Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

586 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
EXAMPLE 12.8 Cooling Moist Air at Constant Volume
An air–water vapor mixture is contained in a rigid, closed vessel with a volume of 35 m
3
at 1.5 bar, 120C, and 10%.
The mixture is cooled at constant volume until its temperature is reduced to 22C. Determine (a) the dew point temperature
corresponding to the initial state, in C, (b) the temperature at which condensation actually begins, in C, and (c) the amount
of water condensed, in kg.
SOLUTION
Known: A rigid, closed tank with a volume of 35 m
3
containing moist air initially at 1.5 bar, 120C, and 10% is cooled
to 22C.
Find: Determine the dew point temperature at the initial state, in C, the temperature at which condensation actually begins,
in C, and the amount of water condensed, in kg.
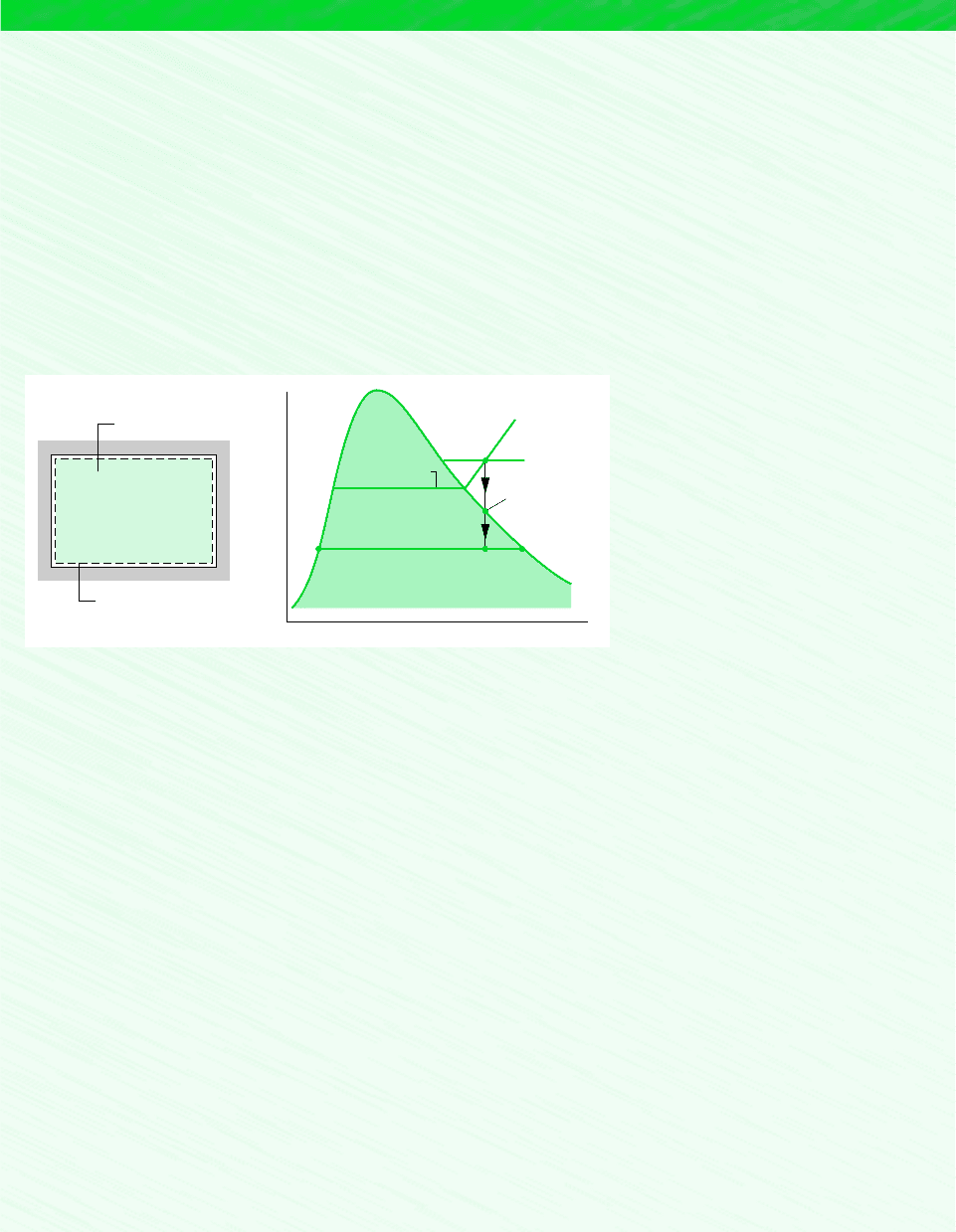
Schematic and Given Data:
T
v
p
v1
T
1
f2 g2
2
1′
1
Condensation
begins
Dew point
temperature
V = 35 m
3
Boundary
Initially moist air
at 1.5 bar, 120°C
= 10%
φ
Figure E12.8
Assumptions:
1. The contents of the tank are taken as a closed system. The system volume remains constant.
2. The gas phase can be treated as an ideal gas mixture. Each mixture component acts as an ideal gas existing alone in the
volume occupied by the gas phase at the mixture temperature.
3. When a liquid water phase is present, the water vapor exists as a saturated vapor at the system temperature. The liquid is
a saturated liquid at the system temperature.
Analysis:
(a) The dew point temperature at the initial state is the saturation temperature corresponding to the partial pressure p
v1
. With
the given relative humidity and the saturation pressure at 120C from Table A-2
Interpolating in Table A-2 gives the dew point temperature as 60C, which is the temperature condensation would begin if the
moist air were cooled at constant pressure.
(b) Whether the water exists as a vapor only, or as liquid and vapor, it occupies the full volume, which remains constant. Ac-
cordingly, since the total mass of the water present is also constant, the water undergoes the constant specific volume process
illustrated on the accompanying T–v diagram. In the process from state 1 to state 1, the water exists as a vapor only. For the
process from state 1 to state 2, the water exists as a two-phase liquid–vapor mixture. Note that pressure does not remain con-
stant during the cooling process from state 1 to state 2.
State 1 on the T–v diagram denotes the state where the water vapor first becomes saturated. The saturation temperature
at this state is denoted as T . Cooling to a temperature less than T would result in condensation of some of the water vapor
present. Since state 1 is a saturated vapor state, the temperature T can be found by interpolating in Table A-2 with the specific
p
v1
f
1
p
g1
10.102 11.9852 0.1985 bar
12.5 Introducing Psychrometric Principles 587
volume of the water at this state. The specific volume of the vapor at state 1 equals the specific volume of the vapor at state 1,
which can be evaluated from the ideal gas equation
Interpolation in Table A-2 with v
v1
v
g
gives T 56C. This is the temperature at which condensation begins.
(c) The amount of condensate equals the difference between the initial and final amounts of water vapor present. The mass
of the water vapor present initially is
The mass of water vapor present finally can be determined from the quality. At the final state, the water forms a two-phase
liquid–vapor mixture having a specific volume of 9.145 m
3
/kg. Using this specific volume value, the quality x
2
of the
liquid–vapor mixture can be found as
where v
f2
and v
g2
are the saturated liquid and saturated vapor specific volumes at T
2
22C, respectively.
Using the quality together with the known total amount of water present, 3.827 kg, the mass of the water vapor contained
in the system at the final state is
The mass of the condensate, m
w2
, is then
When a moist air mixture is cooled at constant mixture volume, the temperature at which condensation begins is not the
dew point temperature corresponding to the initial state. In this case, condensation begins at 56C, but the dew point tem-
perature at the initial state, determined in part (a), is 60C.
m
w2
m
v1
m
v2
3.827 0.681 3.146 kg
m
v2
10.1782 13.8272 0.681 kg
x
2
v
v2
v
f2
v
g2
v
f2
9.145 1.0022 10
3
51.447 1.0022 10
3
0.178
m
v1
V
v
v1
35 m
3
9.145 m
3
/kg
3.827 kg
9.145
m
3
kg
v
v1
1R
M
v
2T
1
p
v1
a
8314
18
N
#
m
kg
#
K
b a
393 K
0.1985 10
5
N/m
2
b
No additional fundamental concepts are required for the study of closed systems involv-
ing mixtures of dry air and water vapor. Example 12.9, which builds on Example 12.8, brings
out some special features of the use of conservation of mass and conservation of energy in
analyzing this kind of system. Similar considerations can be used to study other closed systems
involving moist air.
EXAMPLE 12.9 Evaluating Heat Transfer for Moist Air Cooling at Constant Volume
An air–water vapor mixture is contained in a rigid, closed vessel with a volume of 35 m
3
at 1.5 bar, 120C, and 10%.
The mixture is cooled until its temperature is reduced to 22C. Determine the heat transfer during the process, in kJ.
SOLUTION
Known: A rigid, closed tank with a volume of 35 m
3
containing moist air initially at 1.5 bar, 120C, and 10% is cooled
to 22C.
Find: Determine the heat transfer for the process, in kJ.
Schematic and Given Data: See the figure for Example 12.8.
❶
❶
588 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
Assumptions:
1. The contents of the tank are taken as a closed system. The system volume remains constant.
2. The gas phase can be treated as an ideal gas mixture. Each component acts as an ideal gas existing alone in the volume
occupied by the gas phase at the mixture temperature.
3. When a liquid water phase is present, the water vapor exists as a saturated vapor and the liquid is a saturated liquid, each
at the system temperature.
4. There is no work during the cooling process and no change in kinetic or potential energy.
Analysis: Reduction of the closed system energy balance using assumption 4 results in
or
where
and
In these equations, the subscripts a, v, and w denote, respectively, dry air, water vapor, and liquid water. The specific internal
energy of the water vapor at the initial state can be approximated as the saturated vapor value at T
1
. At the final state, the wa-
ter vapor is assumed to exist as a saturated vapor, so its specific internal energy is u
g
at T
2
. The liquid water at the final state
is saturated, so its specific internal energy is u
f
at T
2
.
Collecting the last three equations
The mass of dry air, m
a
, can be found using the ideal gas equation of state together with the partial pressure of the dry air
at the initial state obtained using p
v1
0.1985 bar from the solution to Example 12.8
Then, evaluating internal energies of dry air and water from Tables A-22 and A-2, respectively
The values for m
v1
, m
v2
, and m
w2
are from the solution to Example 12.8.
The first underlined term in this equation for Q is evaluated with specific internal energies from the ideal gas table for air,
Table A-22. Steam table data are used to evaluate the second underlined term. The different datums for internal energy un-
derlying these tables cancel because each of these two terms involves internal energy differences. Since the specific heat
c
va
for dry air varies only slightly over the interval from 120 to 22C (Table A-20), the specific internal energy change of
the dry air could be evaluated alternatively using a constant c
va
value. This is left as an exercise.
2851.87 1638.28 290.44 9679.63 10,603 kJ
Q 40.389
1210.49 281.12 0.681 12405.72 3.146 192.322 3.827 12529.32
40.389 kg
m
a
p
a1
V
1R
M
a
2
T
1
311.5 0.19852 10
5
N/m
2
4 135 m
3
2
18314
28.97 N
#
m/kg
#
K2 1393 K2
Q m
a
1u
a2
u
a1
2 m
v2
u
g2
m
w2
u
f2
m
v1
u
g1
U
2
m
a
u
a2
m
v2
u
v2
m
w2
u
w2
m
a
u
a2
m
v2
u
g2
m
w2
u
f2
U
1
m
a
u
a1
m
v1
u
v1
m
a
u
a1
m
v1
u
g1
Q U
2
U
1
¢U Q W
0
12.5.5 Evaluating Humidity Ratio Using the
Adiabatic-Saturation Temperature
The humidity ratio of an air–water vapor mixture can be determined, in principle, knowing
the values of three mixture properties: the pressure p, the temperature T, and the adiabatic-
saturation temperature T
as
introduced in this section. The relationship among these quantities
adiabatic-saturation
temperature
❶
❶
12.5 Introducing Psychrometric Principles 589
is obtained by applying conservation of mass and conservation of energy to an adiabatic sat-
uration process (see box).
Equations 12.48 and 12.49 give the humidity ratio in terms of the adiabatic-saturation
temperature and other quantities:
(12.48)
where h
f
and h
g
denote the enthalpies of saturated liquid water and saturated water vapor, re-
spectively, obtained from the steam tables at the indicated temperatures. The enthalpies of
the dry air h
a
can be obtained from the ideal gas table for air. Alternatively, h
a
(T
as
) h
a
(T )
c
pa
(T
as
T ), where c
pa
is an appropriate constant value for the specific heat of dry air. The
humidity ratio appearing in Eq. 12.48 is
(12.49)
where p
g
(T
as
) is the saturation pressure at the adiabatic-saturation temperature and p is the
mixture pressure.
v¿ 0.622
p
g
1T
as
2
p p
g
1T
as
2
v
h
a
1T
as
2 h
a
1T 2 v¿ 3h
g
1T
as
2 h
f
1T
as
24
h
g
1T 2 h
f
1T
as
2
MODELING AN ADIABATIC SATURATION PROCESS
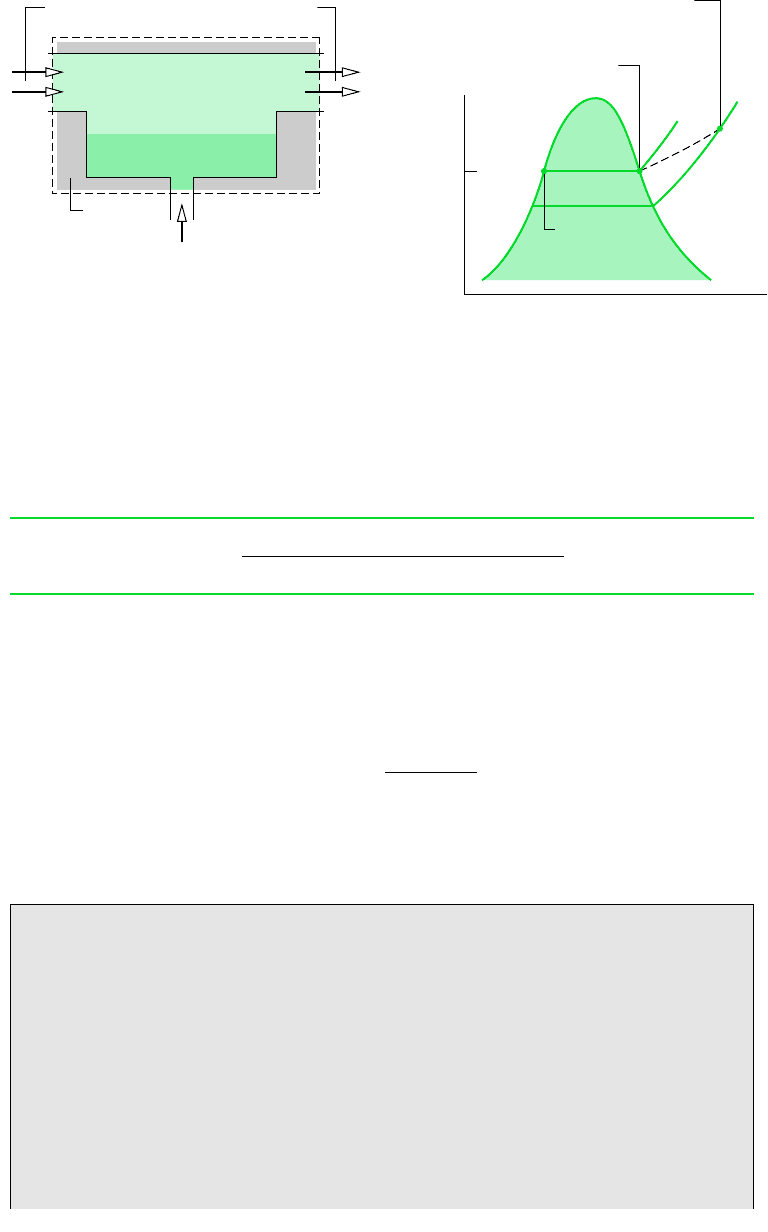
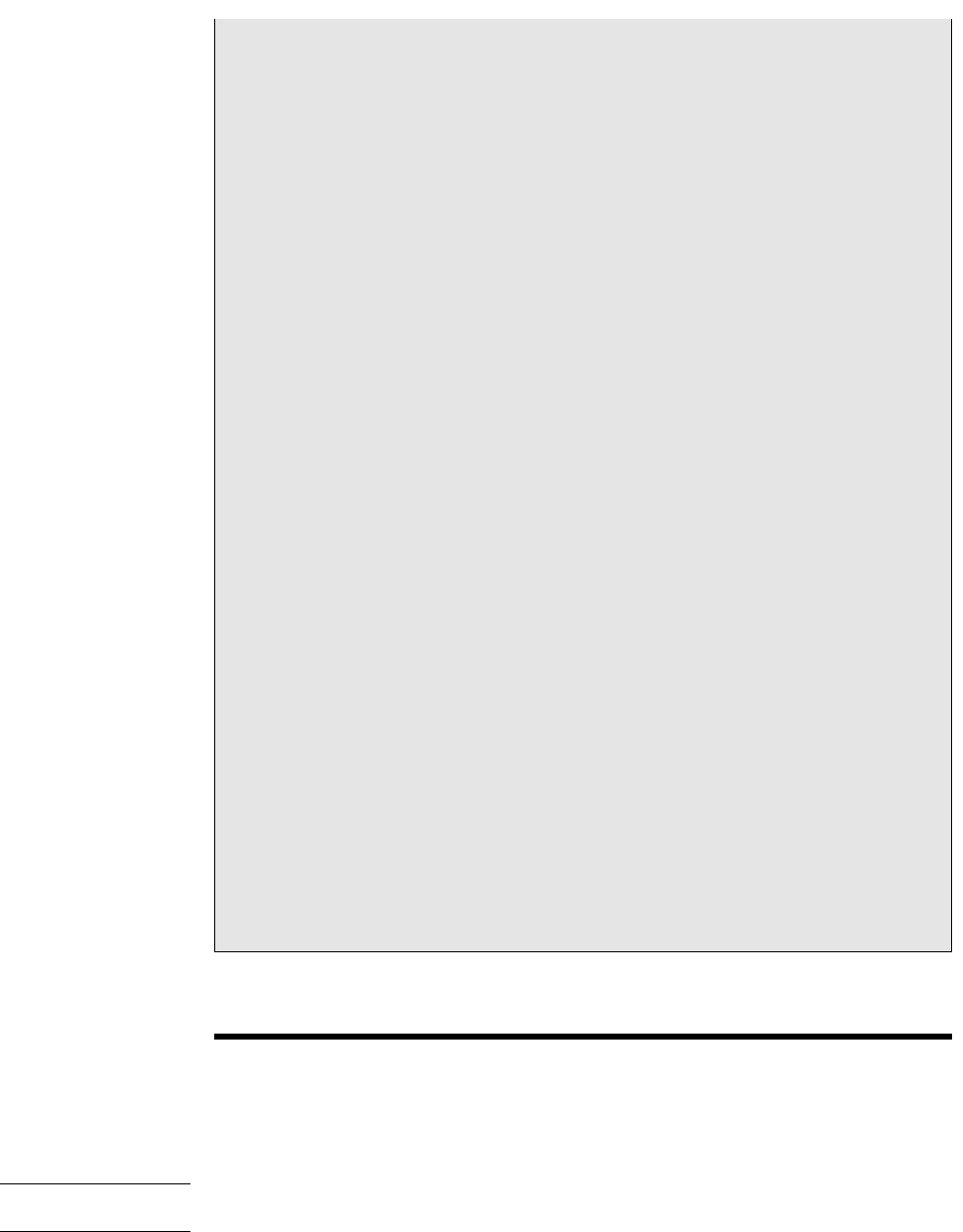
Figure 12.7 shows the schematic and process representations of an adiabatic saturator,
which is a two-inlet, single-exit device through which moist air passes. The device is
assumed to operate at steady state and without significant heat transfer with its sur-
roundings. An air–water vapor mixture of unknown humidity ratio enters the adia-
batic saturator at a known pressure p and temperature T. As the mixture passes through
the device, it comes into contact with a pool of water. If the entering mixture is not
saturated ( 100%), some of the water would evaporate. The energy required to
evaporate the water would come from the moist air, so the mixture temperature would
decrease as the air passes through the duct. For a sufficiently long duct, the mixture
would be saturated as it exits ( 100%). Since a saturated mixture would be achieved
T
T
as
v
p
g
(T
as
)
State of the water vapor
in the incoming moist air stream
State of the water
vapor in the
exiting moist air
stream
State of the
makeup water
Makeup water —
saturated liquid at T
as
,
mass flow rate = m
·
′
v
– m
·
v
Insulation
Moist air
p, T,
ω
Saturated mixture
T
as
, ′, p
ω
m
·
a
m
·
v
m
·
a
m
·
′
v
(a)(b)
Figure 12.7
Adiabatic saturator. (a) Schematic. (b) Process representation.
590 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
without heat transfer with the surroundings, the temperature of the exiting mixture is
the adiabatic-saturation temperature. As indicated on Fig. 12.7, a steady flow of
makeup water at temperature T
as
is added at the same rate at which water is evapo-
rated. The pressure of the mixture is assumed to remain constant as it passes through
the device. Equation 12.48 giving the humidity ratio of the entering moist air in terms
of p, T, and T
as
can be obtained by applying conservation of mass and conservation of
energy to the adiabatic saturator, as follows.
At steady state, the mass flow rate of the dry air entering the device, , must equal
the mass flow rate of the dry air exiting. The mass flow rate of the makeup water is
the difference between the exiting and entering vapor flow rates denoted by and
respectively. These flow rates are labeled on Fig. 12.7a. At steady state, the energy rate
balance reduces to
Several assumptions underlie this expression: Each of the two moist air streams is mod-
eled as an ideal gas mixture of dry air and water vapor. Heat transfer with the sur-
roundings is assumed to be negligible. There is no work , and changes in kinetic
and potential energy are ignored.
Dividing by the mass flow rate of the dry air, , the energy rate balance can be
written on the basis of a unit mass of dry air passing through the device as
(12.50)
where and .
For the exiting saturated mixture, the partial pressure of the water vapor is the satu-
ration pressure corresponding to the adiabatic-saturation temperature, p
g
(T
as
). Accord-
ingly, the humidity ratio ¿ can be evaluated knowing T
as
and the mixture pressure p,as
indicated by Eq. 12.49. In writing Eq. 12.50, the specific enthalpy of the entering water
vapor has been evaluated as that of saturated water vapor at the temperature of the
incoming mixture, in accordance with Eq. 12.47. Since the exiting mixture is saturated,
the enthalpy of the water vapor at the exit is given by the saturated vapor value at T
as
.
The enthalpy of the makeup water is evaluated as that of saturated liquid at T
as
.
When Eq. 12.50 is solved for , Eq. 12.48 results. The details of the solution are
left as an exercise. Although derived with reference to an adiabatic saturator, the rela-
tionship provided by Eq. 12.48 applies generally to moist air mixtures and is not re-
stricted to this type of system or even to control volumes. The relationship allows the
humidity ratio to be determined for any moist air mixture for which the pressure p,
temperature T, and adiabatic-saturation temperature T
as
are known.
v¿ m
#
¿
v
m
#
a
v m
#
v
m
#
a
1h
a
vh
g
2moist air
entering
31v¿ v2h
f
4makeup
water
1h
a
v¿h
g
2moist air
exiting
m
#
a
W
#
cv
1m
#
a
h
a
m
#
v
h
v
2moist air
entering
31m
#
¿
v
m
#
v
2h
w
4makeup
water
1m
#
a
h
a
m
#
¿
v
h
v
2moist air
exiting
m
#
¿
v
,m
#
v
m
#
a
12.6 Psychrometers: Measuring the Wet-Bulb
and Dry-Bulb Temperatures
For moist air mixtures in the normal pressure and temperature ranges of psychrometrics, the
readily-measured wet-bulb temperature is an important parameter.
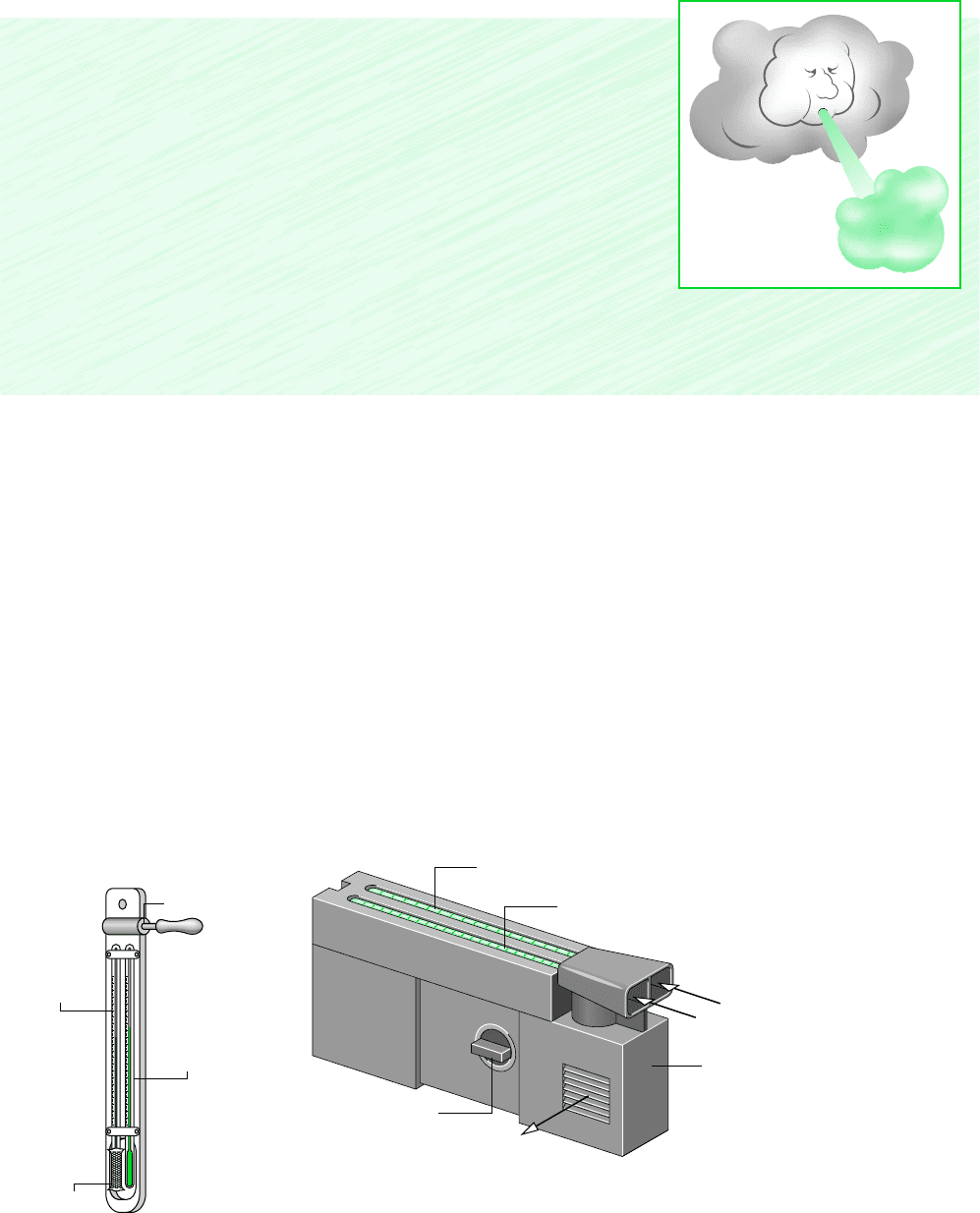
The wet-bulb temperature is read from a wet-bulb thermometer, which is an ordinary
liquid-in-glass thermometer whose bulb is enclosed by a wick moistened with water. The
term dry-bulb temperature refers simply to the temperature that would be measured by a
thermometer placed in the mixture. Often a wet-bulb thermometer is mounted together with
a dry-bulb thermometer to form an instrument called a psychrometer.
wet-bulb temperature
psychrometer
dry-bulb temperature
12.6 Psychrometers: Measuring the Wet-Bulb and Dry-Bulb Temperatures 591
The psychrometer of Fig. 12.8a is whirled in the air whose wet- and dry-bulb temperatures
are to be determined. This induces air flow over the two thermometers. For the psychrometer
of Fig. 12.8b, the air flow is induced by a battery-operated fan. In each type of psychrome-
ter, if the surrounding air is not saturated, water in the wick of the wet-bulb thermometer evap-
orates and the temperature of the remaining water falls below the dry-bulb temperature. Even-
tually a steady-state condition is attained by the wet-bulb thermometer. The wet- and dry-bulb
temperatures are then read from the respective thermometers. The wet-bulb temperature de-
pends on the rates of heat and mass transfer between the moistened wick and the air. Since
these depend in turn on the geometry of the thermometer, air velocity, supply water temper-
ature, and other factors, the wet-bulb temperature is not a mixture property.
For moist air mixtures in the normal pressure and temperature ranges of psychrometric ap-
plications, the adiabatic saturation temperature introduced in Sec. 12.5.5 is closely approximated
by the wet-bulb temperature. Accordingly, the humidity ratio for any such mixture can be
calculated by using the wet-bulb temperature in Eqs. 12.48 and 12.49 in place of the adiabatic-
saturation temperature. Close agreement between the adiabatic-saturation and wet-bulb temper-
atures is not generally found for moist air departing from normal psychrometric conditions.
Air in
Air out
Battery-
operated
fan
Dry-bulb
thermometer
Wet-bulb
thermometer
Switch
(b)
Bearing
Handle
Wet-bulb
thermometer
Dry-bulb
thermometer
Wick
(a)
Figure 12.8
Psychrometers. (a) Sling psychrometer. (b) Aspirating psychrometer.
are better armed to
avoid exposure that
can lead to such seri-
ous medical problems
as frostbite.
The improved
measure was devel-
oped by universities,
international scientific
societies, and govern-
ment in a two-year
effort that led to the
new standard being
adopted in the United States and Canada. Further upgrades
are in the works to include the amount of cloud cover in the
formula, since solar radiation is also an important factor in
how cold it feels.
How Cold is Cold?
Thermodynamics in the News...
The National Weather Service is finding better ways to help
measure our misery during cold snaps so we can avoid
weather dangers. The wind chill index, for many years based
on a single 1945 study, was recently upgraded using new phys-
iological data and computer modeling to better reflect the
perils of cold winds and freezing temperatures.
The new wind chill index is a standardized “temperature”
that accounts for both the actual air temperature and the wind
speed. The formula on which it is based uses measurements of
skin tissue thermal resistance and computer models of the wind
patterns over the human face, together with principles of heat
transfer. Using the new index, an air temperature of 5F and a
wind speed of 25 miles per hour correspond to a wind chill tem-
perature of 40F. The old index assigned a wind chill of only
20F to the same conditions. With the new information, people
592 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
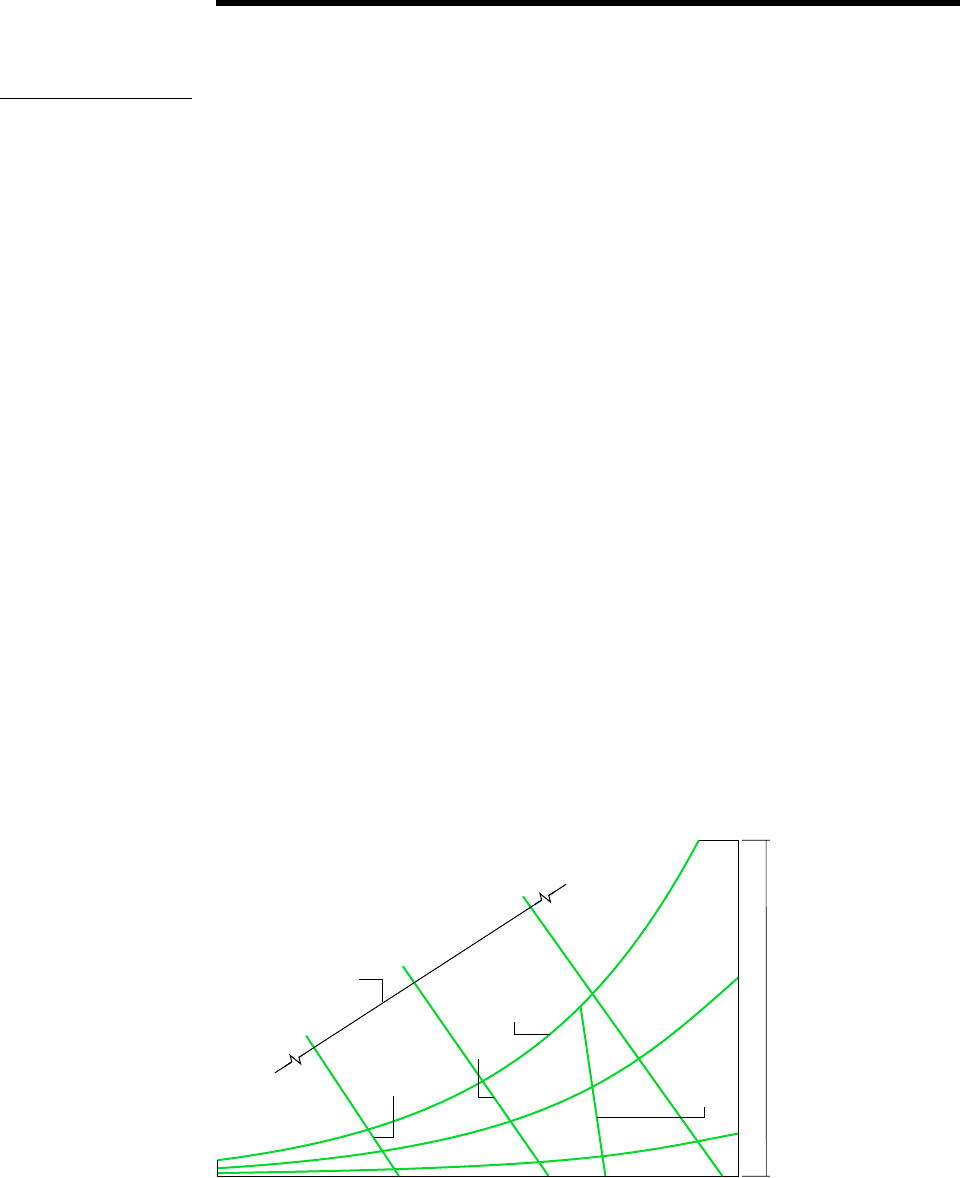
Graphical representations of several important properties of moist air are provided by
psychrometric charts. The main features of one form of chart are shown in Fig. 12.9. A
complete chart in SI units is given in Fig. A-9. This chart is constructed for a mixture
pressure of 1 atm, but charts for other mixture pressures are also available. When the mix-
ture pressure differs only slightly from 1 atm, Figs. A-9 remain sufficiently accurate for
engineering analyses. In this text, such differences are ignored.
Let us consider several features of the psychrometric chart:
Referring to Fig. 12.9, note that the abscissa gives the dry-bulb temperature and the
ordinate provides the humidity ratio. For charts in SI, the temperature is in C and
is expressed in kg, or g, of water vapor per kg of dry air.
Equation 12.43 shows that for fixed mixture pressure there is a direct correspondence
between the partial pressure of the water vapor and the humidity ratio. Accordingly, the
vapor pressure also can be shown on the ordinate, as illustrated on Fig. 12.9.
Curves of constant relative humidity are shown on psychrometric charts. On
Fig. 12.9, curves labeled 100, 50, and 10% are indicated. Since the dew point
is the state where the mixture becomes saturated when cooled at constant vapor
pressure, the dew point temperature corresponding to a given moist air state can be
determined by following a line of constant (constant p
v
) to the saturation line,
100%. The dew point temperature and dry-bulb temperature are identical for
states on the saturation curve.
Psychrometric charts also give values of the mixture enthalpy per unit mass of dry air
in the mixture: h
a
h
v
. In Fig. A-9, the mixture enthalpy has units of kJ per kg of
dry air. The numerical values provided on these charts are determined relative to the
following special reference states and reference values. In Fig. A-9, the enthalpy of the
dry air h
a
is determined relative to a zero value at 0C, and not 0 K as in Table A-22.
Accordingly, in place of Eq. 3.49 used to develop the enthalpy data of Tables A-22,
the following expression is employed to evaluate the enthalpy of the dry air for use
12.7 Psychrometric Charts
psychrometric chart
= 100%
φ
= 50%
φ
= 10%
φ
Volume
per unit
mass of
dry air
T
wb
T
wb
T
wb
Wet-bulb and
dew point
temperature
scales
Scale for the
mixture enthalpy
per unit mass of
dry air
Barometric pressure = 1 atm
Dry-bulb temperature
ω
p
v
Figure 12.9 Psychrometric chart.

12.8 Analyzing Air-Conditioning Processes 593
on the psychrometric chart:
(12.51)
where c
pa
is a constant value for the specific heat c
p
of dry air and T(C) denotes
the temperature in C. In the temperature range of Fig. A-9, c
pa
can be taken as
1.005 kJ/kg · K. On Figs. A-9 the enthalpy of the water vapor h
v
is evaluated as h
g
at
the dry-bulb temperature of the mixture from Table A-2.
Another important parameter on psychrometer charts is the wet-bulb temperature. As
illustrated by Figs. A-9, constant T
wb
lines run from the upper left to the lower right of
the chart. The relationship between the wet-bulb temperature and other chart quantities
is provided by Eq. 12.48. The wet-bulb temperature can be used in this equation in
place of the adiabatic-saturation temperature for the states of moist air located on
Figs. A-9.
Lines of constant wet-bulb temperature are approximately lines of constant mixture
enthalpy per unit mass of dry air. This feature can be brought out by study of the
energy balance for the adiabatic saturator, Eq. 12.50. Since the contribution of the
energy entering the adiabatic saturator with the makeup water is normally much
smaller than that of the moist air, the enthalpy of the entering moist air is very
nearly equal to the enthalpy of the saturated mixture exiting. Accordingly, all states
with the same value of the wet-bulb temperature (adiabatic-saturation temperature)
have nearly the same value for the mixture enthalpy per unit mass of dry air. Al-
though Figs. A-9 ignore this slight effect, some psychrometric charts are drawn to
show the departure of lines of constant wet-bulb temperature from lines of constant
mixture enthalpy.
As shown on Fig. 12.9, psychrometric charts also provide lines representing volume per
unit mass of dry air, Vm
a
. Figure A-9 gives this quantity in units of m
3
/kg. These spe-
cific volume lines can be interpreted as giving the volume of dry air or of water vapor,
per unit mass of dry air, since each mixture component is considered to fill the entire
volume.
The psychrometric chart is easily used. for example. . . a psychrometer indicates
that in a classroom the dry-bulb temperature is 20C and the wet-bulb temperature is 15C.
Locating the mixture state on Fig. A-9 corresponding to the intersection of these tempera-
tures, we read 0.0086 kg(vapor)/kg(dry air) and 60%.
h
a
T
273.15 K
c
pa
dT c
pa
T 1°C2
12.8 Analyzing Air-Conditioning Processes
The purpose of the present section is to study typical air-conditioning processes using
the psychrometric principles developed in this chapter. Specific illustrations are pro-
vided in the form of solved examples involving control volumes at steady state. In
each case, the methodology introduced in Sec. 12.8.1 is employed to arrive at the solu-
tion. To reinforce the psychrometric principles developed in the present chapter, the re-
quired psychrometric parameters are determined in most cases using tabular data provided
in the appendix. It is left as an exercise to check these values by means of a psychro-
metric chart.
594 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
12.8.1 Applying Mass and Energy Balances to
Air-Conditioning Systems
The object of this section is to illustrate the use of the conservation of mass and conserva-
tion of energy principles in analyzing systems involving mixtures of dry air and water vapor
in which a condensed water phase may be present. The same basic solution approach that
has been used in thermodynamic analyses considered thus far is applicable. The only new
aspect is the use of the special vocabulary and parameters of psychrometrics.
Systems that accomplish air-conditioning processes such as heating, cooling, humidifica-
tion, or dehumidification are normally analyzed on a control volume basis. To consider a typ-
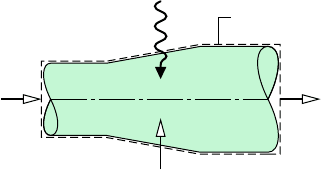
ical analysis, refer to Fig. 12.10, which shows a two-inlet, single-exit control volume at steady
state. A moist air stream enters at 1, a moist air stream exits at 2, and a water-only stream
enters at 3. The water-only stream may be a liquid or a vapor. Heat transfer at the rate
can occur between the control volume and its surroundings. Depending on the application,
the value of might be positive, negative, or zero.
MASS BALANCE. At steady state, the amounts of dry air and water vapor contained within
the control volume cannot vary. Thus, for each component individually it is necessary for the
total incoming and outgoing mass flow rates to be equal. That is
For simplicity, the constant mass flow rate of the dry air is denoted by . The mass flow
rates of the water vapor can be expressed conveniently in terms of humidity ratios as
and . With these expressions, the mass balance for water becomes
(12.52)
When water is added at 3,
2
is greater than
1
.
ENERGY BALANCE. Assuming and ignoring all kinetic and potential energy ef-
fects, the energy rate balance reduces at steady state to
(12.53)
In this equation, the entering and exiting moist air streams are regarded as ideal gas mixtures
of dry air and water vapor.
Equation 12.53 can be cast into a form that is particularly convenient for the analysis of
air-conditioning systems. First, with Eq. 12.47 the enthalpies of the entering and exiting water
vapor can be evaluated as the saturated vapor enthalpies corresponding to the temperatures
T
1
and T
2
, respectively, giving
Then, with and , the equation can be expressed as
(12.54)0 Q
#
cv
m
#
a
1h
a1
v
1
h
g1
2 m
#
w
h
w
m
#
a
1h
a2
v
2
h
g2
2
m
#
v2
v
2
m
#
a
m
#
v1
v
1
m
#
a
0 Q
#
cv
1m
#
a
h
a1
m
#
v1
h
g1
2 m
#
w
h
w
1m
#
a
h
a2
m
#
v2
h
g2
2
0 Q
#
cv
1m
#
a
h
a1
m
#
v1
h
v1
2 m
#
w
h
w
1m
#
a
h
a2
m
#
v2
h
v2
2
W
#
cv
0
m
#
w
m
#
a
1v
2
v
1
2
1water2
m
#
v2
v
2
m
#
a
m
#
v1
v
1
m
#
a
m
#
a
m
#
v1
m
#
w
m
#
v2
1water2
m
#
a1
m
#
a2
1dry air2
Q
#
cv
Q
#
cv
Liquid or vapor, m
·
w
Boundary
Moist air
m
·
a
,
m
·
v1
1
2
3
Moist air
m
·
a
, m
·
v2
Q
·
cv
Figure 12.10 System for conditioning
moist air.
12.8 Analyzing Air-Conditioning Processes 595
Finally, introducing Eq. 12.52, the energy rate balance becomes
(12.55)
The first underlined term of Eq. 12.55 can be evaluated from Tables A-22 giving the ideal
gas properties of air. Alternatively, since relatively small temperature differences are nor-
mally encountered in the class of systems under present consideration, this term can be eval-
uated as h
a1
h
a2
c
pa
(T
1
T
2
), where c
pa
is a constant value for the specific heat of dry
air. The second underlined term of Eq. 12.55 can be evaluated using steam table data to-
gether with known values for
1
and
2
.
MODELING SUMMARY. As suggested by the foregoing development, several simplifying
assumptions are commonly used when analyzing the air-conditioning systems under present
consideration. In addition to the assumption of steady-state operation, one-dimensional flow
is assumed to apply at locations where matter crosses the boundary of the control volume,
and the effects of kinetic and potential energy at these locations are neglected. In most cases
there is no work, except for flow work where matter crosses the boundary. Further simplifi-
cations also may be required in particular cases.
12.8.2 Conditioning Moist Air at Constant Composition
Building air-conditioning systems frequently heat or cool a moist air stream with no change
in the amount of water vapor present. In such cases the humidity ratio remains constant,
while relative humidity and other moist air parameters vary. Example 12.10 gives an ele-
mentary illustration using the methodology of Sec. 12.8.1.
0 Q
#
cv
m
#
a
31h
a1
h
a2
2 v
1
h
g1
1v
2
v
1
2h
w
v
2
h
g2
4
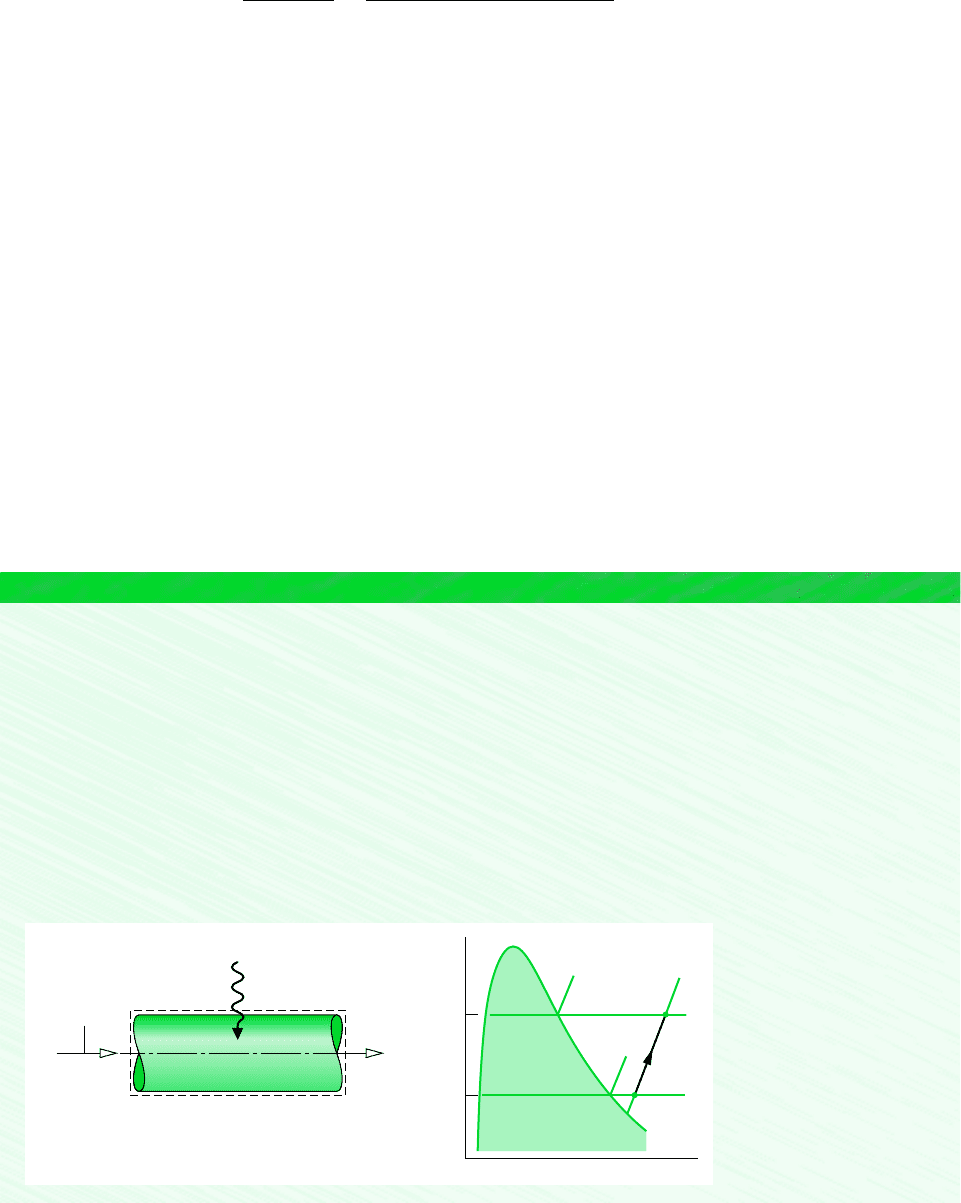
EXAMPLE 12.10 Heating Moist Air in a Duct
Moist air enters a duct at 10C, 80% relative humidity, and a volumetric flow rate of 150 m
3
/min. The mixture is heated as it
flows through the duct and exits at 30C. No moisture is added or removed, and the mixture pressure remains approximately
constant at 1 bar. For steady-state operation, determine (a) the rate of heat transfer, in kJ/min, and (b) the relative humidity
at the exit. Changes in kinetic and potential energy can be ignored.
SOLUTION
Known: Moist air that enters a duct at 10C and 80% with a volumetric flow rate of 150 m
3
/min is heated at constant
pressure and exits at 30C. No moisture is added or removed.
Find: Determine the rate of heat transfer, in kJ/min, and the relative humidity at the exit.
Schematic and Given Data:
T
T
2
T
1
v
p
v
p
g
(T
1
)
p
g
(T
2
)
2
1
Q
·
cv
Boundary
T
2
= 30°C
12
= 150
= 10°C
= 80%
(AV)
1
T
1
1
φ
m
3
___
min
Figure E12.10a
