Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

566 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
where S
i
is the entropy of component i evaluated at the mixture temperature T and partial
pressure p
i
(or at temperature T and total volume V ).
Equation 12.25 can be written on a molar basis as
(12.26)
where is the entropy of the mixture per mole of mixture and is the entropy of component i
per mole of i. Dividing by the total number of moles of mixture gives an expression for the
entropy of the mixture per mole of mixture
(12.27)
The specific entropies of Eqs. 12.26 and 12.27 are evaluated at the mixture temperature T
and the partial pressure p
i
.
WORKING ON A MASS BASIS. In cases where it is convenient to work on a mass basis,
the foregoing expressions would be written with the mass of the mixture, m, and the mass
of component i in the mixture, m
i
, replacing, respectively, the number of moles of mixture,
n, and the number of moles of component i, n
i
. Similarly, the mass fraction of component
i, mf
i
, would replace the mole fraction, y
i
. All specific internal energies, enthalpies, and en-
tropies would be evaluated on a unit mass basis rather than on a per mole basis as above.
The development of the appropriate expressions is left as an exercise. By using the molecu-
lar weight of the mixture or of component i, as appropriate, data can be converted from a
mass basis to a molar basis, or conversely, with relations of the form
(12.28)
for the mixture, and
(12.29)
for component i.
u
i
M
i
u
i
,
h M
i
h
i
,
c
p,i
M
i
c
p,i
,
c
v,i
M
i
c
v,i
,
s
i
M
i
s
i
u Mu,
h Mh,
c
p
Mc
p
,
c
v
Mc
v
,
s Ms
s
i
s
a
j
i1
y
i
s
i
s
i
s
ns n
1
s
1
n
2
s
2
###
n
j
s
j
a
j
i1
n
i
s
i
12.4 Analyzing Systems Involving Mixtures
To perform thermodynamic analyses of systems involving nonreacting ideal gas mixtures re-
quires no new fundamental principles. The conservation of mass and energy principles and
the second law of thermodynamics are applicable in the forms previously introduced. The
only new aspect is the proper evaluation of the required property data for the mixtures in-
volved. This is illustrated in the present section, which deals with two classes of problems
involving mixtures. In Sec. 12.4.1 the mixture is already formed, and we study processes in
which there is no change in composition. Section 12.4.2 considers the formation of mixtures
from individual components that are initially separate.
12.4.1 Mixture Processes at Constant Composition
In the present section we are concerned with the case of ideal gas mixtures undergoing
processes during which the composition remains constant. The number of moles of each com-
ponent present, and thus the total number of moles of mixture, remain the same throughout
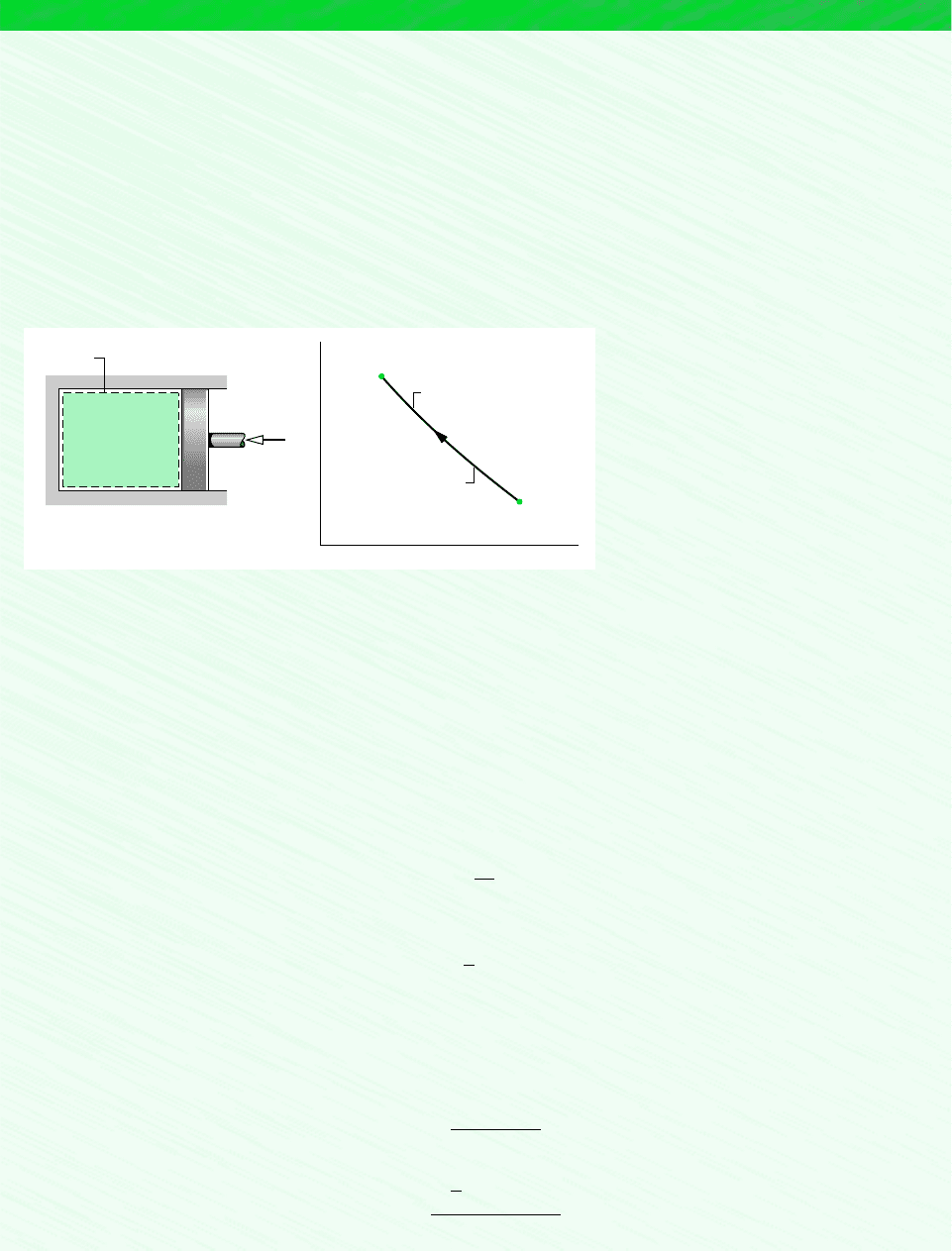
the process. This case is shown schematically in Fig. 12.2, which is labeled with expressions
for U, H, and S of a mixture at the initial and final states of a process undergone by the
mixture. In accordance with the discussion of Sec. 12.3, the specific internal energies and
12.4 Analyzing Systems Involving Mixtures 567
enthalpies of the components are evaluated at the temperature of the mixture. The specific
entropy of each component is evaluated at the mixture temperature and the partial pressure
of the component in the mixture.
The changes in the internal energy and enthalpy of the mixture during the process are
given, respectively, by
(12.30)
(12.31)
where T
1
and T
2
denote the temperature at the initial and final states. Dividing by the num-
ber of moles of mixture, n, expressions for the change in internal energy and enthalpy of the
mixture per mole of mixture result
(12.32)
(12.33)
Similarly, the change in entropy for the mixture is
(12.34)
where p
i1
and p
i2
denote, respectively, the initial and final partial pressures of component i.
Dividing by the total moles of mixture, Eq. 12.34 becomes
(12.35)
Companion expressions for Eqs. 12.30 through 12.35 on a mass basis also can be written.
This is left as an exercise.
The foregoing expressions giving the changes in internal energy, enthalpy, and entropy of
the mixture are written in terms of the respective property changes of the components.
Accordingly, different datums might be used to assign specific enthalpy values to the various
¢s
a
j
i1
y
i
3s
i
1T
2
, p
i2
2 s
i
1T
1
, p
i1
24
S
2
S
1
a
j
i1
n
i
3s
i
1T
2
, p
i2
2 s
i
1T
1
, p
i1
24
¢h
a
j
i1
y
i
3h
i
1T
2
2 h
i
1T
1
24
¢u
a
j
i1
y
i
3u
i
1T
2
2 u
i
1T
1
24
H
2
H
1
a
j
i1
n
i
3h
i
1T
2
2 h
i
1T
1
24
U
2
U
1
a
j
i1
n
i
3u
i
1T
2
2 u
i
1T
1
24
(n
1
, n
2
, …, n
j
)
at
T
1
, p
1
i
Σ
=
j
1
n
i
h
_
i
(T
1
)
i
Σ
=
j
1
n
i
s
_
i
(T
1
, p
i1
)
i
Σ
=
j
1
n
i
h
_
i
(T
2
)
State 1 State 2
(n
1
, n
2
, …, n
j
)
at
T
2
, p
2
H
1
=
i
Σ
=
j
1
n
i
u
_
i
(T
1
)U
1
=
i
Σ
=
j
1
n
i
u
_
i
(T
2
)U
2
=
S
1
=
i
Σ
=
j
1
n
i
s
_
i
(T
2
, p
i2
)S
2
=
H
2
=
Figure 12.2
Process of an ideal gas
mixture.

568 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
components because the datums would cancel when the component enthalpy changes are cal-
culated. Similar remarks apply to the cases of internal energy and entropy.
USING IDEAL GAS TABLES. For several common gases modeled as ideal gases, the quan-
tities and appearing in the foregoing expressions can be evaluated as functions of tem-
perature only from Tables A-22 and A-23. Table A-22 for air gives these quantities on a mass
basis. Table A-23 gives them on a molar basis. The ideal gas tables also can be used to eval-
uate the entropy change. The change in specific entropy of component i required by Eqs. 12.34
and 12.35 can be determined with Eq. 6.21b as
Since the mixture composition remains constant, the ratio of the partial pressures in this
expression is the same as the ratio of the mixture pressures, as can be shown by using
Eq. 12.12 to write
Accordingly, when the composition is constant, the change in the specific entropy of com-
ponent i is simply
(12.36)
where p
1
and p
2
denote, respectively, the initial and final mixture pressures. The terms
of Eq. 12.36 can be obtained as functions of temperature for several common gases from
Table A-23. Table A-22 for air gives versus temperature.
ASSUMING CONSTANT SPECIFIC HEATS. When the component specific heats and
are taken as constants, the specific internal energy, enthalpy, and entropy changes of the mix-
ture and the components of the mixture are given by
(12.37)
(12.38)
(12.39)
where the mixture specific heats and are evaluated from Eqs. 12.23 and 12.24, respec-
tively, using data from Tables A-20 or the literature, as required. The expression for can
be obtained formally by substituting the above expression for into Eq. 12.32 and using
Eq. 12.23 to simplify the result. Similarly, the expressions for and can be obtained by
inserting and into Eqs. 12.33 and 12.35, respectively, and using Eq. 12.24 to simplify.
In the equations for entropy change, the ratio of mixture pressures replaces the ratio of par-
tial pressures as discussed above. Similar expressions can be written for the mixture specific
internal energy, enthalpy, and entropy changes on a mass basis. This is left as an exercise.
USING COMPUTER SOFTWARE. The changes in internal energy, enthalpy, and entropy
required in Eqs. 12.32, 12.33, and 12.35, respectively, also can be evaluated using computer
software. Interactive Thermodynamics: IT provides data for a large number of gases mod-
eled as ideal gases, and its use is illustrated in Example 12.4 below.
The next example illustrates the use of ideal gas mixture relations for analyzing a com-
pression process.
¢s
i
¢h
i
¢s¢h
¢u
i
¢u
c
p
c
v
¢s c
p
ln
T
2
T
1
R ln
p
2
p
1
,
¢s
i
c
p,i
ln
T
2
T
1
R ln
p
2
p
1
¢h c
p
1T
2
T
1
2,
¢h
i
c
p,i
1T
2
T
1
2
¢u c
v
1T
2
T
1
2,
¢u
i
c
v,i
1T
2
T
1
2
c
p,i
c
v,i
s°
s
°
i
¢s
i
s°
i
1T
2
2 s°
i
1T
1
2 R ln
p
2
p
1
p
i2
p
i1
y
i
p
2
y
i
p
1
p
2
p
1
¢s
i
s°
i
1T
2
2 s°
i
1T
1
2 R ln
p
i2
p
i1
h
i
u
i
12.4 Analyzing Systems Involving Mixtures 569
EXAMPLE 12.3 Compressing an Ideal Gas Mixture
A mixture of 0.3 kg of carbon dioxide and 0.2 kg of nitrogen is compressed from p
1
1 bar, T
1
300 K to p
2
3 bars in
a polytropic process for which n 1.25. Determine (a) the final temperature, in K, (b) the work, in kJ, (c) the heat transfer,
in kJ, (d) the change in entropy of the mixture, in kJ/K.
SOLUTION
Known: A mixture of 0.3 kg of CO
2
and 0.2 kg of N
2
is compressed in a polytropic process for which n 1.25. At the
initial state, p
1
1 bar, T
1
300K. At the final state, p
2
3 bar.
Find: Determine the final temperature, in K, the work, in kJ, the heat transfer, kJ, and the entropy change of the mixture in,
kJ/K.
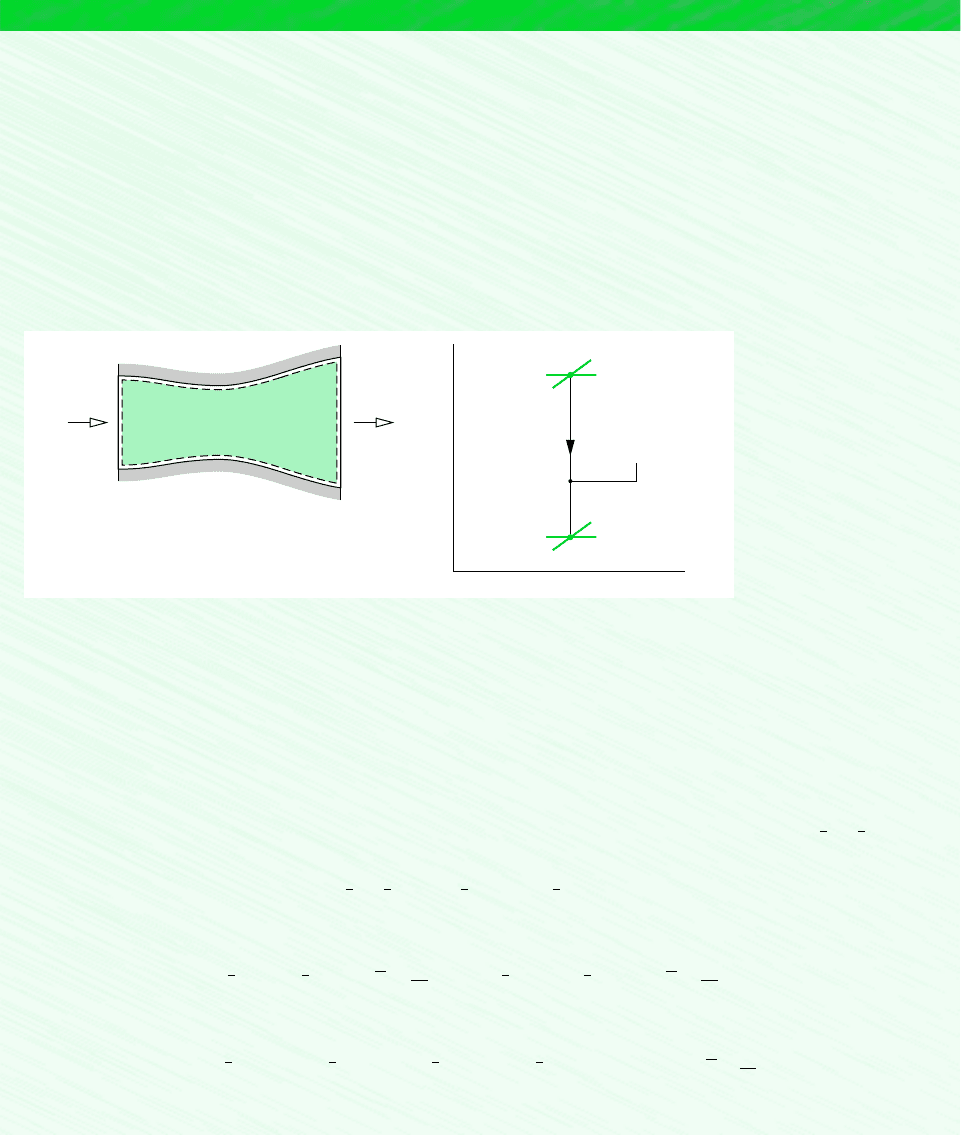
Schematic and Given Data:
p
v
States of the
mixture
n = 1.25
1
2
0.3 kg CO
2
0.2 kg N
2
Boundary
p
1
= 1 bar, T
1
= 300 K,
p
2
= 3 bars
Figure E12.3
Assumptions:
1. As shown in the accompanying figure, the system is the mixture of CO
2
and N
2
. The mixture composition remains con-
stant during the compression.
2. Each mixture component behaves as if it were an ideal gas occupying the entire system volume at the mixture tempera-
ture. The overall mixture acts as an ideal gas.
3. The compression process is a polytropic process for which n 1.25.
4. The changes in kinetic and potential energy between the initial and final states can be ignored.
Analysis:
(a) For an ideal gas, the temperatures and pressures at the end states of a polytropic process are related by Eq. 3.56
Inserting values
(b) The work for the compression process is given by
Introducing pV
n
constant and performing the integration
With the ideal gas equation of state, this reduces to
W
m1R
M21T
2
T
1
2
1 n
W
p
2
V
2
p
1
V
1
1 n
W
2
1
p dV
T
2
300 a
3
1
b
0.2
374 K
T
2
T
1
a
p
2
p
1
b
1n 12
n

570 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
The mass of the mixture is m 0.3 0.2 0.5 kg. The apparent molecular weight of the mixture can be calculated
using M mn, where n is the total number of moles of mixture. With Eq. 12.1, the numbers of moles of CO
2
and N
2
are,
respectively
The total number of moles of mixture is then n 0.0139 kmol. The apparent molecular weight of the mixture is M
0.50.0139 35.97.
Calculating the work
where the minus sign indicates that work is done on the mixture, as expected.
(c) With assumption 4, the closed system energy balance can be placed in the form
where U is the change in internal energy of the mixture.
The change in internal energy of the mixture equals the sum of the internal energy changes of the components. With
Eq. 12.30
This form is convenient because Table A-23 gives internal energy values for N
2
and CO
2
, respectively, on a molar basis. With
values from this table
Inserting values for U and W into the expression for Q
where the minus sign signifies a heat transfer from the system.
(d) The change in entropy of the mixture equals the sum of the entropy changes of the components. With Eq. 12.34
where and are evaluated using Eq. 12.36 and values of for N
2
and CO
2
from Table A-23. That is
Entropy decreases in the process because entropy is transferred from the system accompanying heat transfer.
In view of the relatively small temperature change, the changes in the internal energy and entropy of the mixture can be
evaluated alternatively using the constant specific heat relations, Eqs. 12.37 and 12.39, respectively. In these equations,
and are specific heats for the mixture determined using Eqs. 12.23 and 12.24 together with appropriate specific heat
values for the components chosen from Table A-20.
Since the composition remains constant, the ratio of mixture pressures equals the ratio of partial pressures, so Eq. 12.36
can be used to evaluate the component specific entropy changes required here.
c
p
c
v
.0231 kJ/K
0.0071
a198.105 191.682 8.314
3
1
b
¢S 0.0068
a222.475 213.915 8.314
3
1
b
s
°¢s
CO
2
¢s
N
2
¢S n
CO
2
¢s
CO
2
n
N
2
¢s
N
2
Q 26.3 34.21 7.91 kJ
26.3
kJ
¢U 10.0068219198 69392 10.0071217770 62292
¢U n
CO
2
3u
CO
2
1T
2
2 u
CO
2
1T
1
24 n
N
2
3u
N
2
1T
2
2 u
N
2
1T
1
24
Q ¢U W
34.21
kJ
W
10.5
kg2 a
8.314 kJ
35.97 kg
#
°K
b
1374 K 300 K2
1 1.25
n
CO
2
0.3
44
0.0068 kmol,
n
N
2
0.2
28
0.0071 kmol
❷
❶
❷
❶
12.4 Analyzing Systems Involving Mixtures 571
The next example illustrates the application of ideal gas mixture principles for the analy-
sis of a mixture expanding isentropically through a nozzle. The solution features the use of
table data and IT as an alternative.
EXAMPLE 12.4 Gas Mixture Expanding Isentropically through a Nozzle
A gas mixture consisting of CO
2
and O
2
with mole fractions 0.8 and 0.2, respectively, expands isentropically and at steady
state through a nozzle from 700 K, 5 bars, 3 m/s to an exit pressure of 1 bar. Determine (a) the temperature at the nozzle exit,
in K, (b) the entropy changes of the CO
2
and O
2
from inlet to exit, in (c) the exit velocity, in m/s.
SOLUTION
Known: A gas mixture consisting of CO
2
and O
2
in specified proportions expands isentropically through a nozzle from spec-
ified inlet conditions to a given exit pressure.
Find: Determine the temperature at the nozzle exit, in K, the entropy changes of the CO
2
and O
2
from inlet to exit, in
and the exit velocity, in m/s.
Schematic and Given Data:
kJ/kmol
#
K,
kJ/kmol
#
K,
T
s
T
1
= 700 K
2
1
V
1
p
1
T
1
= 3 m/s
= 5 bars
= 700 K
p
2
=
1 bar
1
2
p
1
= 5 bars
T
2
= ?
p
2
= 1 bar
States of the mixture
Figure E12.4
Assumptions:
1. The control volume shown by the dashed line on the accompanying figure operates at steady state.
2. The mixture composition remains constant as the mixture expands isentropically through the nozzle. The overall mixture
and each mixture component act as ideal gases. The state of each component is defined by the temperature and the partial
pressure of the component.
3. The change in potential energy between inlet and exit can be ignored.
Analysis:
(a) The temperature at the exit can be determined using the fact that the expansion occurs isentropically: . As
there is no change in the specific entropy of the mixture between inlet and exit, Eq. 12.35 can be used to write
(a)
The change in specific entropy of each component can be determined using Eq. 12.36. Thus, Eq. (a) becomes
On rearrangement
The sum of mole fractions equals unity, so the coefficient of the last term on the right side is
1y
O
2
y
CO
2
2 1.
y
O
2
s °
O
2
1T
2
2 y
CO
2
s °
CO
2
1T
2
2 y
O
2
s °
O
2
1T
1
2 y
CO
2
s °
CO
2
1T
1
2 1y
O
2
y
CO
2
2 R ln
p
2
p
1
y
O
2
cs °
O
2
1T
2
2 s°
O
2
1T
1
2 R ln
p
2
p
1
d y
CO
2
cs °
CO
2
1T
2
2 s °
CO
2
1T
1
2 R ln
p
2
p
1
d 0
s
2
s
1
y
O
2
¢s
O
2
y
CO
2
¢s
CO
2
0
s
2
s
1
0

572 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
Introducing given data, and values of for O
2
and CO
2
at T
1
700 K from Table A-23
or
To determine the temperature T
2
requires an iterative approach with the above equation: A final temperature T
2
is assumed,
and the values for O
2
and CO
2
are found from Table A-23. If these two values do not satisfy the equation, another tem-
perature is assumed. The procedure continues until the desired agreement is attained. In the present case
Linear interpolation between these values gives T
2
517.6 K.
Alternative Solution:
Alternatively, the following IT program can be used to evaluate T
2
without resorting to iteration with table data. In the pro-
gram, yO2 denotes the mole fraction of O
2
, p1
_
O2 denotes the partial pressure of O
2
at state 1, s1
_
O2 denotes the entropy
per mole of O
2
at state 1, and so on.
T1 = 700 // K
p1 = 5 // bar
p2 = 1 // bar
yO2 = 0.2
yCO2 = 0.8
p1_O2 = yO2 * p1
p1_CO2 = yCO2 * p1
p2_O2 = yO2 * p2
p2_CO2 = yCO2 * p2
s1_O2 = s_TP(“O2”,T1,p1_O2)
s1_CO2 = s_TP(“CO2”,T1,p1_CO2)
s2_O2 = s_TP(“O2”,T2,p2_O2)
s2_CO2 = s_TP(“CO2”,T2,p2_CO2)
// When expressed in terms of these quantities, Eq. (a) takes the form
yO2 * (s2_O2 – s1_O2) + yCO2 * (s2_CO2 – s1_CO2) = 0
Using the Solve button, the result is T
2
517.6 K, which agrees with the value obtained using table data. Note that IT provides
the value of specific entropy for each component directly and does not return of the ideal gas tables.
(b) The change in the specific entropy for each of the components can be determined using Eq. 12.36. For O
2
Inserting values for O
2
from Table A-23
Similarly, with CO
2
data from Table A-23
0.92 kJ/kmol
#
K
236.365 250.663 8.314 ln
10.22
¢s
CO
2
s °
CO
2
1T
2
2 s °
CO
2
1T
1
2 R ln
p
2
p
1
¢s
O
2
221.667 231.358 8.314 ln 10.22 3.69 kJ/kmol
#
K
s
°
¢s
O
2
s °
O
2
1T
2
2 s °
O
2
1T
1
2 R ln
p
2
p
1
s °
at T 520 K:
0.21221.8122 0.81236.5752 233.62
at T 510 K:
0.21221.2062 0.81235.7002 232.80
s
°
0.2s
°
O
2
1T
2
2 0.8s °
CO
2
1T
2
2 233.42 kJ/kmol
#
K
0.2s
°
O
2
1T
2
2 0.8s °
CO
2
1T
2
2 0.21231.3582 0.81250.6632 8.314 ln
1
5
s
°
❷
❶
12.4 Analyzing Systems Involving Mixtures 573
(c) Reducing the energy rate balance for the one-inlet, one-exit control volume at steady state
where h
1
and h
2
are the enthalpy of the mixture, per unit mass of mixture, at the inlet and exit, respectively. Solving for V
2
The term (h
1
h
2
) in the expression for V
2
can be evaluated as
where M is the apparent molecular weight of mixture, and the molar specific enthalpies of O
2
and CO
2
are from Table A-23.
With Eq. 12.9, the apparent molecular weight of the mixture is
Then, with enthalpy values at T
1
700 K and T
2
517.6 K from Table A-23
Finally,
Parts (b) and (c) can be solved alternatively using IT. These parts also can be solved using a constant c
p
together with
Eqs. 12.38 and 12.39. Inspection of Table A-20 shows that the specific heats of CO
2
and O
2
increase only slightly with
temperature over the interval from 518 to 700 K, and so suitable constant values of c
p
for the components and the overall
mixture can be readily determined. These alternative solutions are left as exercises.
Each component experiences an entropy change as it passes from inlet to exit. The increase in entropy of the oxygen and
the decrease in entropy of the carbon dioxide are due to an entropy transfer accompanying heat transfer from the CO
2
to
the O
2
as they expand through the nozzle. However, as indicated by Eq. (a), there is no change in the entropy of the mixture
as it expands through the nozzle.
Note the use of unit conversion factors in the calculation of V
2
.
V
2
B
a3
m
s
b
2
2 a194.7
kJ
kg
b`
1
kg
#
m/s
2
1 N
``
10
3
N
#
m
1 kJ
`
624 m/s
194.7 kJ/kg
h
1
h
2
1
41.6
30.2121,184 15,3202 0.8127,125 18,46824
M 0.81442 0.21322 41.6 kg/kmol
h
1
h
2
h
1
h
2
M
1
M
3y
O
2
1h
1
h
2
2
O
2
y
CO
2
1h
1
h
2
2
CO
2
4
V
2
2V
2
1
21h
1
h
2
2
0 h
1
h
2
V
2
1
V
2
2
2
❶
❸
❸
❷
12.4.2 Mixing of Ideal Gases
Thus far, we have considered only mixtures that have already been formed. Now let us take
up cases where ideal gas mixtures are formed by mixing gases that are initially separate.
Such mixing is irreversible because the mixture forms spontaneously, and a work input from
the surroundings would be required to separate the gases and return them to their respective
initial states. In this section, the irreversibility of mixing is demonstrated through calcula-
tions of the entropy production.
Three factors contribute to the production of entropy in mixing processes:
1. The gases are initially at different temperatures.
2. The gases are initially at different pressures.
3. The gases are distinguishable from one another.
574 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
rates are higher, and
the proportion of out-
side air is greater than
provided in the build-
ings where the travel-
ers live and work. Still,
the National Academy
of Sciences and the
American Society of
Heating, Refrigerating,
and Air Conditioning
Engineers are calling
for greater use of high-
grade filters that can remove virtually all of the bacteria and
fungus from the air. Such filters are already used in hospital
operating room ventilation systems.
Thermodynamics in the News...
About half the air we breathe on some airplanes is fresh air,
and the rest is recirculated. This has some passengers and
flight attendants worried that recycled air is making them sick.
The debate rages, and the Federal Aviation Agency is listen-
ing closely as engineers, scientists, and health professionals
try to sort out the facts.
According to a study reported in the Journal of the
American Medical Society, while people on airplanes seem to
get more colds, recirculating cabin air doesn’t appear to make
the situation worse. Surveys show that travelers on flights with
recirculated air or on flights with fresh air report about the
same incidence of colds within a week of flying. In both cases,
cold incidence is much greater than among those who have
not flown recently.
Aircraft industry sources point out that airplane air is filtered.
They claim that airplane filtration systems are better, air change
EXAMPLE 12.5 Adiabatic Mixing at Constant Total Volume
Two rigid, insulated tanks are interconnected by a valve. Initially 0.79 kmol of nitrogen at 2 bars and 250 K fills one tank.
The other tank contains 0.21 kmol of oxygen at 1 bar and 300 K. The valve is opened and the gases are allowed to mix until
a final equilibrium state is attained. During this process, there are no heat or work interactions between the tank contents and
the surroundings. Determine (a) the final temperature of the mixture, in K, (b) the final pressure of the mixture, in atm, (c) the
amount of entropy produced in the mixing process, in kJ/K.
SOLUTION
Known: Nitrogen and oxygen, initially separate at different temperatures and pressures, are allowed to mix without heat or
work interactions with the surroundings until a final equilibrium state is attained.
Find: Determine the final temperature of the mixture, in K, the final pressure of the mixture, in bars, and the amount of
entropy produced in the mixing process, in kJ/K.
Schematic and Given Data:
Valve
Initially 0.21 kgmol
of O
2
at 1 bar
and 300°K
Initially 0.79 kgmol
of N
2
at 2 bars
and 250°K
Insulation
Figure E12.5
Assumptions:
1. The system is taken to be the nitrogen and the oxygen together.
2. When separate, each of the gases behaves as an ideal gas. The final mix-
ture also acts as an ideal gas. Each mixture component occupies the total
volume and exhibits the mixture temperature.
3. No heat or work interactions occur with the surroundings, and there are
no changes in kinetic and potential energy.
Is Stale Airplane Air Making Us Sick?
Entropy is produced when any of these factors is present during a mixing process. This is
illustrated in the next example, where different gases, initially at different temperatures and
pressures, are mixed.
12.4 Analyzing Systems Involving Mixtures 575
Analysis:
(a) The final temperature of the mixture can be determined from an energy balance. With assumption 3, the closed system
energy balance reduces to
The initial internal energy of the system, U
1
, equals the sum of the internal energies of the two gases when separate
where T
N
2
250K is the initial temperature of the nitrogen and T
O
2
300K is the initial temperature of the oxygen. The
final internal energy of the system, U
2
, equals the sum of the internal energies of the two gases evaluated at the final mixture
temperature T
2
Collecting the last three equations
The temperature T
2
can be determined using specific internal energy data from Table A-23 and an iterative procedure like
that employed in part (a) of Example 12.4. However, since the specific heats of N
2
and O
2
vary little over the temperature in-
terval from 250 to 300K, the solution can be conducted accurately on the basis of constant specific heats. Hence, the fore-
going equation becomes
Solving for T
2
Selecting c
v
values for N
2
and O
2
from Table A-20 at the average of the initial temperatures of the gases, 275 K, and us-
ing the respective molecular weights to convert to a molar basis
Substituting values into the expression for T
2
(b) The final mixture pressure p
2
can be determined using the ideal gas equation of state, where n is the total
number of moles of mixture and V is the total volume occupied by the mixture. The volume V is the sum of the volumes of
the two tanks, obtained with the ideal gas equation of state as follows
V
n
N
2
RT
N
2
p
N
2
n
O
2
RT
O
2
p
O
2
p
2
nRT
2
V,
261°K
T
2
10.79
kmol2 a
20.82 kJ
kmol
#
K
b
1250 K2 10.21 kmol2 a
20.99 kJ
kmol
#
K
b
1300 K2
10 .79 kmol2 a20.82
kJ
kmol
#
K
b 10.21 kmol2 a20.99
kJ
kmol
#
K
b
c
v,O
2
a32.0
kg
kmol
b
a
.656 kJ
kg
#
K
b 20.99
kJ
kmol
#
°K
c
v,N
2
a28.01
kg
kmol
b
a
.743 kJ
kg
#
K
b 20.82
kJ
kmol
#
°K
T
2
n
N
2
c
v,N
2
T
N
2
n
O
2
c
v,O
2
T
O
2
n
N
2
c
v,N
2
n
O
2
c
v,O
2
n
N
2
c
v,N
2
1T
2
T
N
2
2 n
O
2
c
v,O
2
1T
2
T
O
2
2 0
n
N
2
3u
N
2
1T
2
2 u
N
2
1T
N
2
24 n
O
2
3u
O
2
1T
2
2 u
O
2
1T
O
2
24 0
U
2
n
N
2
u
N
2
1T
2
2 n
O
2
u
O
2
1T
2
2
U
1
n
N
2
u
N
2
1T
N
2
2 n
O
2
u
O
2
1T
O
2
2
¢U Q
0
W
0
or
U
2
U
1
0
