Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

656 Chapter 13 Reacting Mixtures and Combustion
Assuming the environment consists of an ideal gas mixture, the oxygen would enter at its
condition within the environment: temperature T
0
and partial pressure where is the
mole fraction of the oxygen in the exergy reference environment. The fuel and oxygen react
completely within the cell to produce carbon dioxide and water vapor, which exit in sepa-
rate streams at their conditions within the environment: temperature T
0
and the partial pres-
sures and respectively. The reaction is given by
(13.29)
For steady-state operation, the energy rate balance for a control volume enclosing the fuel
cell reduces to give
(13.30)
Kinetic and potential energy effects are regarded as negligible. Since the fuel cell is at steady
state, its volume does not change with time, so no portion of is required to displace the
environment. Thus, Eq. 13.30 gives the work developed by the combined system of system
plus environment. Heat transfer is assumed to occur with the environment only at tempera-
ture T
0
. An entropy rate balance for the control volume enclosing the fuel cell takes the form
(13.31)
Eliminating the heat transfer rate between Eqs. 13.30 and 13.31 results in
(13.32)
T
0
cs
F
aa
b
4
b s
O
2
as
CO
2
b
2
s
H
2
O
d T
0
s
#
cv
n
#
F
W
#
cv
n
#
F
ch
F
aa
b
4
b h
O
2
ah
CO
2
b
2
h
H
2
O
d
0
Q
#
cv
n
#
F
T
0
s
F
aa
b
4
b s
O
2
as
CO
2
b
2
s
H
2
O
s
#
cv
n
#
F
W
#
cv
W
#
cv
n
#
F
Q
#
cv
n
#
F
h
F
aa
b
4
b h
O
2
ah
CO
2
b
2
h
H
2
O
C
a
H
b
aa
b
4
b O
2
S aCO
2
b
2
H
2
O
y
e
H
2
O
p
0
,y
e
CO
2
p
0
y
e
O
2
y
e
O
2
p
0
,
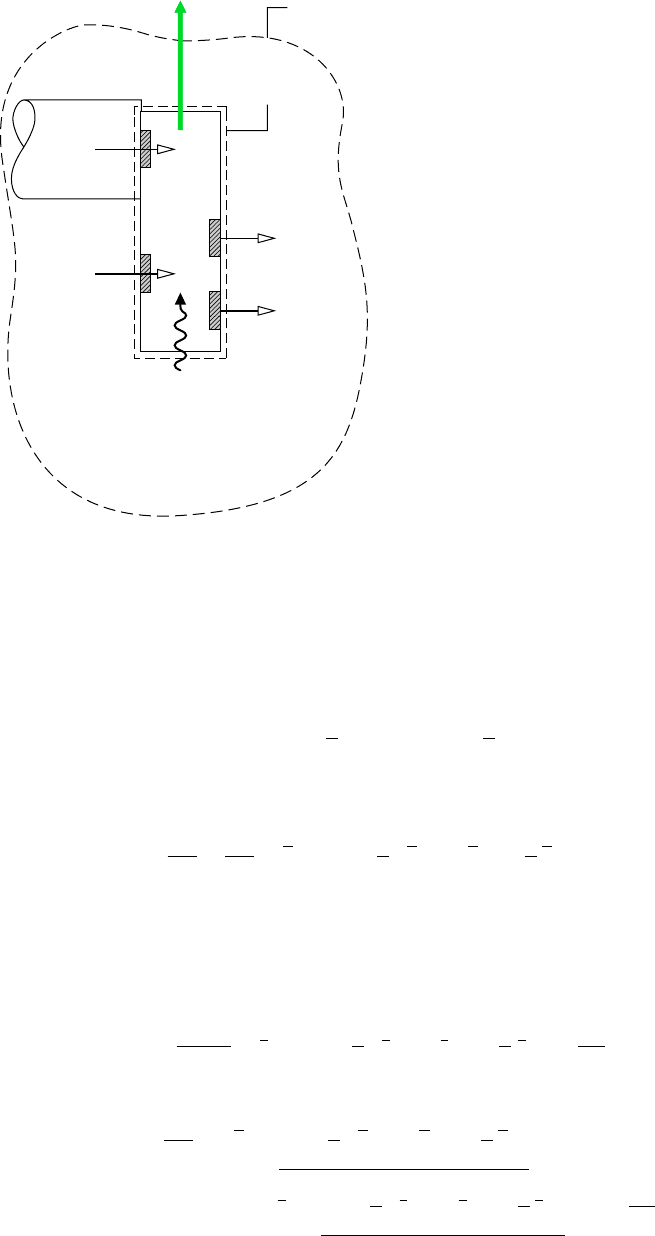
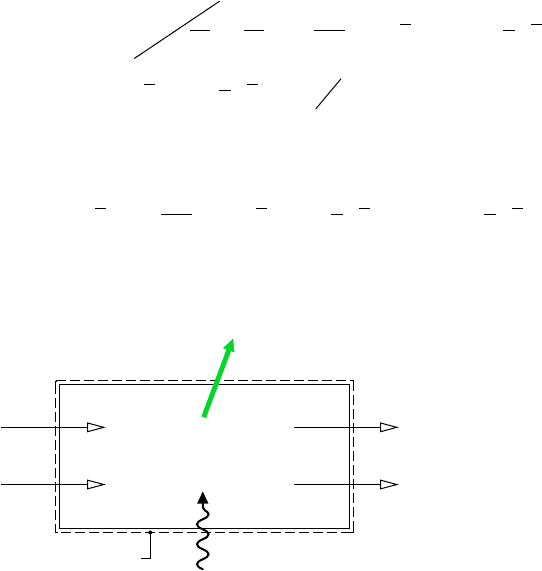
Work
Boundary of
combined system
Boundary of the cell
Heat transfer
with environment
Environment at T
0
, p
0
C
a
H
b
at
T
0
, p
0
T
0
CO
2
at T
0
, y
CO
2
p
0
e
O
2
at T
0
, y
O
2
p
0
e
H
2
O at T
0
, y
H
2
O
p
0
e
Figure 13.4 Illustration used to intro-
duce the fuel chemical exergy concept.
In Eq. 13.32, the specific enthalpy and entropy of the fuel are evaluated at T
0
and p
0
.
The values of the specific enthalpies in the first underlined term can be determined know-
ing only the temperature T
0
. The specific entropies in the second underlined term can be
determined knowing the temperature, pressure, and composition of the environment. Ac-
cordingly, once the environment is specified, all enthalpy and entropy terms of Eq. 13.32
can be regarded as known and independent of the nature of the processes occurring within
the control volume. The term depends explicity on the nature of such processes,
however. In accordance with the second law, is positive whenever internal irre-
versibilities are present, vanishes in the limiting case of no irreversibilities, and is never
negative. The maximum theoretical value for the work developed is obtained when no
irreversibilities are present. Setting to zero in Eq. 13.32, the following expression
for the chemical exergy results
(13.33)
The superscript ch is used to distinguish this contribution to the exergy magnitude from the
thermomechanical exergy introduced in Chap. 7.
WORKING EQUATIONS. For computational convenience, the chemical exergy given by
Eq. 13.33 can be written as Eq. 13.35 by replacing the specific entropy terms for O
2
,CO
2
,
and H
2
O using the following expression obtained by reduction of Eq. 6.21b
(13.34)
The first term on the right is the absolute entropy at T
0
and p
0
, and is the mole fraction of
component i in the environment.
Applying Eq. 13.34, Eq. 13.33 becomes
(13.35)
The specific enthalpy terms are determined using the enthalpies of formation for the re-
spective substances. The specific entropies appearing in the equation are absolute entropies
determined as described in Sec. 13.5. The logarithmic term normally contributes only a few
percent to the chemical exergy magnitude.
Equation 13.35 can be expressed alternatively in terms of the Gibbs functions of the re-
spective substances as
(13.36)
RT
0
ln c
1y
e
O
2
2
ab
4
1y
e
CO
2
2
a
1y
e
H
2
O
2
b
2
d
e
ch
cg
F
aa
b
4
b g
O
2
ag
CO
2
b
2
g
H
2
O1g2
d 1T
0
, p
0
2
RT
0
ln c
1y
e
O
2
2
ab
4
1y
e
CO
2
2
a
1y
e
H
2
O
2
b
2
d
T
0
cs
F
aa
b
4
b s
O
2
as
CO
2
b
2
s
H
2
O1g2
d 1T
0
, p
0
2
e
ch
ch
F
aa
b
4
b h
O
2
ah
CO
2
b
2
h
H
2
O1g2
d 1T
0
, p
0
2
y
i
e
s
i
1T
0
, y
e
i
p
0
2 s
i
1T
0
, p
0
2 R ln y
e
i
T
0
cs
F
aa
b
4
b s
O
2
as
CO
2
b
2
s
H
2
O
d
e
ch
ch
F
aa
b
4
b h
O
2
ah
CO
2
b
2
h
H
2
O
d
T
0
s
#
cv
T
0
s
#
cv
T
0
s
#
cv
13.6 Introducing Chemical Exergy 657
658 Chapter 13 Reacting Mixtures and Combustion
The specific Gibbs functions are evaluated at the temperature T
0
and pressure p
0
of the en-
vironment. These terms can be determined with Eq. 13.28 as
(13.37)
where is the Gibbs function of formation. For the special case where T
0
and p
0
are the
same as T
ref
and p
ref
, respectively, the second term on the right of Eq. 13.37 vanishes and the
specific Gibbs function is just the Gibbs function of formation. Finally, note that the under-
lined term of Eq. 13.36 can be written more compactly as the negative of the change
in Gibbs function for the reaction, Eq. 13.29, regarding each substance as separate at tem-
perature T
0
and pressure p
0
.
CHEMICAL EXERGY OF OTHER SUBSTANCES
The method introduced above for evaluating the chemical exergy of pure hydrocarbons
also can be used in principle for substances other than hydrocarbons: The chemical exergy
is the maximum theoretical work that could be developed by a fuel cell into which a sub-
stance of interest enters at T
0
, p
0
and reacts completely with environmental components to
produce environmental components. All environmental components involved enter and exit
the cell at their conditions within the environment. By describing the environment appro-
priately, this method can be applied to all substances of practical interest.
2
In the follow-
ing discussion, we limit consideration to the compounds CO, H
2
O, N
2
,O
2
, and CO
2
, for
these participate in the elementary combustion reactions serving as the focus of the pres-
ent chapter. Of these five compounds, only carbon monoxide is not among the substances
present in the environment we have been considering. Let us take up the compounds in the
order listed.
Paralleling the development of Eq. 13.36 for the case of pure carbon monoxide, at
T
0
, p
0
, the reaction within the cell is and the chemical exergy is
given by
(13.38)
If carbon monoxide is not pure but a component of an ideal gas mixture at T
0
, p
0
, each
component i of the mixture would enter the cell at temperature T
0
and the respective
partial pressure y
i
p
0
. The contribution of carbon monoxide to the chemical exergy of
the mixture, per mole of CO, is then given by Eq. 13.38 with the mole fraction of car-
bon monoxide in the mixture, y
CO
, appearing in the numerator of the logarithmic term
that then reads This consideration is important when evaluating the
exergy of combustion products involving carbon monoxide.
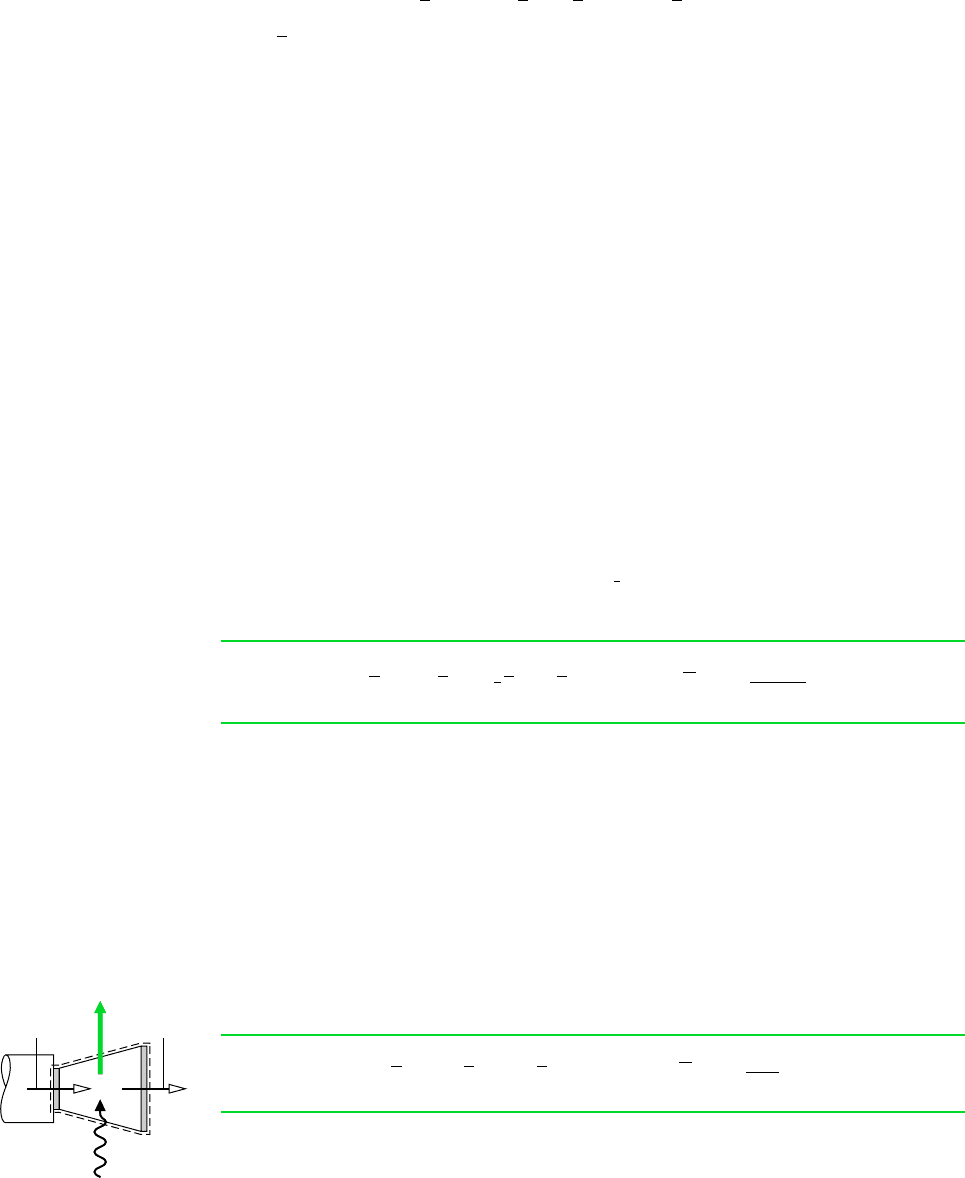
Next, consider the case of pure water at T
0
, p
0
. Water is present as a vapor within the
environment under present consideration but normally is a liquid when at T
0
, p
0
. Thus,
water would enter the cell as a liquid at T
0
, p
0
and exit as a vapor at T
0
, with no
cell reaction occurring. The chemical exergy is
(13.39)
e
ch
H
2
O
3g
H
2
O1l2
g
H
2
O1g2
41T
0
, p
0
2 RT
0
ln a
1
y
e
H
2
O
b
y
e
H
2
O
p
0
,
ln3y
CO
1y
e
O
2
2
1
2
y
e
CO
2
4.
e
ch
CO
3g
CO
1
2
g
O
2
g
CO
2
41T
0
, p
0
2 RT
0
ln c
1y
e
O
2
2
1
2
y
e
CO
2
d
CO
1
2
O
2
S CO
2
,
¢G:
g
o
f
g 1T
0
, p
0
2 g °
f
3g 1T
0
, p
0
2 g 1T
ref
, p
ref
24
2
For further discussion see M. J. Moran, Availability Analysis: A Guide to Efficient Energy Use, ASME Press,
New York, 1989, pp. 169–170.
H
2
O (l)
at
T
0
, p
0
H
2
O(g)
at
T
0
, y
H
2
O
p
0
e
T
0
13.7 Standard Chemical Exergy 659
Next, consider N
2
,O
2
,CO
2
, each pure at T
0
, p
0
. Nitrogen, oxygen, and carbon dioxide
are present within the environment, and normally are gases when at T
0
, p
0
. For each
case, the gas would enter the cell at T
0
, p
0
and exit at T
0
, y
e
p
0
, where y
e
is the mole
fraction of N
2
,O
2
, or CO
2
in the environment, as appropriate. No cell reaction would
occur. The chemical exergy is given simply by a logarithmic term of the form
(13.40)
Finally, for an ideal gas mixture at T
0
, p
0
consisting only of substances present as gases
in the environment, the chemical exergy is obtained by summing the contributions of
each of the components. The result, per mole of mixture, is
(13.41a)
where y
i
and denote, respectively, the mole fraction of component i in the mixture at
T
0
, p
0
and in the environment. Expressing the logarithmic term as and
introducing Eq. 13.40 for each gas, Eq. 13.41a can be written alternatively as
(13.41b)
The development of Eqs. 13.38 through 13.41 is left as an exercise. Subsequent examples
and end-of-chapter problems illustrate their use.
e
ch
a
i
y
i
e
ch
i
RT
0
a
i
y
i
ln y
i
1ln11
y
i
e
2 ln y
i
2
y
e
i
e
ch
RT
0
a
i
y
i
ln a
y
i
y
e
i
b
e
ch
RT
0
ln a
1
y
e
b
N
2
at
T
0
, p
0
N
2
at
T
0
, y
N
2
p
0
e
T
0
13.7 Standard Chemical Exergy
The exergy reference environments used thus far to calculate exergy values are adequate for
a wide range of practical applications, including combustion. However, for many cases of
interest the environment must be extended to include other substances. In applications in-
volving coal, for example, sulfur dioxide or some other sulfur-bearing compound would gen-
erally appear among the environmental components. Furthermore, once the environment is
determined, a series of calculations would be required to obtain exergy values for the sub-
stances of interest. These complexities can be sidestepped by using a table of standard
chemical exergies.
Standard chemical exergy values are based on a standard exergy reference environment
exhibiting standard values of the environmental temperature T
0
and pressure p
0
such as
298.15 K and 1 atm, respectively. The exergy reference environment also consists of a set
of reference substances with standard concentrations reflecting as closely as possible
the chemical makeup of the natural environment. To exclude the possibility of develop-
ing work from interactions among parts of the environment, these reference substances must
be in equilibrium mutually.
The reference substances generally fall into three groups: gaseous components of the
atmosphere, solid substances from the Earth’s crust, and ionic and nonionic substances
from the oceans. A common feature of standard exergy reference environments is a gas
phase, intended to represent air, that includes N
2
,O
2
,CO
2
,H
2
O(g), and other gases. The
standard chemical exergy
660 Chapter 13 Reacting Mixtures and Combustion
ith gas present in this gas phase is assumed to be at temperature T
0
and the partial pressure
Two alternative standard exergy reference environments are commonly used, called here
Model I and Model II. For each of these models, Table A-26 gives values of the standard
chemical exergy for several substances, in units of kJ/kmol, together with a brief description
of the underlying rationale. The methods employed to determine the tabulated standard chem-
ical exergy values are detailed in the references accompanying the tables. Only one of the
two models should be used in a particular analysis.
The use of a table of standard chemical exergies often simplifies the application of ex-
ergy principles. However, the term “standard” is somewhat misleading, for there is no one
specification of the environment that suffices for all applications. Still, chemical exergies
calculated relative to alternative specifications of the environment are generally in good
agreement. For a broad range of engineering applications, the convenience of using stan-
dard values generally outweighs the slight lack of accuracy that might result. In particular,
the effect of slight variations in the values of T
0
and p
0
about their standard values can be
neglected.
STANDARD CHEMICAL EXERGY OF A HYDROCARBON: C
a
H
b
In principle, the standard chemical exergy of a substance not present in the environment can
be evaluated by considering an idealized reaction of the substance involving other substances
for which the chemical exergies are known. To illustrate this for the case of a pure hydro-
carbon fuel C
a
H
b
at T
0
, p
0
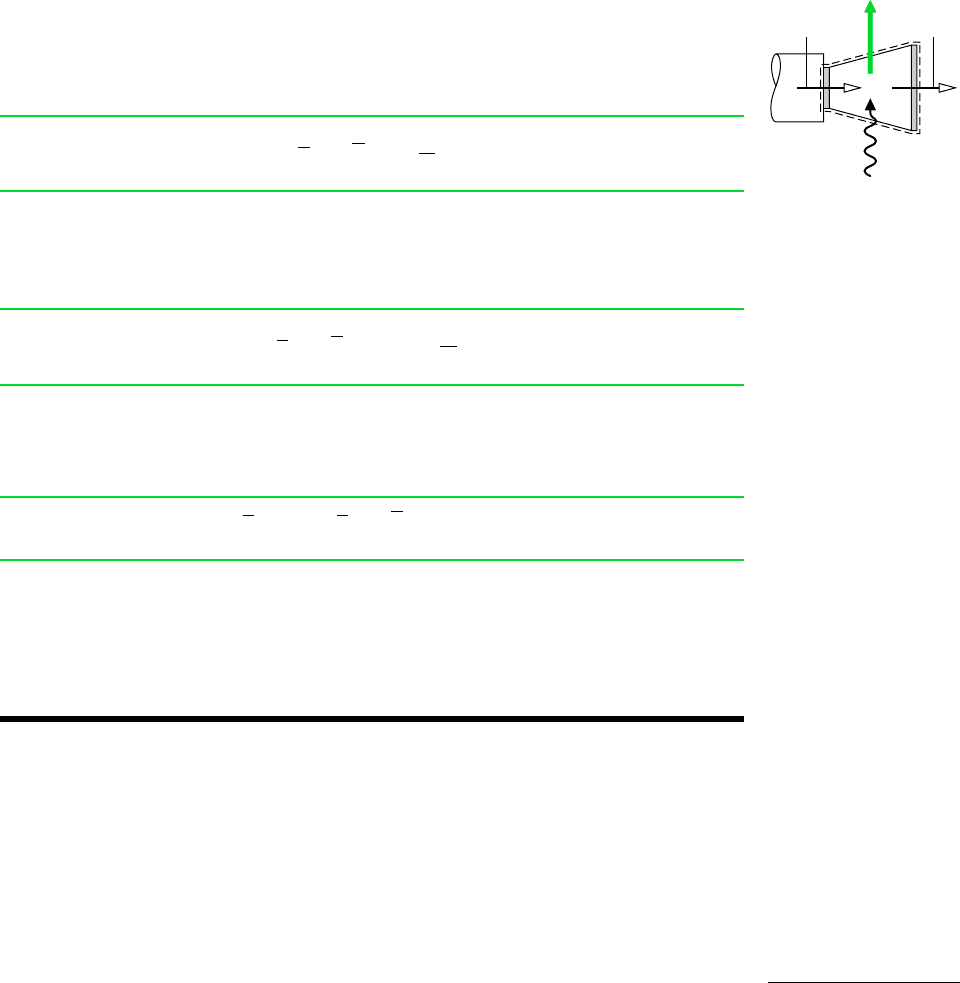
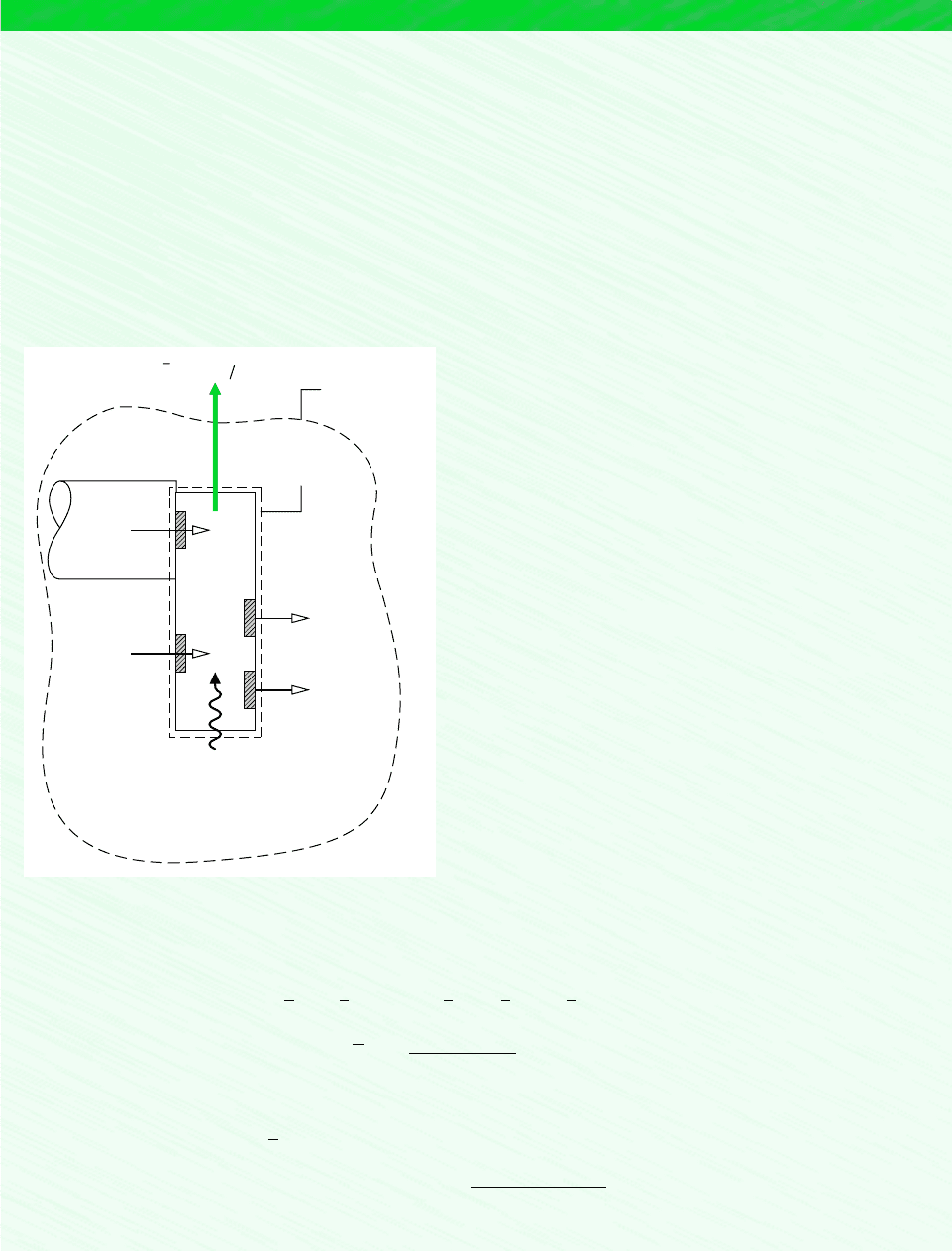
, refer to the control volume at steady state shown in Fig. 13.5
where the fuel reacts with oxygen to form carbon dioxide and liquid water according to
Eq. 13.29. All substances are assumed to enter and exit at T
0
, p
0
and heat transfer occurs only
at temperature T
0
.
Assuming no irreversibilities, an exergy rate balance reads
where, as before, the subscript F denotes the fuel. Solving for the fuel chemical exergy, we
obtain
(13.42)e
ch
F
a
W
#
cv
n
#
F
b
rev
int
ae
ch
CO
2
a
b
2
b e
ch
H
2
O1l2
aa
b
2
b e
ch
O
2
ae
ch
CO
2
a
b
2
b e
ch
H
2
O1l2
E
#
0
d
0
a
j
c1
T
0
T
j
d
0
a
Q
#
j
n
#
F
b a
W
#
cv
n
#
F
b
rev
int
e
ch
F
aa
b
4
b e
ch
O
2
p
e
i
y
e
i
p
0
.
C
a
H
b
at T
0
, p
0
O
2
at T
0
, p
0
CO
2
at T
0
, p
0
H
2
O (l)
at T
0
, p
0
T
0
Q
cv
·
W
cv
·
Figure 13.5 Reactor
used to introduce the stan-
dard chemical exergy of
C
a
H
b
.

13.7 Standard Chemical Exergy 661
3
See, for example, A. Bejan, G. Tsatsaronis, and M. J. Moran, Thermal Design and Optimization,Wiley,
New York, 1996, Sec. 3.4.3.
Paralleling the development of Eq. 13.32 for the case of Fig. 13.5, we also have
(13.43)
The underlined term in Eq. 13.43 is recognized from Sec. 13.2 as the molar higher heat-
ing value (T
0
, p
0
). Introducing Eq. 13.43 into Eq. 13.42, we have
(13.44a)
Equations 13.42 and 13.43 can be expressed alternatively in terms of molar Gibbs functions
as follows
(13.44b)
With Eqs. 13.44, the standard chemical exergy of the hydrocarbon C
a
H
b
can be calcu-
lated using the standard chemical exergies of O
2
,CO
2
,and H
2
O(l), together with selected
property data: the higher heating value and absolute entropies, or Gibbs functions. for
example. . .
consider the case of methane, CH
4
, and T
0
298.15 K (25C), p
0
1 atm.
For this application we can use Gibbs function data directly from Table A-25, and standard
chemical exergies for CO
2
,H
2
O(l), and O
2
from Table A-26 (Model II), since each source
corresponds to 298 K, 1 atm. With a 1, b 4, Eq. 13.44b gives 831,680 kJ/kmol. This
agrees with the value listed for methane in Table A-26 for Model II.
We conclude the present discussion by noting special aspects of Eqs. 13.44:
First, Eq. 13.44a requires the higher heating value and the absolute entropy of the fuel.
When data from property compilations are lacking, as in the cases of coal, char, and
fuel oil, the approach of Eq. 13.44a can be invoked using a measured or estimated
heating value and an estimated value of the fuel absolute entropy determined with
procedures discussed in the literature.
3
Next, note that the first term of Eq. 13.44b involving the Gibbs functions has the same
form as the first term of Eq. 13.36, except here liquid water appears. Also, the first term
of Eq. 13.44b can be written more compactly as the negative of the change in
Gibbs function for the reaction.
Finally, note that only the underlined terms of Eqs. 13.44 require chemical exergy data
relative to the model selected for the exergy reference environment.
In Example 13.12 we compare the use of Eq. 13.36 and Eq. 13.44b for evaluating the
chemical exergy of a pure hydrocarbon fuel.
¢G:
ae
ch
CO
2
a
b
2
b e
ch
H
2
O1l2
aa
b
4
b e
ch
O
2
e
ch
F
cg
F
aa
b
4
b g
O
2
ag
CO
2
b
2
g
H
2
O1l2
d1T
0
, p
0
2
ae
ch
CO
2
a
b
2
b e
ch
H
2
O1l2
aa
b
4
b e
ch
O
2
e
ch
F
HHV1T
0
, p
0
2 T
0
cs
F
aa
b
4
b s
O
2
as
CO
2
b
2
s
H
2
O1l2
d1T
0
, p
0
2
HHV
T
0
cs
F
aa
b
4
b s
O
2
as
CO
2
b
2
s
H
2
O1l2
d1T
0
, p
0
2
a
W
#
cv
n
#
F
b
rev
int
ch
F
aa
b
4
b h
O
2
ah
CO
2
b
2
h
H
2
O1l2
d1T
0
, p
0
2
662 Chapter 13 Reacting Mixtures and Combustion
EXAMPLE 13.12 Evaluating the Chemical Exergy of Octane
Determine the chemical exergy of liquid octane at 25C, 1 atm, in kJ/kg. (a) Using Eq. 13.36, evaluate the chemical exergy
for an environment consisting of a gas phase at 25C, 1 atm obeying the ideal gas model with the following composition on
a molar basis: N
2
, 75.67%; O
2
, 20.35%; H
2
O, 3.12%; CO
2
, 0.03%; other, 0.83%. (b) Evaluate the chemical exergy using
Eq. 13.44b and standard chemical exergies from Table A-26 (Model II).
SOLUTION
Known: The fuel is liquid octane.
Find: Determine the chemical exergy (a) using Eq. 13.36 relative to an environment consisting of a gas phase at 25C, 1 atm
with a specified composition, (b) using Eq. 13.44b and standard chemical exergies.
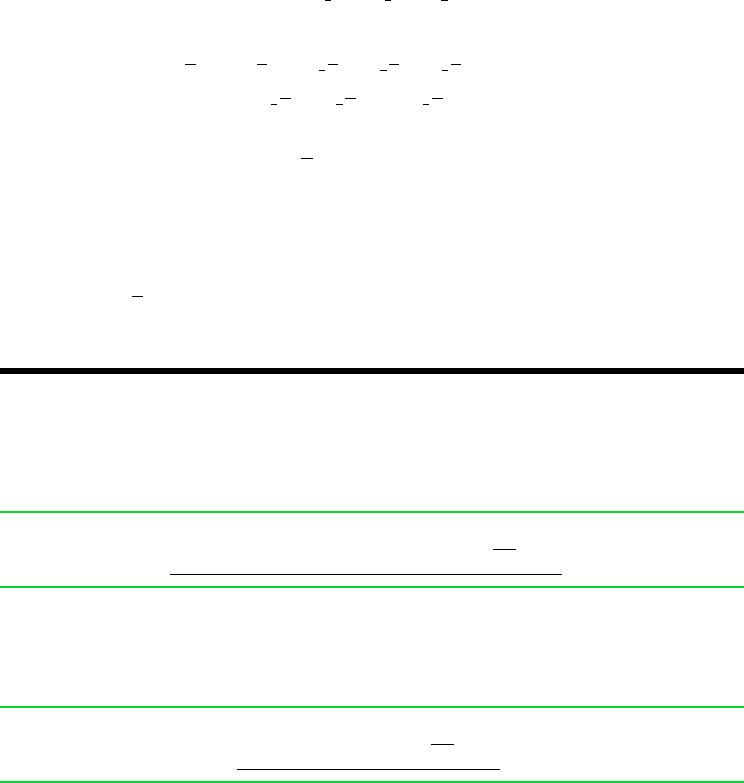
Schematic and Given Data:
Boundary of
combined system
Boundary of the cell
Heat transfer
with environment
C
8
H
18
at
T
0
, p
0
T
0
CO
2
at T
0
, y
CO
2
p
0
e
O
2
at T
0
, y
O
2
p
0
e
H
2
O at T
0
, y
H
2
O
p
0
e
e
ch
= (W
cv
n
F
)
max
·
·
Environment:
T
0
p
0
y
N
2
y
O
2
y
H
2
O
(g)
y
CO
2
e
e
e
e
= 25°C
= 1 atm
= 0.7567
= 0.2035
= 0.0312
= 0.0003
Figure E13.12
Assumptions: As shown in Fig. E13.12, the environment for
part (a) consists of an ideal gas mixture with the molar analysis:
N
2
, 75.67%; O
2
, 20.35%; H
2
O, 3.12%; CO
2
, 0.03%; other, 0.83%.
For part (b), Model II of Table A-26 applies.
Analysis: Complete combustion of liquid octane with O
2
is described by
(a) For this reaction equation, Eq. 13.36 takes the form
Since T
0
T
ref
and p
0
p
ref
, the required specific Gibbs functions are just the Gibbs functions of formation from Table
A-25. With the given composition of the environment and data from Table A-25, the above equation gives
5,218,960 188,883 5,407,843 kJ/kmol
8.3141298.152 ln c
10.20352
12.5
10.00032
8
10.03122
9
d
e
ch
36610 12.5102 81394,3802 91228,59024
R
T
0
ln c
1y
e
O
2
2
12.5
1y
e
CO
2
2
8
1y
e
H
2
O1g2
2
9
d
e
ch
3g
C
8
H
18
1l2
12.5 g
O
2
8 g
CO
2
9 g
H
2
O1g2
41T
0
, p
0
2
C
8
H
18
1l2 12.5O
2
S 8CO
2
9H
2
O
❶
❷

13.7 Standard Chemical Exergy 663
Dividing by the molecular weight, the chemical exergy is obtained on a unit mass basis
(b) Using coefficients from the reaction equation above, Eq. 13.44b reads
With data from Table A-25 and Model II of Table A-26, the above equation gives
As expected, this agrees closely with the value listed for octane in Table A-26 (Model II). Dividing by the molecular weight,
the chemical exergy is obtained on a unit mass basis
The chemical exergies determined with the two approaches used in parts (a) and (b) closely agree.
A molar analysis of the environment of part (a) on a dry basis reads, O
2
: 21%, N
2
,CO
2
and the other dry components:
79%. This is consistent with the dry air analysis used throughout the chapter. The water vapor present in the assumed en-
vironment corresponds to the amount of vapor that would be present were the gas phase saturated with water at the spec-
ified temperature and pressure.
The value of the logarithmic term of Eq. 13.36 depends on the composition of the environment. In the present case, this
term contributes about 3% to the magnitude of the chemical exergy. The contribution of the logarithmic term is usually
small. In such instances, a satisfactory approximation to the chemical exergy can be obtained by omitting the term.
The terms of Eqs. 13.36 and 13.44b involving the Gibbs functions have the same form, except appears in Eq. 13.36
for water vapor and in Eq. 13.44b for liquid water.
g
H
2
O
e
ch
5,413,705
114.22
47,397 kJ/kg
5,296,270 117,435 5,413,705 kJ/kmol
8119,8702 919002 12.5139702
e
ch
36610 12.5102 81394,3802 91237,18024
8
e
ch
CO
2
9 e
ch
H
2
O1l2
12.5 e
ch
O
2
e
ch
3g
C
8
H
18
1l2
12.5 g
O
2
8 g
CO
2
9 g
H
2
O1l2
41T
0
, p
0
2
e
ch
5,407,843
114 .22
47,346 kJ/kg
❸
❶
❷
❸
STANDARD CHEMICAL EXERGY OF OTHER SUBSTANCES
By paralleling the development given above for hydrocarbon fuels leading to Eq. 13.44b, we
can in principle determine the standard chemical exergy of any substance not present in the
environment. With such a substance playing the role of the fuel in the previous development,
we consider a reaction of the substance involving other substances for which the standard
chemical exergies are known, and write
(13.45)
where G is the change in Gibbs function for the reaction, regarding each substance as sep-
arate at temperature T
0
and pressure p
0
. The underlined term corresponds to the underlined
term of Eq. 13.44b and is evaluated using the known standard chemical exergies, together
with the n’s giving the moles of these reactants and products per mole of the substance whose
chemical exergy is being evaluated.
for example. . . consider the case of ammonia, NH
3
, and T
0
298.15 K (25C),
p
0
1 atm. Letting NH
3
play the role of the hydrocarbon in the development leading to
e
ch
¢G
a
P
ne
ch
a
R
ne
ch
664 Chapter 13 Reacting Mixtures and Combustion
Eq. 13.44b, we can consider any reaction of NH
3
involving other substances for which the
standard chemical exergies are known. For the reaction
Eq. 13.45 takes the form
Using Gibbs function data from Table A-25, and standard chemical exergies for O
2
,N
2
, and
H
2
O(l) from Table A-26 (Model II), kJ/kmol. This agrees closely with the
value for ammonia listed in Table A-26 for Model II.
Finally, note that Eq. 13.41b is valid for mixtures containing gases other than those pres-
ent in the reference environment, for example gaseous fuels. This equation also can be applied
to mixtures (and solutions) that do not adhere to the ideal gas model. In all such applica-
tions, the terms are selected from a table of standard chemical exergies.e
ch
i
e
ch
NH
3
337,910
1
2
e
ch
N
2
3
2
e
ch
H
2
O1l2
3
4
e
ch
O
2
e
ch
NH
3
3g
NH
3
3
4
g
O
2
1
2
g
N
2
3
2
g
H
2
O1l2
41T
0
, p
0
2
NH
3
3
4
O
2
S
1
2
N
2
3
2
H
2
O
13.8 Exergy Summary
The exergy associated with a specified state of a system is the sum of two contributions: the
thermomechanical contribution introduced in Chap. 7 and the chemical contribution intro-
duced in this chapter. On a unit mass basis, the total exergy is
(13.46)
where the underlined term is the thermomechanical contribution (Eq. 7.9) and e
ch
is the chem-
ical contribution evaluated as in Sec. 13.6 or 13.7. Similarly, the specific flow exergy asso-
ciated with a specified state is the sum
(13.47)
where the underlined term is the thermomechanical contribution (Eq. 7.20) and e
ch
is the
chemical contribution.
When evaluating the thermomechanical contributions, we can think of bringing the system
without change in composition from the specified state to T
0
, p
0
, the condition where the sys-
tem is in thermal and mechanical equilibrium with the environment. Depending on the nature
of the system, this may be a hypothetical condition.
When a difference in exergy or flow exergy between states of the same composition is
evaluated, the chemical contribution cancels, leaving just the difference in the thermome-
chanical contributions. For such a calculation, it is unnecessary to evaluate the chemical ex-
ergy explicitly. However, for many evaluations it is necessary to account explicitly for the
chemical exergy contribution. This is brought out in subsequent examples.
ILLUSTRATIONS
The following two examples illustrate the exergy principles considered above. In Exam-
ple 13.13, Eq. 13.47 is applied to evaluate the specific flow exergy of a steam leak.
e
f
h h
0
T
0
1s s
0
2
V
2
2
gz e
ch
e 1u u
0
2 p
0
1v v
0
2 T
0
1s s
0
2
V
2
2
gz e
ch
total exergy

13.8 Exergy Summary 665
EXAMPLE 13.13 Evaluating the Flow Exergy of a Steam Leak
Steam at 5 bar, 240C leaks from a line in a vapor power plant. Evaluate the flow exergy of the steam, in kJ/kg, relative to
an environment at 25C, 1 atm in which the mole fraction of water vapor is
SOLUTION
Known: Water vapor at a known state is specified. The environment is also described.
Find: Determine the flow exergy of the water vapor, in kJ/kg.
Assumptions:
1. The environment consists of a gas phase that obeys the ideal gas model. The mole fraction of water vapor in the environ-
ment is 0.0303.
2. Neglect the effects of motion and gravity.
Analysis: With assumption 2, the specific flow exergy is given by Eq. 13.47 as
The underlined term is the thermomechanical contribution to the flow exergy, evaluated as in Chap. 7. With data from the
steam tables, and noting that water is a liquid at T
0
, p
0
where h
0
and s
0
are approximated as the saturated liquid values at T
0
.
The chemical exergy contribution to the flow exergy relative to the specified environment is evaluated using Eq. 13.39.
With data from Table A-25
Adding the thermomechanical and chemical contributions, the flow exergy of steam at the specified state is
In this case, the chemical exergy contributes less than 1% to the total flow exergy magnitude.
e
f
789.7 4.1 793.8 kJ/kg
73.1 kJ/kmol
18 kg/kmol
4.1 kJ/kg
1
18
e3237,180 1228,59024 18.314212982 ln a
1
0.0303
bf
e
ch
1
M
e3g
H
2
O1l2
g
H
2
O1g2
41T
0
, p
0
2 RT
0
ln a
1
y
e
H
2
O
bf
789.7 kJ/kg
h h
0
T
0
1s s
0
2 12939.9 104.92 29817.2307 0.36742
e
f
1h h
0
2 T
0
1s s
0
2
e
ch
y
e
H
2
O
0.0303.
In Example 13.14, we evaluate the flow exergy of combustion products.
EXAMPLE 13.14 Evaluating the Flow Exergy of Combustion Products
Methane gas enters a reactor and burns completely with 140% theoretical air. Combustion products exit as a mixture at tem-
perature T and a pressure of 1 atm. For T 480 and 1560 K, evaluate the flow exergy of the combustion products, in kJ per
kmol of fuel. Perform calculations relative to an environment consisting of an ideal gas mixture at 25C, 1 atm with the molar
analysis, y
e
N
2
0.7567, y
e
O
2
0.2035, y
e
H
2
O
0.0303, y
e
CO
2
0.0003.
