Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.


626 Chapter 13 Reacting Mixtures and Combustion
patterns indicate that
this leads to heavy
rainfall and flooding
in some areas, while
dry conditions and
dust storms occur in
other areas.
Soot is turning out to be more significant than previously
thought, experts say. Based on the computer models, soot
seems to have as much of an effect on climate as carbon
dioxide, although the atmospheric mechanisms are different.
Industrialized nations produce the most carbon dioxide,
whereas developing nations tend to emit more soot into the
atmosphere. These new findings suggest that curbing both
sources of atmospheric emission might be needed to address
climate change concerns.
Airborne Soot Adds to Weather Woes,
Some Say
Thermodynamics in the News...
According to a new study, the weather in northern Africa, India,
China, and the southern United States is being affected by soot
from cooking fires and diesel engines. Like global warming
associated with carbon dioxide, researchers believe that soot
may contribute significantly to climate change.
Widespread use of fuels such as coal, cow dung, crop
residue, and wood in developing countries produces vast
amounts of unburned black carbon, or soot. Diesel engine
exhaust also contains soot. The sooty carbon particles are
thought to increase the absorption of sunlight in the upper
atmosphere, causing hotter upper air and less sunlight to
reach the ground. With uneven warming, the air becomes un-
stable and more clouds form. Computer models of weather
EXAMPLE 13.2 Using a Dry Product Analysis
Methane, CH
4
, is burned with dry air. The molar analysis of the products on a dry basis is CO
2
, 9.7%; CO, 0.5%; O
2
, 2.95%;
and N
2
, 86.85%. Determine (a) the air–fuel ratio on both a molar and a mass basis, (b) the percent theoretical air, (c) the dew
point temperature of the products, in C, if the mixture were cooled at 1 atm.
SOLUTION
Known: Methane is burned with dry air. The molar analysis of the products on a dry basis is provided.
Find: Determine (a) the air–fuel ratio on both a molar and a mass basis, (b) the percent theoretical air, and (c) the dew point
temperature of the products, in C, if cooled at 1 atm.
Assumptions:
1. Each mole of oxygen in the combustion air is accompanied by 3.76 moles of nitrogen, which is inert.
2. The products form an ideal gas mixture.
Analysis:
(a) The solution is conveniently conducted on the basis of 100 kmol of dry products. The chemical equation then reads
In addition to the assumed 100 lbmol of dry products, water must be included as a product.
Applying conservation of mass to carbon, hydrogen, and oxygen, respectively
Solving this set of equations gives a 10.2, b 23.1, c 20.4. The balanced chemical equation is
10.2CH
4
23.11O
2
3.76N
2
2S 9.7CO
2
0.5CO 2.95O
2
86.85N
2
20.4H
2
O
O:
19.72122 0.5 212.952 c 2b
H:
2 c 4a
C:
9.7 0.5 a
aCH
4
b1O
2
3.76N
2
2S 9.7CO
2
0.5CO 2.95O
2
86.85N
2
cH
2
O
❶
❷
13.1 Introducing Combustion 627
On a molar basis, the air–fuel ratio is
On a mass basis
(b) The balanced chemical equation for the complete combustion of methane with the theoretical amount of air is
The theoretical air–fuel ratio on a molar basis is
The percent theoretical air is then found from
(c) To determine the dew point temperature requires the partial pressure of the water vapor p
v
. The partial pressure p
v
is found
from p
v
y
v
p, where y
v
is the mole fraction of the water vapor in the combustion products and p is 1 atm.
Referring to the balanced chemical equation of part (a), the mole fraction of the water vapor is
Thus, p
v
0.169 atm 0.1712 bar. Interpolating in Table A-2, T 57C.
The solution could be obtained on the basis of any assumed amount of dry products—for example, 1 lbmol. With some
other assumed amount, the values of the coefficients of the balanced chemical equation would differ from those obtained
in the solution, but the air–fuel ratio, the value for the percent of theoretical air, and the dew point temperature would be
unchanged.
The three unknown coefficients, a, b, and c, are evaluated here by application of conservation of mass to carbon, hydro-
gen, and oxygen. As a check, note that the nitrogen also balances
This confirms the accuracy of both the given product analysis and the calculations conducted to determine the unknown
coefficients.
If the products of combustion were cooled at constant pressure below the dew point temperature of 134F, some conden-
sation of the water vapor would occur.
N:
b13.762 86.85
y
v
20.4
100 20.4
0.169
10.78 kmol 1air2
kmol 1fuel2
9.52 kmol 1air2
kmol 1fuel2
1.13 1113%2
% theoretical air
1AF
2
1AF2
theo
1AF2
theo
214.762
1
9.52
kmol 1air2
kmol 1fuel2
CH
4
21O
2
3.76N
2
2S CO
2
2H
2
O 7.52N
2
AF 110.782 a
28.97
16.04
b 19.47
kg 1air2
kg 1fuel2
AF
23.114.762
10.2
10.78
kmol 1air2
kmol 1fuel2
❶
❷
❸
❸
In Example 13.3, a fuel mixture having a known molar analysis is burned with air, giv-
ing products with a known dry analysis.

628 Chapter 13 Reacting Mixtures and Combustion
EXAMPLE 13.3 Burning Natural Gas with Excess Air
A natural gas has the following molar analysis: CH
4
, 80.62%; C
2
H
6
, 5.41%; C
3
H
8
, 1.87%; C
4
H
10
, 1.60%; N
2
, 10.50%. The gas is
burned with dry air, giving products having a molar analysis on a dry basis: CO
2
, 7.8%; CO, 0.2%; O
2
,7%; N
2
, 85%. (a) Deter-
mine the air–fuel ratio on a molar basis. (b) Assuming ideal gas behavior for the fuel mixture, determine the amount of products
in kmol that would be formed from 100 m
3
of fuel mixture at 300 K and 1 bar. (c) Determine the percent of theoretical air.
SOLUTION
Known: A natural gas with a specified molar analysis burns with dry air giving products having a known molar analysis on
a dry basis.
Find: Determine the air–fuel ratio on a molar basis, the amount of products in kmol that would be formed from 100 m
3
of
natural gas at 300 K and 1 bar, and the percent of theoretical air.
Assumptions:
1. Each mole of oxygen in the combustion air is accompanied by 3.76 moles of nitrogen, which is inert.
2. The fuel mixture can be modeled as an ideal gas.
Analysis:
(a) The solution can be conducted on the basis of an assumed amount of fuel mixture or on the basis of an assumed amount
of dry products. Let us illustrate the first procedure, basing the solution on 1 kmol of fuel mixture. The chemical equation
then takes the form
The products consist of b kmol of dry products and c kmol of water vapor, each per kmol of fuel mixture.
Applying conservation of mass to carbon
Solving gives b 12.931. Conservation of mass for hydrogen results in
which gives c 1.93. The unknown coefficient a can be found from either an oxygen balance or a nitrogen balance. Apply-
ing conservation of mass to oxygen
giving a 2.892.
The balanced chemical equation is then
The air–fuel ratio on a molar basis is
(b) By inspection of the chemical reaction equation, the total amount of products is b c 12.931 1.93 14.861 kmol
of products per kmol of fuel. The amount of fuel in kmol, n
F
, present in 100 m
3
of fuel mixture at 300 K and 1 bar can be
determined from the ideal gas equation of state as
110
5
N/m
2
21100 m
3
2
18314 N
#
m/ kmol
#
K21300 K2
4.01 kmol 1fuel2
n
F
pV
RT
AF
12.892214.762
1
13.77
kmol 1air2
kmol 1fuel2
1.93H
2
O
2.8921O
2
3.76N
2
2S 12.93110.078CO
2
0.002CO 0.07O
2
0.85N
2
2
10.8062CH
4
0.0541C
2
H
6
0.0187C
3
H
8
0.0160C
4
H
10
0.1050N
2
2
12.9313210.0782 0.002 210.0724 1.93 2a
2c 410.80622 610.05412 810.01872 1010.01602
b10.078 0.0022 0.8062 210.05412 310.01872 410.01602
a1O
2
3.76N
2
2S b10.078CO
2
0.002CO 0.07O
2
0.85N
2
2 cH
2
O
10.8062CH
4
0.0541C
2
H
6
0.0187C
3
H
8
0.0160C
4
H
10
0.1050N
2
2
❶

13.2 Conservation of Energy—Reacting Systems 629
Accordingly, the amount of product mixture that would be formed from 100 m
3
of fuel mixture is (14.861)(4.01) 59.59
kmol of product gas.
(c) The balanced chemical equation for the complete combustion of the fuel mixture with the theoretical amount of air is
The theoretical air–fuel ratio on a molar basis is
The percent theoretical air is then
A check on both the accuracy of the given molar analyses and the calculations conducted to determine the unknown co-
efficients is obtained by applying conservation of mass to nitrogen. The amount of nitrogen in the reactants is
The amount of nitrogen in the products is (0.85)(12.931) 10.99 kmolkmol of fuel. The difference can be attributed to
round-off.
0.105 13.76212.8922 10.98 kmol
kmol of fuel
% theoretical air
13.77 kmol 1air2
kmol 1fuel2
9.52 kmol 1air2
kmol 1fuel2
1.45 1145%2
1AF
2
theo
214.762
1
9.52
kmol 1air2
kmol 1fuel2
21O
2
3.76N
2
2S 1.0345CO
2
1.93H
2
O 7.625N
2
10.8062CH
4
0.0541C
2
H
6
0.0187C
3
H
8
0.0160C
4
H
10
0.1050N
2
2
❶
13.2 Conservation of Energy—
Reacting Systems
The objective of the present section is to illustrate the application of the conservation of en-
ergy principle to reacting systems. The forms of the conservation of energy principle intro-
duced previously remain valid whether or not a chemical reaction occurs within the system.
However, the methods used for evaluating the properties of reacting systems differ somewhat
from the practices used to this point.
13.2.1 Evaluating Enthalpy for Reacting Systems
In each of the tables of thermodynamic properties used thus far, values for the specific in-
ternal energy, enthalpy, and entropy are given relative to some arbitrary datum state where
the enthalpy (or alternatively the internal energy) and entropy are set to zero. This approach
is satisfactory for evaluations involving differences in property values between states of the
same composition, for then arbitrary datums cancel. However, when a chemical reaction oc-
curs, reactants disappear and products are formed, so differences cannot be calculated for all
substances involved. For reacting systems, it is necessary to evaluate h, u, and s in such a
way that there are no subsequent ambiguities or inconsistencies in evaluating properties. In
this section, we will consider how this is accomplished for h and u. The case of entropy is
handled differently and is taken up in Sec. 13.5.
An enthalpy datum for the study of reacting systems can be established by assigning
arbitrarily a value of zero to the enthalpy of the stable elements at a state called the
standard reference state and defined by T
ref
298.15 K (25C) and p
ref
1 atm. Note
that only stable elements are assigned a value of zero enthalpy at the standard state. The
term stable simply means that the particular element is in a chemically stable form. For
standard reference state
630 Chapter 13 Reacting Mixtures and Combustion
example, at the standard state the stable forms of hydrogen, oxygen, and nitrogen are H
2
,
O
2
, and N
2
and not the monatomic H, O, and N. No ambiguities or conflicts result with
this choice of datum.
ENTHALPY OF FORMATION. Using the datum introduced above, enthalpy values can be
assigned to compounds for use in the study of reacting systems. The enthalpy of a compound
at the standard state equals its enthalpy of formation, symbolized The enthalpy of
formation is the energy released or absorbed when the compound is formed from its elements,
the compound and elements all being at T
ref
and p
ref
. The enthalpy of formation is usually
determined by application of procedures from statistical thermodynamics using observed
spectroscopic data.
The enthalpy of formation also can be found in principle by measuring the heat transfer
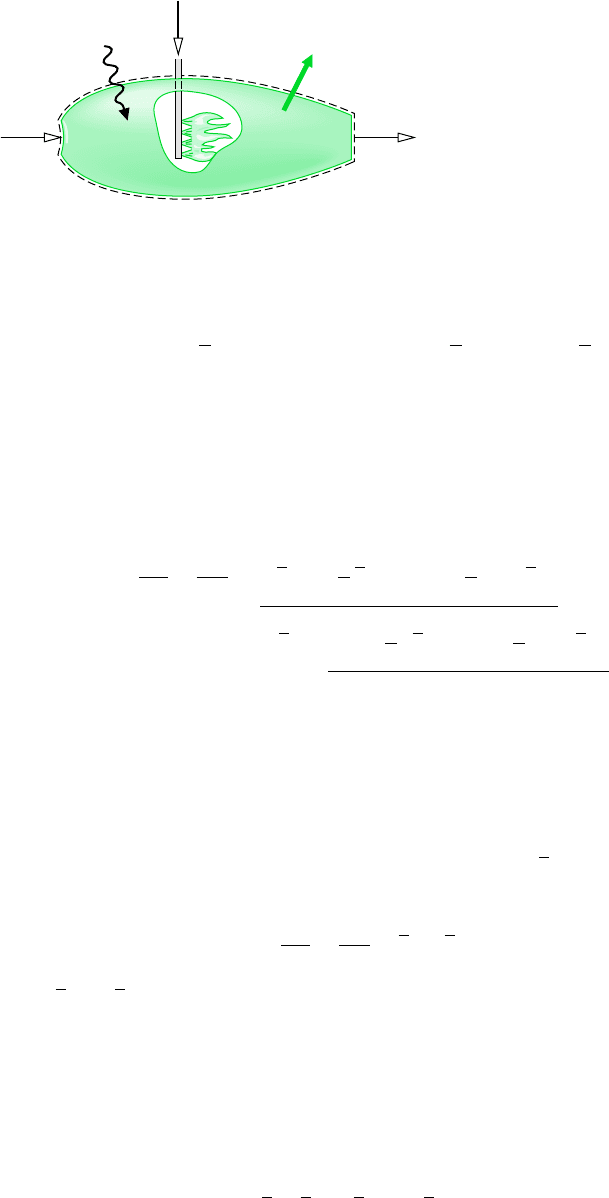
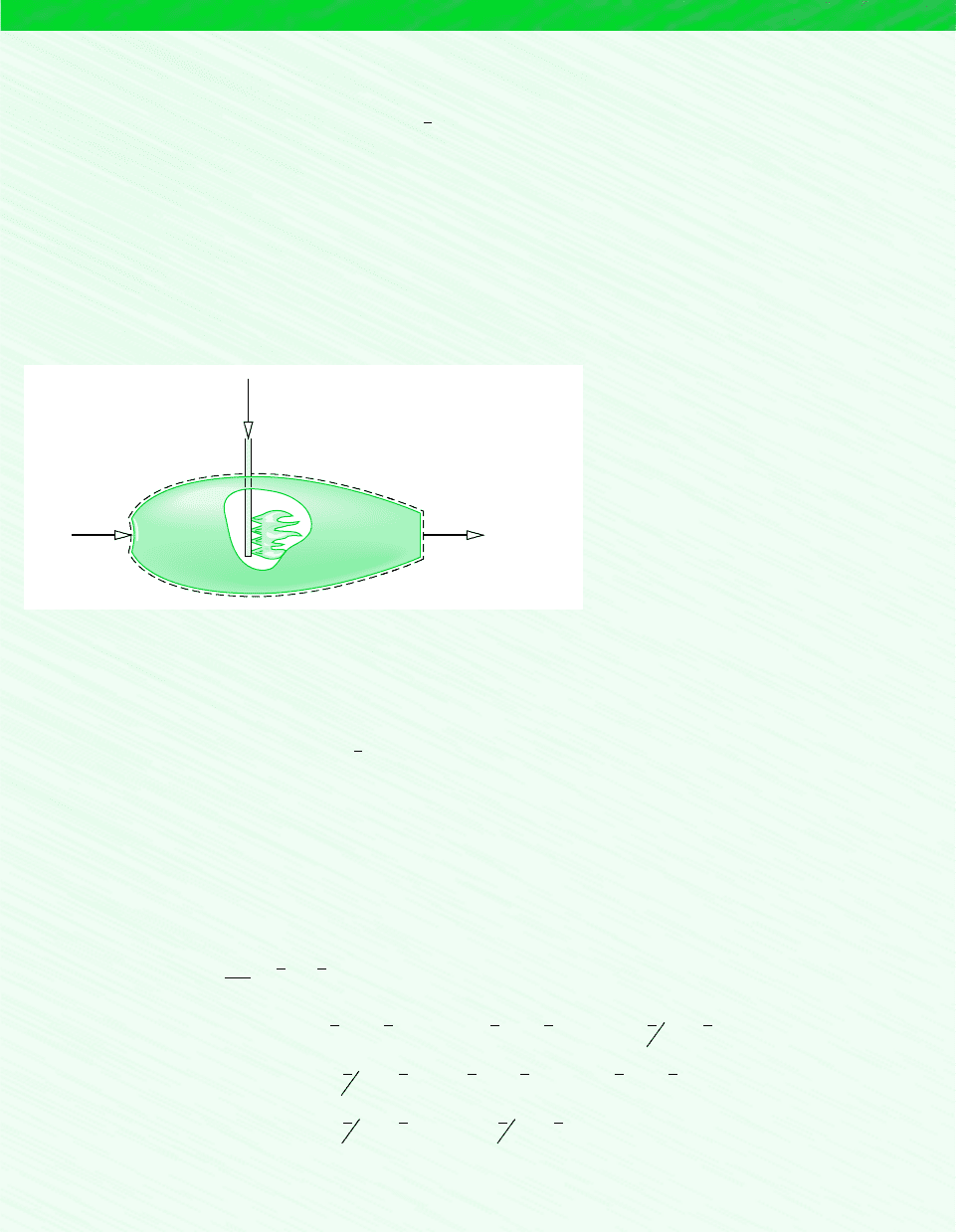
in a reaction in which the compound is formed from the elements. for example . . .
consider the simple reactor shown in Fig. 13.1, in which carbon and oxygen each enter at
T
ref
and p
ref
and react completely at steady state to form carbon dioxide at the same temperature
and pressure. Carbon dioxide is formed from carbon and oxygen according to
(13.6)
This reaction would be exothermic, so for the carbon dioxide to exit at the same tempera-
ture as the entering elements, there would be a heat transfer from the reactor to its sur-
roundings. The rate of heat transfer and the enthalpies of the incoming and exiting streams
are related by the energy rate balance
where and h denote, respectively, mass flow rate and specific enthalpy. In writing this
equation, we have assumed no work and negligible effects of kinetic and potential en-
ergy. For enthalpies on a molar basis, the energy rate balance appears as
where and denote, respectively, the molar flow rate and enthalpy per mole. Solving for
the specific enthalpy of carbon dioxide and noting from Eq. 13.6 that all molar flow rates
are equal
(13.7)
Since carbon and oxygen are stable elements at the standard state, and Eq. 13.7
becomes
(13.8)
Accordingly, the value assigned to the specific enthalpy of carbon dioxide at the standard
state, the enthalpy of formation, equals the heat transfer, per mole of CO
2
, between the re-
actor and its surroundings. If the heat transfer could be measured accurately, it would be
found to equal 393,520 kJ per kmol of carbon dioxide formed.
Table A-25 give values of the enthalpy of formation for several compounds in units of
kJ/kmol. In this text, the superscript is used to denote properties at 1 atm. For the case
of the enthalpy of formation, the reference temperature T
ref
is also intended by this symbol.
The values of listed in Table A-25 for CO
2
correspond to those given in the previous
example.
h
°
f
h
CO
2
Q
#
cv
n
#
CO
2
h
C
h
O
2
0,
h
CO
2
Q
#
cv
n
#
CO
2
n
#
C
n
#
CO
2
h
C
n
#
O
2
n
#
CO
2
h
O
2
Q
#
cv
n
#
CO
2
h
C
h
O
2
hn
#
0 Q
#
cv
n
#
C
h
C
n
#
O
2
h
O
2
n
#
CO
2
h
CO
2
W
#
cv
m
#
0 Q
#
cv
m
#
C
h
C
m
#
O
2
h
O
2
m
#
CO
2
h
CO
2
C O
2
S CO
2
h°
f
.
C
T
ref
, p
ref
O
2
T
ref
, p
ref
CO
2
T
ref
, p
ref
Figure 13.1 Reactor
used to discuss the
enthalpy of formation
concept.
enthalpy of formation

13.2 Conservation of Energy—Reacting Systems 631
The sign associated with the enthalpy of formation values appearing in Tables A-25 cor-
responds to the sign convention for heat transfer. If there is heat transfer from a reactor in
which a compound is formed from its elements (an exothermic reaction as in the previous
example), the enthalpy of formation has a negative sign. If a heat transfer to the reactor is
required (an endothermic reaction), the enthalpy of formation is positive.
EVALUATING ENTHALPY. The specific enthalpy of a compound at a state other than the
standard state is found by adding the specific enthalpy change between the standard state
and the state of interest to the enthalpy of formation
(13.9)
That is, the enthalpy of a compound is composed of associated with the formation of the
compound from its elements, and associated with a change of state at constant compo-
sition. An arbitrary choice of datum can be used to determine since it is a difference at
constant composition. Accordingly, can be evaluated from tabular sources such as the
steam tables, the ideal gas tables when appropriate, and so on. Note that as a consequence
of the enthalpy datum adopted for the stable elements, the specific enthalpy determined from
Eq. 13.9 is often negative.
Tables A-25 provide two values of the enthalpy of formation of water. One is for liq-
uid water and the other is for water vapor. Under equilibrium conditions, water exists only
as a liquid at 25C and 1 atm. The vapor value listed is for a hypothetical ideal gas state
in which water is a vapor at 25C and 1 atm. The difference between the two enthalpy of
formation values is given closely by the enthalpy of vaporization at T
ref
(13.10)
Similar considerations apply to other substances for which liquid and vapor values for are
listed in Tables A-25.
13.2.2 Energy Balances for Reacting Systems
Several considerations enter when writing energy balances for systems involving combus-
tion. Some of these apply generally, without regard for whether combustion takes place. For
example, it is necessary to consider if significant work and heat transfers take place and if
the respective values are known or unknown. Also, the effects of kinetic and potential en-
ergy must be assessed. Other considerations are related directly to the occurrence of com-
bustion. For example, it is important to know the state of the fuel before combustion occurs.
Whether the fuel is a liquid, a gas, or a solid is important. It is necessary to consider whether
the fuel is premixed with the combustion air or the fuel and air enter a reactor separately. The
state of the combustion products also must be assessed. It is important to know whether the
products of combustion are a gaseous mixture or whether some of the water formed on
combustion has condensed.
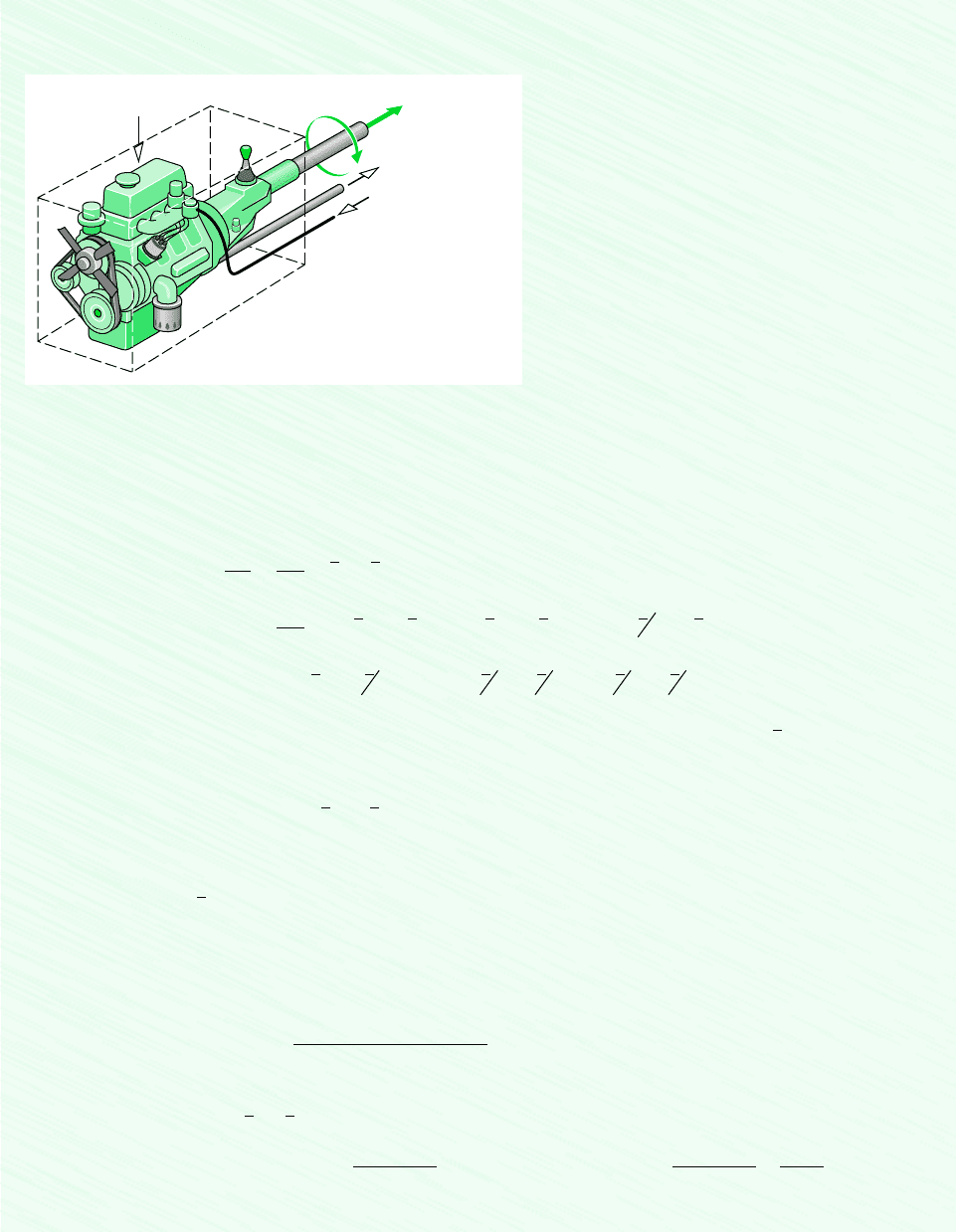
CONTROL VOLUMES AT STEADY STATE
To illustrate the many considerations involved when writing energy balances for reacting
systems, we consider special cases of broad interest, highlighting the underlying as-
sumptions. Let us begin by considering the steady-state reactor shown in Fig. 13.2, in
h
o
f
h°
f
1g2 h°
f
1l2 h
fg
h
fg
¢h
¢h,
¢h
,
h°
f
,
h
1T, p2 h°
f
3h1T, p2 h1T
ref
, p
ref
24 h°
f
¢h
¢h
METHODOLOGY
UPDATE
When applying Eq. 13.9 to
water vapor, we use the
vapor value of the en-
thalpy of formation of wa-
ter,
–
h from Table A-25
together with
–
h for water
vapor from the ideal gas
table, Table A-23.
°
f
1g2,
632 Chapter 13 Reacting Mixtures and Combustion
which a hydrocarbon fuel C
a
H
b
burns completely with the theoretical amount of air
according to
(13.11)
The fuel enters the reactor in a stream separate from the combustion air, which is regarded
as an ideal gas mixture. The products of combustion also are assumed to form an ideal gas
mixture. Kinetic and potential energy effects are ignored.
With the foregoing idealizations, the mass and energy rate balances for the two-inlet,
single-exit reactor can be used to obtain the following equation on a per mole of fuel
basis:
(13.12a)
where denotes the molar flow rate of the fuel. Note that each coefficient on the right side
of this equation is the same as the coefficient of the corresponding substance in the reaction
equation.
The first underlined term on the right side of Eq. 13.12a is the enthalpy of the exiting
gaseous products of combustion per mole of fuel. The second underlined term on the right
side is the enthalpy of the combustion air per mole of fuel. Note that the enthalpies of the
combustion products and the air have been evaluated by adding the contribution of each com-
ponent present in the respective ideal gas mixtures. The symbol denotes the molar en-
thalpy of the fuel. Equation 13.12a can be expressed more concisely as
(13.12b)
where and denote, respectively, the enthalpies of the products and reactants per mole
of fuel.
EVALUATING ENTHALPY TERMS. Once the energy balance has been written, the next step
is to evaluate the individual enthalpy terms. Since each component of the combustion prod-
ucts is assumed to behave as an ideal gas, its contribution to the enthalpy of the products de-
pends solely on the temperature of the products, T
P
. Accordingly, for each component of the
products, Eq. 13.9 takes the form
(13.13)
h h°
f
3h1T
P
2 h1T
ref
24
h
R
h
P
Q
#
cv
n
#
F
W
#
cv
n
#
F
h
P
h
R
h
F
n
#
F
h
F
caa
b
4
b h
O
2
aa
b
4
b 3.76h
N
2
d
Q
#
cv
n
#
F
W
#
cv
n
#
F
cah
CO
2
b
2
h
H
2
O
aa
b
4
b 3.76h
N
2
d
C
a
H
b
aa
b
4
b 1O
2
3.76N
2
2S aCO
2
b
2
H
2
O aa
b
4
b 3.76N
2
Air at
T
A
Combustion products
at T
P
W
cv
·
Q
cv
·
C
a
H
b
at
T
F
Figure 13.2 Reactor at
steady state.

13.2 Conservation of Energy—Reacting Systems 633
EXAMPLE 13.4 Analyzing an Internal Combustion Engine
Liquid octane enters an internal combustion engine operating at steady state with a mass flow rate of 1.8 10
3
kg/s and is
mixed with the theoretical amount of air. The fuel and air enter the engine at 25C and 1 atm. The mixture burns completely
and combustion products leave the engine at 890 K. The engine develops a power output of 37 kW. Determine the rate of heat
transfer from the engine, in kW, neglecting kinetic and potential energy effects.
SOLUTION
Known: Liquid octane and the theoretical amount of air enter an internal combustion engine operating at steady state in sep-
arate streams at 25C, 1 atm. Combustion is complete and the products exit at 890 K. The power developed by the engine and
fuel mass flow rate are specified.
Find: Determine the rate of heat transfer from the engine, in kW.
In Eq. 13.13, is the enthalpy of formation from Table A-25. The second term accounts
for the change in enthalpy from the temperature T
ref
to the temperature T
P
. For several com-
mon gases, this term can be evaluated from tabulated values of enthalpy versus temperature
in Tables A-23. Alternatively, the term can be obtained by integration of the ideal gas spe-
cific heat obtained from Tables A-21 or some other source of data. A similar approach
would be employed to evaluate the enthalpies of the oxygen and nitrogen in the combus-
tion air. For these
(13.14)
where T
A
is the temperature of the air entering the reactor. Note that the enthalpy of forma-
tion for oxygen and nitrogen is zero by definition and thus drops out of Eq. 13.14 as indi-
cated. The evaluation of the enthalpy of the fuel is also based on Eq. 13.9. If the fuel can be
modeled as an ideal gas, the fuel enthalpy is obtained using an expression of the same form
as Eq. 13.13 with the temperature of the incoming fuel replacing T
P
.
With the foregoing considerations, Eq. 13.12a takes the form
(13.15a)
The terms set to zero in this expression are the enthalpies of formation of oxygen and
nitrogen.
Equation 13.15a can be written more concisely as
(13.15b)
where i denotes the incoming fuel and air streams and e the exiting combustion products.
The coefficients n
i
and n
e
correspond to the respective coefficients of the reaction equation
giving the moles of reactants and products per mole of fuel, respectively. Although Eqs. 13.15
have been developed with reference to the reaction of Eq. 13.11, equations having the same
general forms would be obtained for other combustion reactions.
In Examples 13.4 and 13.5, the energy balance is applied together with tabular property
data to analyze control volumes at steady state involving combustion.
Q
#
cv
n
#
F
W
#
cv
n
#
F
a
P
n
e
1h°
f
¢h2
e
a
R
n
i
1h°
f
¢h2
i
1h°
f
¢h2
F
aa
b
4
b 1h°
f
0
¢h2
O
2
aa
b
4
b 3.761h°
f
0
¢h2
N
2
Q
#
cv
n
#
F
W
#
cv
n
#
F
a1h°
f
¢h2
CO
2
b
2
1h°
f
¢h2
H
2
O
aa
b
4
b 3.761h°
f
0
¢h2
N
2
h h°
f
0
3h1T
A
2 h1T
ref
24
c
p
h°
f
634 Chapter 13 Reacting Mixtures and Combustion
Schematic and Given Data:
Drive
shaft
Combustion products
at 1140°F
50 hp
Fuel at 77°F, 1 atm
Air at 77°F, 1 atm
Figure E13.4
Assumptions:
1. The control volume identified by a dashed line on
the accompanying figure operates at steady state.
2. Kinetic and potential energy effects can be ignored.
3. The combustion air and the products of combus-
tion each form ideal gas mixtures.
4. Each mole of oxygen in the combustion air is ac-
companied by 3.76 moles of nitrogen. The nitrogen is
inert and combustion is complete.
Analysis: The balanced chemical equation for complete combustion with the theoretical amount of air is obtained from the
solution to Example 13.1 as
The energy rate balance reduces, with assumptions 1–3, to give
where each coefficient is the same as the corresponding term of the balanced chemical equation and Eq. 13.9 has been used
to evaluate enthalpy terms. The enthalpy of formation terms for oxygen and nitrogen are zero, and for each of the
reactants, because the fuel and combustion air enter at 25C.
With the enthalpy of formation for C
8
H
18
(l) from Table A-25
With enthalpy of formation values for CO
2
and H
2
O(g) from Table A-25, and enthalpy values for N
2
,H
2
O, and CO
2
from
Table A-23
Using the molecular weight of the fuel from Table A-1, the molar flow rate of the fuel is
Inserting values into the expression for the rate of heat transfer
23.3 kW
37 kW c1.58 10
5
kmol 1fuel2
s
d34,069,466 1249,91024 a
kJ
kmol 1fuel2
b a
1 kW
1 kJ/s
b
Q
#
cv
W
#
cv
n
#
F
1h
P
h
R
2
n
#
F
1.8 10
3
kg 1fuel2/s
114.22 lb1fuel2
kmol1fuel2
1.58 10
5
kmol1fuel2/s
4,069,466 kJ/kmol
47326,568 8,6694
h
P
83393,520 136,876 936424 93241,820 131,429 9,90424
h
R
1h°
f
2
C
8
H
18
1l2
249,910 kJ/kmol 1fuel2
¢h
0
53h
°
f
¢h 4
0
C
8
H
18
1l2
12.53h°
f
0
¢h 4
0
O
2
473h°
f
0
¢h 4
0
N
2
6
W
#
cv
n
#
F
583h°
f
¢h 4
CO
2
93h°
f
¢h 4
H
2
O1g2
473h°
f
0
¢h 4
N
2
6
Q
#
cv
n
#
F
W
#
cv
n
#
F
h
P
h
R
C
8
H
18
12.5O
2
47N
2
S 8CO
2
9H
2
O 47N
2
13.2 Conservation of Energy—Reacting Systems 635
EXAMPLE 13.5 Analyzing a Combustion Chamber
Methane gas at 400 K and 1 atm enters a combustion chamber, where it is mixed with air entering at 500 K and 1 atm. The
products of combustion exit at 1800 K and 1 atm with the product analysis given in Example 13.2. For operation at steady
state, determine the rate of heat transfer from the combustion chamber in kJ per kmol of fuel. Neglect kinetic and potential
energy effects. The average value for the specific heat of methane between 298 and 400 K is 38 kJ/kmol K.
SOLUTION
Known: Methane gas, air, and combustion products enter and exit a combustion chamber at steady state in separate streams
with specified temperatures and a pressure of 1 atm. An analysis of the dry products of combustion is provided.
Find: Determine the rate of heat transfer, in kJ per kmol of fuel.
Schematic and Given Data:
#
c
p
Air at
500 K, 1 atm
Combustion products
1800 K, 1 atm
CH
4
(g)
400 K, 1 atm
Figure E13.5
Assumptions:
1. The control volume identified by a dashed line on the accompanying figure operates at steady state with
2. Kinetic and potential energy effects can be ignored.
3. The fuel is modeled as an ideal gas with The combustion air and the products of combustion each
form ideal gas mixtures.
4. Each mole of oxygen in the combustion air is accompanied by 3.76 moles of nitrogen, which is inert.
Analysis: When expressed on a per mole of fuel basis, the balanced chemical equation obtained in the solution to Example 13.2
takes the form
The energy rate balance reduces with assumptions 1–3 to give
where each coefficient in the equation is the same as the coefficient of the corresponding term of the balanced chemical equa-
tion and Eq. 13.9 has been used to evaluate the enthalpy terms. The enthalpies of formation for oxygen and nitrogen are set
to zero.
2.2651h
°
f
0
¢h2
O
2
8.5151h°
f
0
¢h2
N
2
4
8.5151h
°
f
0
¢h2
N
2
21h°
f
¢h2
H
2
O1g2
4 31h°
f
¢h2
CH
4
30.9511h°
f
¢h2
CO
2
0.0491h°
f
¢h2
CO
0.2891h°
f
0
¢h2
O
2
Q
#
cv
n
#
F
h
P
h
R
CH
4
2.265O
2
8.515N
2
S 0.951CO
2
0.049CO 0.289O
2
8.515N
2
2H
2
O
c
p
38 kJ/kmol
#
K.
W
#
cv
0.
