Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

Design & Open Ended Problems: Exploring Engineering Practice
556 Chapter 11 Thermodynamic Relations
10 bar, 250 K and a molar flow rate of 6 kmol/h. The mixture
exits the compressor at 100 bar. During compression, the tem-
perature of the mixture departs from 250 K by no more than
0.1 K. The power required by the compressor is reported to be
6 kW. Can this value be correct? Explain. Ignore kinetic and
potential energy effects. Assume the mixture is modeled as an
ideal solution. For the pure components at 250 K:
h (kJ/kg) s (kJ/kg K)
10 bar 100 bar 10 bar 100 bar
Methane 506.0 358.6 10.003 8.3716
Nitrogen 256.18 229.68 5.962 5.188
#
exergy is stored by compressing the nitrogen. When the vehi-
cle accelerates again, the gas expands and returns some exergy
to the hydraulic fluid which is in communication with the ve-
hicle’s drive train, thereby assisting the vehicle to accelerate.
In a proposal for one such device, the nitrogen operates in the
range 50–150 bar and 200–350 K. Develop a thermodynamic
model of the accumulator and use the model to assess its suit-
ability for vehicle deceleration/acceleration.
11.4D To investigate liquid–vapor phase transition behavior,
construct a p–v diagram for water showing isotherms in the
range 0.7 T
R
1.2 by solving the van der Waals equation
of state for pressure and specific volume at constant tempera-
ture. Superimpose the vapor dome on the diagram using satu-
rated liquid and saturated vapor data from the steam tables. In-
terpret the behavior of the various isotherms in the liquid and
vapor regions on the diagram. Referring to the literature as nec-
essary, explain the behavior of the isotherms in the two-phase,
liquid–vapor region, carefully distinguishing among stable,
metastable, and unstable states. Write a paper discussing the
plot and summarizing your findings.
11.5D In the experiment for the regelation of ice, a small-
diameter wire weighted at each end is draped over a block of
ice. The loaded wire is observed to cut slowly through the ice
without leaving a trace. In one such set of experiments, a
weighted 1.00-mm diameter wire is reported to have passed
through 0C ice at a rate of 54 mm/h. Perform the regelation
experiment and propose a plausible explanation for this
phenomenon.
11.6D During a phase change from liquid to vapor at fixed
pressure, the temperature of a binary nonazeotropic solution
such as an ammonia–water solution increases rather than re-
mains constant as for a pure substance. This attribute is ex-
ploited in both the Kalina power cycle and in the Lorenz re-
frigeration cycle. Write a report assessing the status of
technologies based on these cycles. Discuss the principal ad-
vantages of using binary nonazeotropic solutions. What are
11.104 The departure of a binary solution from ideal solution
behavior is gauged by the activity coefficient,
i
a
i
y
i
, where
a
i
is the activity of component i and y
i
is its mole fraction in
the solution (i 1, 2). Introducing Eq. 11.140, the activity coef-
ficient can be expressed alternatively as Using this
expression together with the Gibbs–Duhem equation, derive the
following relation among the activity coefficients and the mole
fractions for a solution at temperature T and pressure p:
How might this expression be used?
ay
1
0 ln g
1
0y
1
b
p, T
ay
2
0 ln g
2
0y
2
b
p, T
g
i
f
i
y
i
f
°
i
.
11.1D Compressed natural gas (CNG) is being used as a fuel
to replace gasoline for automobile engines. Aluminum cylin-
ders wrapped in a fibrous composite can provide lightweight,
economical, and safe on-board storage. The storage vessels
should hold enough CNG for 100 to 125 miles of urban travel,
at storage pressures up to 20 Mpa, and with a maximum total
mass of 70 kg. Adhering to applicable standards, specify both
the size and number of cylinders that would meet the above
design constraints.
11.2D Develop the preliminary design of a thermal storage sys-
tem that would recover automobile engine waste heat for later
use in improving the engine cold-start performance. Among
the specifications are: Reliable operation down to an ambient
temperature of 30C, a storage duration of 16 hours, and no
more than 15 minutes of urban driving to return the storage
medium to its maximum temperature of 200C. Specify the
storage medium and determine whether the medium should be
charged by the engine exhaust gases, the engine coolant, or
some combination. Explain how the system would be config-
ured and where it would be located in the automobile.
11.3D Figure P11.3D shows the schematic of a hydraulic ac-
cumulator in the form of a cylindrical pressure vessel with a
piston separating a hydraulic fluid from a charge of nitrogen
gas. The device has been proposed as a means for storing some
of the exergy of a decelerating vehicle as it comes to rest. The
Hydraulic
fluid
Nitrogen
gas
Piston
Figure P11.3D
Design & Open Ended Problems: Exploring Engineering Practice 557
11.8D An inventor has proposed a new type of marine engine
fueled by fresh water stored on board and seawater drawn in
from the surrounding ocean. At steady state, the engine would
develop power from freshwater and seawater streams, each en-
tering the engine at the ambient temperature and pressure. A
single mixed stream would be discharged at the ambient tem-
perature and pressure. Critically evaluate this proposal.
11.9D Small Power Plants Pack Punch (see box Sec. 11.5).
Using manufacturer’s data, specify a natural gas-fueled mi-
croturbine cogeneration system to provide for the needs of a
hospital in your locale. Write a report including at least three
references.
some of the main design issues related to their use in power
and refrigeration systems?
11.7D The Servel refrigerator works on an absorption principle
and requires no moving parts. An energy input by heat trans-
fer is used to drive the cycle, and the refrigerant circulates due
to its natural buoyancy. This type of refrigerator is commonly
employed in mobile applications, such as recreational vehicles.
Liquid propane is burned to provide the required energy input
during mobile operation, and electric power is used when the
vehicle is parked and can be connected to an electrical outlet.
Investigate the principles of operation of commercially avail-
able Servel-type systems, and study their feasibility for solar-
activated operation. Consider applications in remote locations
where electricity or gas is not available. Write a report sum-
marizing your findings.

558
12
C
H
A
P
T
E
R
Ideal Gas
Mixture and
Psychrometric
Applications
12.1 Describing Mixture Composition
To specify the state of a mixture requires the composition and the values of two independ-
ent intensive properties such as temperature and pressure. The object of the present section
is to consider ways for describing mixture composition. In subsequent sections, we show
how mixture properties other than composition can be evaluated.
Consider a closed system consisting of a gaseous mixture of two or more components.
The composition of the mixture can be described by giving the mass or the number of moles
of each component present. With Eq. 1.10, the mass, the number of moles, and the molecu-
lar weight of a component i are related by
(12.1)n
i
m
i
M
i
ENGINEERING CONTEXT Many systems of interest involve gas
mixtures of two or more components. To apply the principles of thermodynamics introduced
thus far to these systems requires that we evaluate properties of the mixtures. Means are
available for determining the properties of mixtures from the mixture composition and the
properties of the individual pure components from which the mixtures are formed. Methods
for this purpose are discussed both in Chap. 11 and in the present chapter.
The objective of the present chapter is to study mixtures where the overall mixture
and each of its components can be modeled as ideal gases. General ideal gas mixture
considerations are provided in the first part of the chapter. Understanding the behavior of
ideal gas mixtures of air and water vapor is prerequisite to considering air-conditioning
processes in the second part of the chapter. In those processes, we sometimes must con-
sider the presence of liquid water as well. We will also need to know how to handle ideal
gas mixtures when we study the subjects of combustion and chemical equilibrium in
Chapters 13 and 14, respectively.
IDEAL GAS MIXTURES:
GENERAL CONSIDERATIONS
chapter objective
12.1 Describing Mixture Composition 559
where m
i
is the mass, n
i
is the number of moles, and M
i
is the molecular weight of compo-
nent i, respectively. When m
i
is expressed in terms of the kilogram, n
i
is in kmol. However,
any unit of mass can be used in this relationship.
The total mass of the mixture, m, is the sum of the masses of its components
(12.2)
The relative amounts of the components present in the mixture can be specified in terms
of mass fractions. The mass fraction mf
i
of component i is defined as
(12.3)
A listing of the mass fractions of the components of a mixture is sometimes referred to as
a gravimetric analysis.
Dividing each term of Eq. 12.2 by the total mass of mixture m and using Eq. 12.3
(12.4)
That is, the sum of the mass fractions of all the components in a mixture is equal to unity.
The total number of moles in a mixture, n, is the sum of the number of moles of each of
its components
(12.5)
The relative amounts of the components present in the mixture also can be described in
terms of mole fractions. The mole fraction y
i
of component i is defined as
(12.6)
A listing of the mole fractions of the components of a mixture may be called a molar analysis.
An analysis of a mixture in terms of mole fractions is also called a volumetric analysis.
Dividing each term of Eq. 12.5 by the total number of moles of mixture n and using
Eq. 12.6
(12.7)
That is, the sum of the mole fractions of all the components in a mixture is equal to unity.
The apparent (or average) molecular weight of the mixture, M, is defined as the ratio of
the total mass of the mixture, m, to the total number of moles of mixture, n
(12.8)
M
m
n
1
a
j
i1
y
i
y
i
n
i
n
n n
1
n
2
###
n
j
a
j
i1
n
i
1
a
j
i1
mf
i
mf
i
m
i
m
m m
1
m
2
###
m
j
a
j
i1
m
i
mass fractions
mole fractions
apparent molecular
weight
molar analysis
volumetric analysis
gravimetric analysis

560 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
TABLE 12.1 Approximate Composition of Dry Air
Mole Fraction
Component (%)
Nitrogen 78.08
Oxygen 20.95
Argon 0.93
Carbon dioxide 0.03
Neon, helium, methane, and others 0.01
EXAMPLE 12.1 Converting Mole Fractions to Mass Fractions
The molar analysis of the gaseous products of combustion of a certain hydrocarbon fuel is CO
2
, 0.08; H
2
O, 0.11; O
2
, 0.07;
N
2
, 0.74. (a) Determine the apparent molecular weight of the mixture. (b) Determine the composition in terms of mass frac-
tions (gravimetric analysis).
dry air
Equation 12.8 can be expressed in a convenient alternative form. With Eq. 12.2, it
becomes
Introducing m
i
n
i
M
i
from Eq. 12.1
Finally, with Eq. 12.6, the apparent molecular weight of the mixture can be calculated as a
mole-fraction average of the component molecular weights
(12.9)
for example. . .
consider the case of air. A sample of atmospheric air contains sev-
eral gaseous components including water vapor and contaminants such as dust, pollen, and
pollutants. The term dry air refers only to the gaseous components when all water vapor and
contaminants have been removed. The molar analysis of a typical sample of dry air is given
in Table 12.1. Selecting molecular weights for nitrogen, oxygen, argon, and carbon dioxide
from Table A-1, and neglecting the trace substances neon, helium, etc., the apparent molec-
ular weight of dry air obtained from Eq. 12.9 is
This value, which is the entry for air in Tables A-1, would not be altered significantly if the
trace substances were also included in the calculation.
Next, we consider two examples illustrating, respectively, the conversion from an analy-
sis in terms of mole fractions to an analysis in terms of mass fractions, and conversely.
28.97 kg/kmol
M 0.7808128.022 0.2095132.002 0.0093139.942 0.0003144.012
M
a
j
i1
y
i
M
i
M
n
1
M
1
n
2
M
2
###
n
j
M
j
n
M
m
1
m
2
###
m
j
n
12.1 Describing Mixture Composition 561
SOLUTION
Known: The molar analysis of the gaseous products of combustion of a hydrocarbon fuel is given.
Find: Determine (a) the apparent molecular weight of the mixture, (b) the composition in terms of mass fractions.
Analysis:
(a) Using Eq. 12.9 and approximate molecular weights from Table A-1
(b) Equations 12.1, 12.3, and 12.6 are the key relations required to determine the composition in terms of mass fractions.
Although the actual amount of mixture is not known, the calculations can be based on any convenient amount. Let us base
the solution on 1 kmol of mixture. Then, with Eq. 12.6 the amount n
i
of each component present in kmol is numerically equal
to the mole fraction, as listed in column (ii) of the accompanying table. Column (iii) of the table gives the respective molec-
ular weights of the components.
Column (iv) of the table gives the mass m
i
of each component, in kg per kmol of mixture, obtained with Eq. 12.1 in the
form m
i
M
i
n
i
. The values of column (iv) are obtained by multiplying each value of column (ii) by the corresponding value
of column (iii). The sum of the values in column (iv) is the mass of the mixture: kg of mixture per kmol of mixture. Note
that this sum is just the apparent mixture molecular weight determined in part (a). Finally, using Eq. 12.3, column (v) gives
the mass fractions as a percentage. The values of column (v) are obtained by dividing the values of column (iv) by the column
(iv) total and multiplying by 100.
28.46 kg/kmol
M 0.081442 0.111182 0.071322 0.741282
(i) (ii)
a
(iii) (iv)
b
(v)
Component n
i
M
i
m
i
mf
i
(%)
CO
2
0.08 44 3.52 12.37
H
2
O 0.11 18 1.98 6.96
O
2
0.07 32 2.24 7.87
N
2
0.74 28 20.72 72.80
1.00 28.46 100.00
a
Entries in this column have units of kmol per kmol of mixture. For example,
the first entry is 0.08 kmol of CO
2
per kmol of mixture.
b
Entries in this column have units of kg per kmol of mixture. For example, the
first entry is 3.52 kg of CO
2
per kmol of mixture. The column sum, 28.46, has
units of kg of mixture per kmol of mixture.
If the solution to part (b) were conducted on the basis of some other assumed amount of mixture—for example, 100 kmol
the same result for the mass fractions would be obtained, as can be verified.
❶
❶
EXAMPLE 12.2 Converting Mass Fractions to Mole Fractions
A gas mixture has the following composition in terms of mass fractions: H
2
, 0.10; N
2
, 0.60; CO
2
, 0.30. Determine (a) the
composition in terms of mole fractions and
(b) the apparent molecular weight of the mixture.
SOLUTION
Known: The gravimetric analysis of a gas mixture is known.
Find: Determine the analysis of the mixture in terms of mole fractions (molar analysis) and the apparent molecular weight
of the mixture.
562 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
Analysis:
(a) Equations 12.1, 12.3, and 12.6 are the key relations required to determine the composition in terms of mole fractions.
Although the actual amount of mixture is not known, the calculation can be based on any convenient amount. Let us base
the solution on 100 kg. Then, with Eq. 12.3, the amount m
i
of each component present, in kg, is equal to the mass fraction
multiplied by 100 kg. The values are listed in column (ii) of the accompanying table. Column (iii) of the table gives the
respective molecular weights of the components.
Column (iv) of the table gives the amount n
i
of each component, in kmol per 100 kg of mixture, obtained using Eq. 12.1.
The values of column (iv) are obtained by dividing each value of column (ii) by the corresponding value of column (iii). The
sum of the values of column (iv) is the total amount of mixture, in kmol per 100 kg of mixture. Finally, using Eq. 12.6, column
(v) gives the mole fractions as a percentage. The values of column (v) are obtained by dividing the values of column (iv) by
the column (iv) total and multiplying by 100.
(i) (ii)
a
(iii) (iv)
b
(v)
Component m
i
M
i
n
i
y
i
(%)
H
2
10 2 5.00 63.9
N
2
60 28 2.14 27.4
CO
2
30 44 0.68 8.7
100 7.82 100.0
a
Entries in this column have units of kg per 100 kg of mixture. For example, the
first entry is 10 kg of H
2
per 100 kg of mixture.
b
Entries in this column have units of kmol per 100 kg of mixture. For example,
the first entry is 5.00 kmol of H
2
per 100 kg of mixture. The column sum, 7.82,
has units of kmol of mixture per 100 kg of mixture.
(b) The apparent molecular weight of the mixture can be found by using Eq. 12.9 and the calculated mole fractions. The
value can be determined alternatively by using the column (iv) total giving the total amount of mixture in kmol per 100 kg
of mixture. Thus, with Eq. 12.8
If the solution to part (a) were conducted on the basis of some other assumed amount of mixture, the same result for the
mass fractions would be obtained, as can be verified.
Although H
2
has the smallest mass fraction, its mole fraction is the largest.
M
m
n
100 kg
7 .82 kmol
12.79
kg
kmol
❶
❷
❶
❷
12.2 Relating p, V, and T for
Ideal Gas Mixtures
The definitions given in Sec. 12.1 apply generally to mixtures. In the present section we are
concerned only with ideal gas mixtures and introduce two models used in conjunction with
this idealization: the Dalton model and the Amagat model.
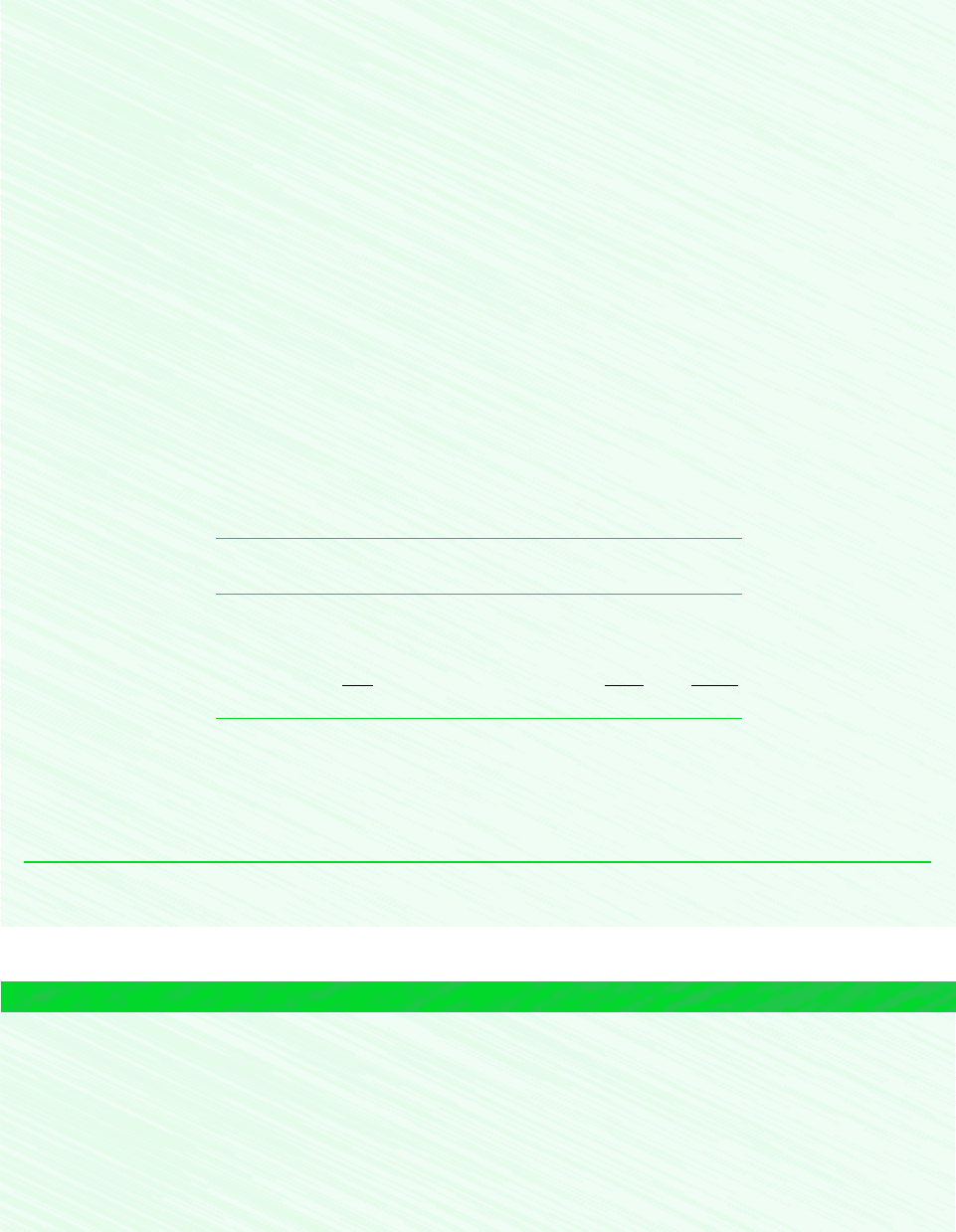
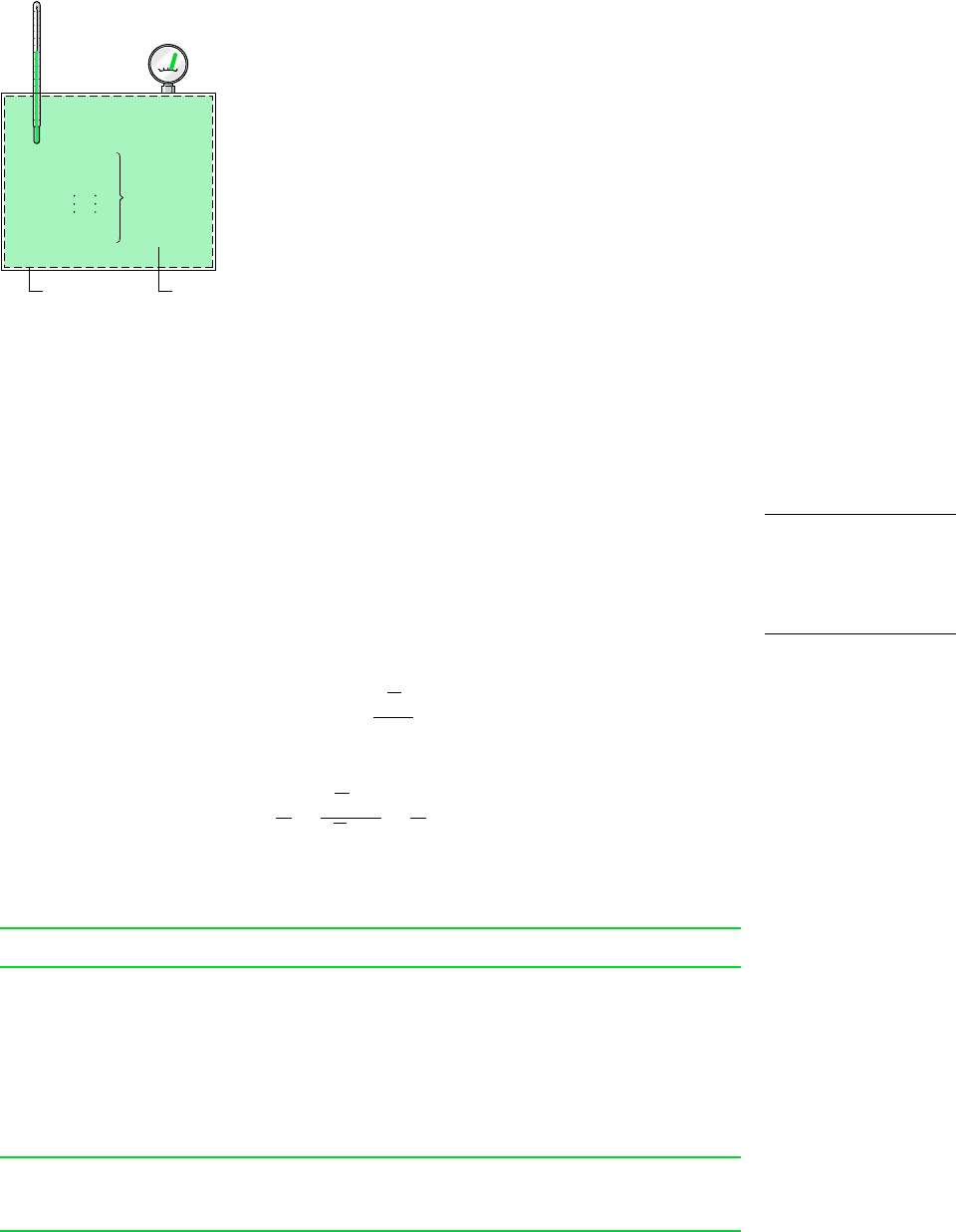
Consider a system consisting of a number of gases contained within a closed vessel of
volume V as shown in Fig. 12.1. The temperature of the gas mixture is T and the pressure is
p. The overall mixture is considered an ideal gas, so p, V, T, and the total number of moles
of mixture n are related by the ideal gas equation of state
(12.10)
With reference to this system, let us consider in turn the Dalton and Amagat models.
p n
RT
V
12.2 Relating p, V, and T for Ideal Gas Mixtures 563
DALTON MODEL. The Dalton model is consistent with the concept of an ideal gas as be-
ing made up of molecules that exert negligible forces on one another and whose volume is
negligible relative to the volume occupied by the gas (Sec. 3.5). In the absence of signifi-
cant intermolecular forces, the behavior of each component would be unaffected by the pres-
ence of the other components. Moreover, if the volume occupied by the molecules is a very
small fraction of the total volume, the molecules of each gas present may be regarded as free
to roam throughout the full volume. In keeping with this simple picture, the Dalton model
assumes that each mixture component behaves as an ideal gas as if it were alone at the
temperature T and volume V of the mixture.
It follows from the Dalton model that the individual components would not exert the mix-
ture pressure p but rather a partial pressure. As shown below, the sum of the partial pressures
equals the mixture pressure. The partial pressure of component i, p
i
, is the pressure that n
i
moles
of component i would exert if the component were alone in the volume V at the mixture tem-
perature T. The partial pressure can be evaluated using the ideal gas equation of state
(12.11)
Dividing Eq. 12.11 by Eq. 12.10
Thus, the partial pressure of component i can be evaluated in terms of its mole fraction y
i
and the mixture pressure p
(12.12)
To show that the sum of partial pressures equals the mixture pressure, sum both sides of
Eq. 12.12 to obtain
Since the sum of the mole fractions is unity (Eq. 12.7), this becomes
(12.13)p
a
j
i1
p
i
a
j
i1
p
i
a
j
i1
y
i
p p
a
j
i1
y
i
p
i
y
i
p
p
i
p
n
i
RT
V
nRT
V
n
i
n
y
i
p
i
n
i
RT
V
Gas 1 : n
1
Gas 2 : n
2
n moles
mixture
Pressure = p
Temperature = T
Gas j : n
j
Volume = VBoundary
Figure 12.1 Mixture of several gases.
Dalton model
partial pressure
564 Chapter 12 Ideal Gas Mixture and Psychrometric Applications
AMAGAT MODEL.
The underlying assumption of the Amagat model is that each mixture
component behaves as an ideal gas as if it existed separately at the pressure p and tempera-
ture T of the mixture. The volume that n
i
moles of component i would occupy if the com-
ponent existed at p and T is called the partial volume, V
i
, of component i. As shown below,
the sum of the partial volumes equals the total volume. The partial volume can be evaluated
using the ideal gas equation of state
(12.14)
Dividing Eq. 12.14 by the total volume V
Thus, the partial volume of component i also can be evaluated in terms of its mole fraction
y
i
and the total volume
(12.15)
This relationship between volume fraction and mole fraction underlies the use of the term
volumetric analysis as signifying an analysis of a mixture in terms of mole fractions.
To show that the sum of partial volumes equals the total volume, sum both sides of
Eq. 12.15 to obtain
Since the sum of the mole fractions equals unity, this becomes
(12.16)
CLOSING COMMENT. The Dalton and Amagat models are special cases, respectively, of
the additive pressure and additive volume rules introduced in Sec. 11.8, which do not require
the assumption of the ideal gas model. The concept of an ideal gas mixture is a special case
of the ideal solution concept introduced in Sec. 11.9.
V
a
j
i1
V
i
a
j
i1
V
i
a
j
i1
y
i
V V
a
j
i1
y
i
V
i
y
i
V
V
i
V
n
i
RT
p
nRT
p
n
i
n
y
i
V
i
n
i
RT
p
12.3 Evaluating U, H, S, and Specific Heats
To apply the conservation of energy principle to a system involving an ideal gas mixture re-
quires evaluation of the internal energy, enthalpy, or specific heats of the mixture at various
states. Similarly, to conduct an analysis using the second law normally requires the entropy
of the mixture. The objective of the present section is to develop means to evaluate these
properties for ideal gas mixtures.
EVALUATING U AND H. Consider a closed system consisting of an ideal gas mixture. Ex-
tensive properties of the mixture, such as U, H, or S, can be found by adding the contribu-
tion of each component at the condition at which the component exists in the mixture. Let
us apply this model to internal energy and enthalpy.
Amagat model
partial volume
12.3 Evaluating U, H, S, and Specific Heats 565
Since the internal energy and enthalpy of ideal gases are functions of temperature only,
the values of these properties for each component present in the mixture are determined by
the mixture temperature alone. Accordingly
(12.17)
(12.18)
where U
i
and H
i
are the internal energy and enthalpy, respectively, of component i evaluated
at the mixture temperature.
Equations 12.17 and 12.18 can be rewritten on a molar basis as
(12.19)
and
(12.20)
where and are the specific internal energy and enthalpy of the mixture per mole of mixture,
and and are the specific internal energy and enthalpy of component i per mole of i.
Dividing by the total number of moles of mixture n gives expressions for the specific inter-
nal energy and enthalpy of the mixture per mole of mixture, respectively
(12.21)
(12.22)
Each of the molar internal energy and enthalpy terms appearing in Eqs. 12.19 through 12.22
is evaluated at the mixture temperature only.
EVALUATING c
v
AND c
P
. Differentiation of Eqs. 12.21 and 12.22 with respect to temper-
ature results, respectively, in the following expressions for the specific heats and of the
mixture on a molar basis
(12.23)
(12.24)
That is, the mixture specific heats and are mole-fraction averages of the respective com-
ponent specific heats. The specific heat ratio for the mixture is
EVALUATING S. The entropy of a mixture can be found, as for U and H, by adding the
contribution of each component at the condition at which the component exists in the mix-
ture. The entropy of an ideal gas depends on two properties, not on temperature alone as for
internal energy and enthalpy. Accordingly, for the mixture
(12.25)S S
1
S
2
###
S
j
a
j
i1
S
i
k c
p
c
v
.
c
v
c
p
c
p
a
j
i1
y
i
c
p,i
c
v
a
j
i1
y
i
c
v,i
c
p
c
v
h
a
j
i1
y
i
h
i
u
a
j
i1
y
i
u
i
h
i
u
i
hu
nh n
1
h
1
n
2
h
2
###
n
j
h
j
a
j
i1
n
i
h
i
nu n
1
u
1
n
2
u
2
###
n
j
u
j
a
j
i1
n
i
u
i
H H
1
H
2
###
H
j
a
j
i1
H
i
U U
1
U
2
###
U
j
a
j
i1
U
i
