuel cell
