Moran M.J., Shapiro H.N. Fundamentals of Engineering Thermodynamics
Подождите немного. Документ загружается.

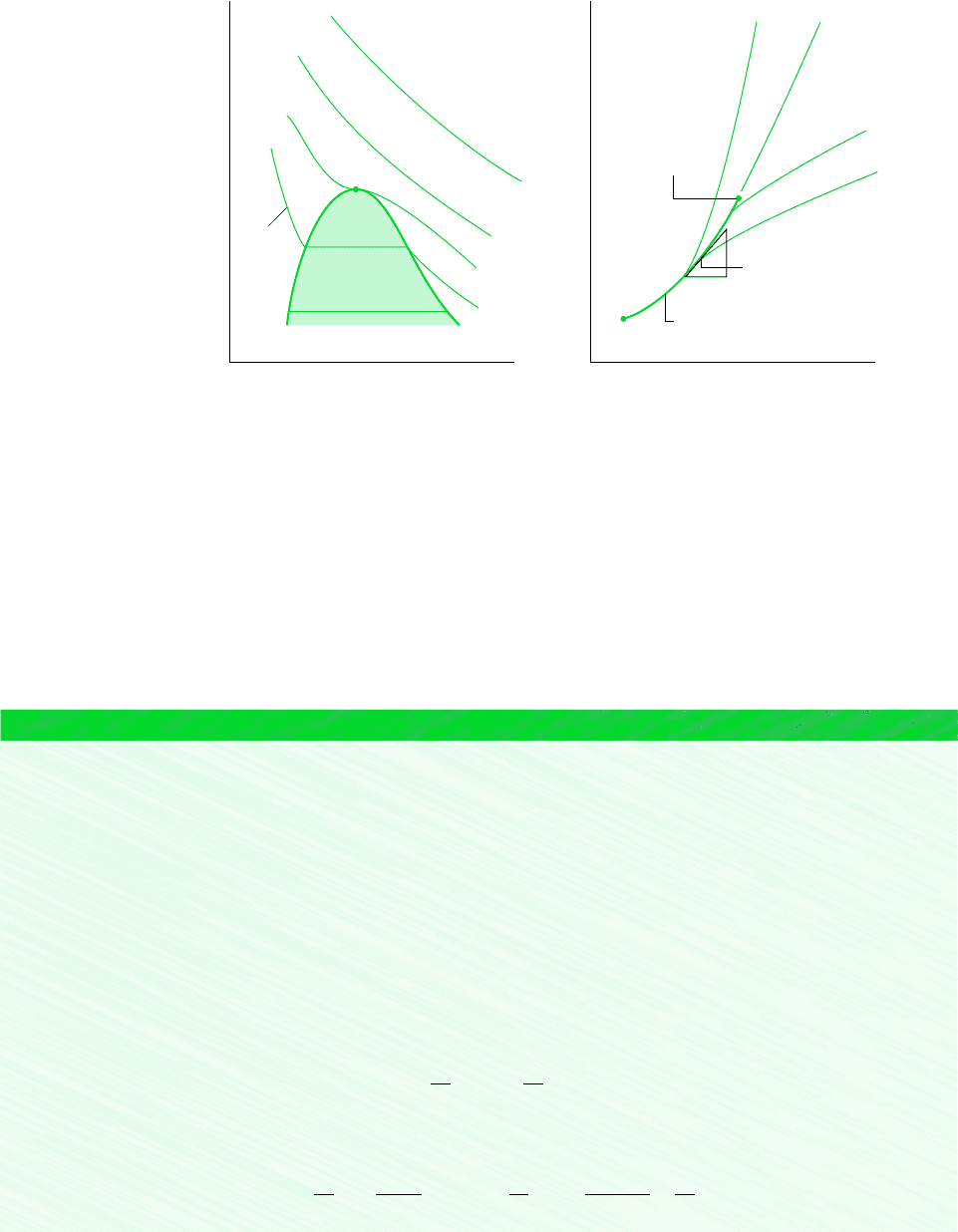
496 Chapter 11 Thermodynamic Relations
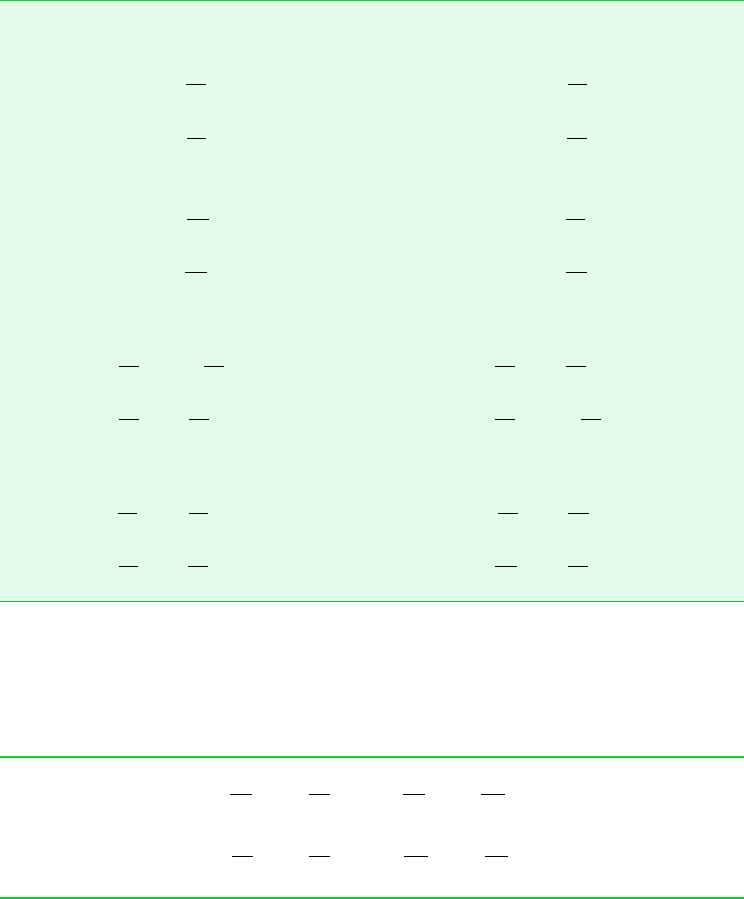
only. Hence, the slopes of the isometrics passing through the two-phase states corresponding
to a specified temperature are all equal, being given by the slope of the saturation curve at
that temperature, denoted simply as (dpdT )
sat
. For these two-phase states, ( p T )
v
(dpdT )
sat
.
In this section, important aspects of functions of two variables have been introduced.
The following example illustrates some of these ideas using the van der Waals equation
of state.
00
p
v
(a)
p
T
(b)
Critical
point
Liquid
Triple
point
Locus of saturation states
Vapor
Isometric
Isometric
v < v
c
v = v
c
v > v
c
∂p
–––
∂T
(
(
v
> 0
∂p
–––
∂v
(
(
T
< 0
∂p
–––
∂v
(
(
T
= 0
∂p
–––
∂v
(
(
T
< 0
∂p
–––
∂T
(
(
v
> 0
dp
–––
dT
(
(
sat
T < T
c
T = T
c
T > T
c
Isotherm
Isotherm
Critical
point
(∂p/∂v)
T
= 0
Liquid-vapor
Figure 11.1 Diagrams used to discuss (0p0v)
T
and (0p0T)
v
. (a) p–v diagram.
(b) Phase diagram.
EXAMPLE 11.2 Applying Mathematical Relations
For the van der Waals equation of state, (a) determine an expression for the exact differential dp, (b) show that the mixed sec-
ond partial derivatives of the result obtained in part (a) are equal, and (c) develop an expression for the partial derivative
(0v0T)
p
.
SOLUTION
Known: The equation of state is the van der Waals equation.
Find: Determine the differential dp, show that the mixed second partial derivatives of dp are equal, and develop an expres-
sion for (0v0T )
p
.
Analysis:
(a) By definition, the differential of a function p p(T, v) is
The partial derivatives appearing in this expression obtained from the van der Waals equation expressed as p
RT(v b) av
2
are
M a
0p
0T
b
v
R
v b
,
N a
0p
0v
b
T
RT
1v b2
2
2a
v
3
dp a
0p
0T
b
v
dT a
0p
0v
b
T
dv
11.3 Developing Property Relations 497
In this section, several important property relations are developed, including the expressions
known as the Maxwell relations. The concept of a fundamental thermodynamic function is
also introduced. These results, which are important for subsequent discussions, are obtained
for simple compressible systems of fixed chemical composition using the concept of an exact
differential.
11.3.1 Principal Exact Differentials
The principal results of this section are obtained using Eqs. 11.18, 11.19, 11.22, and 11.23.
The first two of these equations are derived in Sec. 6.3.1, where they are referred to as the
Accordingly, the differential takes the form
(b) Calculating the mixed second partial derivatives
Thus, the mixed second partial derivatives are equal, as expected.
(c) An expression for (0v0T )
p
can be derived using Eqs. 11.15 and 11.16. Thus, with x p, y v, and z T, Eq. 11.16
gives
or
Then, with x T, y p, and z v, Eq. 11.15 gives
Combining these results
The numerator and denominator of this expression have been evaluated in part (a), so
which is the desired result.
Since the van der Waals equation is cubic in specific volume, it can be solved for v (T, p) at only certain states. Part (c)
shows how the partial derivative (0v0T )
p
can be evaluated at states where it exists.
a
0v
0T
b
p
R
1v b2
3RT
1v b2
2
2a
v
3
4
a
0v
0T
b
p
10p
0T 2
v
10p
0v2
T
a
0T
0p
b
v
1
10p
0T 2
v
a
0v
0T
b
p
1
10p
0v2
T
10T
0p2
v
a
0v
0T
b
p
a
0p
0v
b
T
a
0T
0p
b
v
1
a
0N
0T
b
v
0
0T
ca
0p
0v
b
T
d
v
R
1v b2
2
a
0M
0v
b
T
0
0v
ca
0p
0T
b
v
d
T
R
1v b2
2
dp a
R
v b
b dT c
RT
1v b2
2
2a
v
3
d dv
❶
❶
11.3 Developing Property Relations
T ds equations. For present purposes, it is convenient to express them as
(11.18)
(11.19)
The other two equations used to obtain the results of this section involve, respectively, the
specific Helmholtz function defined by
(11.20)
and the specific Gibbs function g defined by
(11.21)
The Helmholtz and Gibbs functions are properties because each is defined in terms of
properties. From inspection of Eqs. 11.20 and 11.21, the units of and g are the same
as those of u and h. These two new properties are introduced solely because they con-
tribute to the present discussion, and no physical significance need be attached to them
at this point.
Forming the differential d
Substituting Eq. 11.18 into this gives
(11.22)
Similarly, forming the differential dg
Substituting Eq. 11.19 into this gives
(11.23)
11.3.2 Property Relations from Exact Differentials
The four differential equations introduced above, Eqs. 11.18, 11.19, 11.22, and 11.23, pro-
vide the basis for several important property relations. Since only properties are involved,
each is an exact differential exhibiting the general form dz M dx N dy considered in
Sec. 11.2. Underlying these exact differentials are, respectively, functions of the form
u(s, v), h(s, p), (v, T), and g(T, p). Let us consider these functions in the order given.
The differential of the function u u(s, v) is
By comparison with Eq. 11.18, we conclude that
(11.24)
(11.25) p a
0u
0v
b
s
T a
0u
0s
b
v
du a
0u
0s
b
v
ds a
0u
0v
b
s
dv
dg v dp s dT
dg dh d1Ts2 dh T ds s dT
dc p dv s dT
dc du d1Ts2 du T ds s dT
g h Ts
c u Ts
dh T ds v dp
du T ds p dv
Helmholtz function
Gibbs function
498 Chapter 11 Thermodynamic Relations
11.3 Developing Property Relations 499
Maxwell relations
The differential of the function h h(s, p) is
By comparison with Eq. 11.19, we conclude that
(11.26)
(11.27)
Similarly, the coefficients p and s of Eq. 11.22 are partial derivatives of (v, T)
(11.28)
(11.29)
and the coefficients v and s of Eq. 11.23 are partial derivatives of g(T, p)
(11.30)
(11.31)
As each of the four differentials introduced above is exact, the second mixed partial de-
rivatives are equal. Thus, in Eq. 11.18, T plays the role of M in Eq. 11.14b and p plays the
role of N in Eq. 11.14b, so
(11.32)
In Eq. 11.19, T and v play the roles of M and N in Eq. 11.14b, respectively. Thus
(11.33)
Similarly, from Eqs. 11.22 and 11.23 follow
(11.34)
(11.35)
Equations 11.32 through 11.35 are known as the Maxwell relations.
a
0v
0T
b
p
a
0s
0p
b
T
a
0p
0T
b
v
a
0s
0v
b
T
a
0T
0p
b
s
a
0v
0s
b
p
a
0T
0v
b
s
a
0p
0s
b
v
s a
0g
0T
b
p
v a
0g
0p
b
T
s a
0c
0T
b
v
p a
0c
0v
b
T
v a
0h
0p
b
s
T a
0h
0s
b
p
dh a
0h
0s
b
p
ds a
0h
0p
b
s
dp
500 Chapter 11 Thermodynamic Relations
TABLE 11.1 Summary of Property Relations from Exact Differentials
Basic relations:
from u u(s, v) from h h(s, p)
(11.24) (11.26)
(11.25) (11.27)
from (v, T ) from g g(T, p)
(11.28) (11.30)
(11.29) (11.31)
Maxwell relations:
(11.32) (11.34)
(11.33) (11.35)
Additional relations:
(11.36)
a
0c
0T
b
v
a
0g
0T
b
p
a
0h
0p
b
s
a
0g
0p
b
T
a
0u
0v
b
s
a
0c
0v
b
T
a
0u
0s
b
v
a
0h
0s
b
p
a
0v
0T
b
p
a
0s
0p
b
T
a
0T
0p
b
s
a
0v
0s
b
p
a
0p
0T
b
v
a
0s
0v
b
T
a
0T
0v
b
s
a
0p
0s
b
v
s a
0g
0T
b
p
s a
0c
0T
b
v
v a
0g
0p
b
T
p a
0c
0v
b
T
v a
0h
0p
b
s
p a
0u
0v
b
s
T a
0h
0s
b
p
T a
0u
0s
b
v
Since each of the properties T, p, v, s appears on the left side of two of the eight equa-
tions, Eqs. 11.24 through 11.31, four additional property relations can be obtained by equat-
ing such expressions. They are
(11.36)
Equations 11.24 through 11.36, which are listed in Table 11.1 for ease of reference, are
16 property relations obtained from Eqs. 11.18, 11.19, 11.22, and 11.23, using the concept
of an exact differential. Since Eqs. 11.19, 11.22, and 11.23 can themselves be derived from
Eq. 11.18, the important role of the first TdSequation in developing property relations is
apparent.
The utility of these 16 property relations is demonstrated in subsequent sections of this
chapter. However, to give a specific illustration at this point, suppose the partial deriva-
tive ( s v)
T
involving entropy is required for a certain purpose. The Maxwell relation
Eq. 11.34 would allow the derivative to be determined by evaluating the partial derivative
( p T)
v
, which can be obtained using p–v–T data only. Further elaboration is provided
in Example 11.3.
00
00
a
0h
0p
b
s
a
0g
0p
b
T
,
a
0c
0T
b
v
a
0g
0T
b
p
a
0u
0s
b
v
a
0h
0s
b
p
,
a
0u
0v
b
s
a
0c
0v
b
T
11.3 Developing Property Relations 501
EXAMPLE 11.3 Applying the Maxwell Relations
Evaluate the partial derivative (0s0v)
T
for water vapor at a state fixed by a temperature of 240C and a specific volume of
0.4646 m
3
/kg. (a) Use the Redlich–Kwong equation of state and an appropriate Maxwell relation. (b) Check the value obtained
using steam table data.
SOLUTION
Known: The system consists of a fixed amount of water vapor at 240C and 0.4646 m
3
/kg.
Find: Determine the partial derivative (0s0v)
T
employing the Redlich–Kwong equation of state, together with a Maxwell
relation. Check the value obtained using steam table data.
Assumptions:
1. The system consists of a fixed amount of water at a known equilibrium state.
2. Accurate values for (0s0T)
v
in the neighborhood of the given state can be determined from the Redlich–Kwong equation
of state.
Analysis:
(a) The Maxwell relation given by Eq. 11.34 allows (0s0v)
T
to be determined from the p–v–T relationship. That is
The partial derivative (0p0T )
v
obtained from the Redlich–Kwong equation, Eq. 11.7, is
At the specified state, the temperature is 513 K and the specific volume on a molar basis is
From Table A-24
Substituting values into the expression for (0p0T )
v
Accordingly
a
0s
0v
b
T
1.0043
kJ
m
3
#
K
1.0043
kJ
m
3
#
K
a1004.3
N
#
m
m
3
#
K
b `
1 kJ
10
3
N
#
m
`
a
0p
0T
b
v
a8314
N
#
m
kmol
#
K
b
18.372 0.02112
m
3
kmol
142.59 bar a
m
3
kmol
b
2
1K2
1
2
2 a8.372
m
2
kmol
b a8.3931
m
3
kmol
b 1513 K2
3
2
`
10
5
N/m
2
1 bar
`
a 142.59 bar
a
m
3
kmol
b
2
1K2
1
2
,
b 0.0211
m
3
kmol
v
0.4646
m
3
kg
a
18.02 kg
kmol
b 8.372
m
3
kmol
a
0p
0T
b
v
R
v b
a
2v 1v b2T
3
2
a
0s
0v
b
T
a
0p
0T
b
v
502 Chapter 11 Thermodynamic Relations
(b) A value for (0s0v)
T
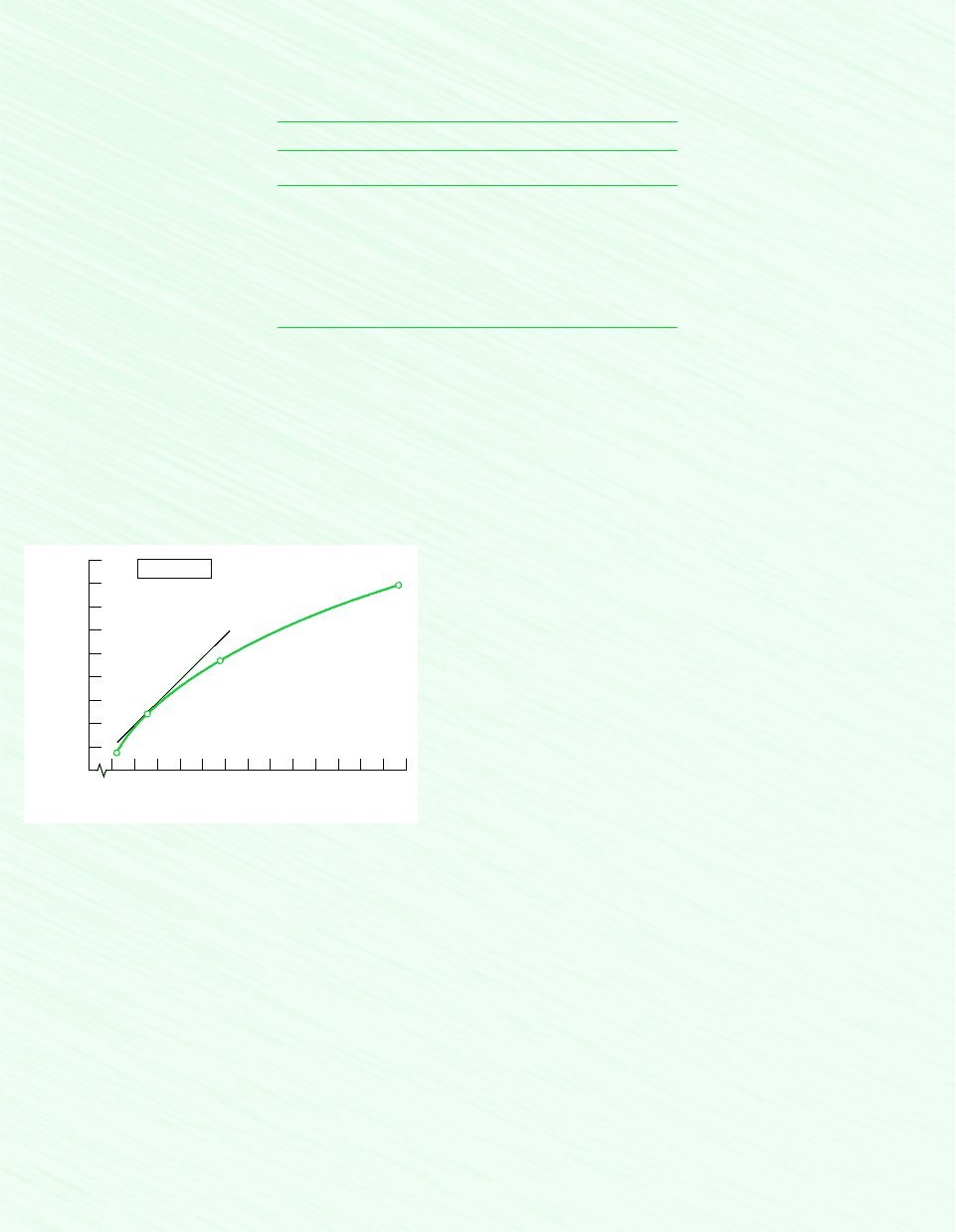
can be estimated using a graphical approach with steam table data, as follows: At 240C, Table A-4
provides the values for specific entropy s and specific volume v tabulated below
T 240C
p (bar) s (kJ/kg K) (m
3
/kg)
1.0 7.9949 2.359
1.5 7.8052 1.570
3.0 7.4774 0.781
5.0 7.2307 0.4646
7.0 7.0641 0.3292
10.0 6.8817 0.2275
With the values for s and v listed in the table, the plot in Fig. E11.3a giving s versus v can be prepared. Note that a line repre-
senting the tangent to the curve at the given state is shown on the plot. The pressure at this state is 5 bar. The slope of the tan-
gent is (0s0v)
T
1.0 kJ/m
3
K. Thus, the value of (0s0v)
T
obtained using the Redlich–Kwong equation agrees closely with
the result determined graphically using steam table data.
#
#
T = 240°C
7.5
7.0
0.5
7 bar
5 bar
3 bar
1.5 bar
1.0
Specific volume, m
3
/kg
1.5
Specific entropy, kJ/kg·K
Figure E11.3a
Alternative Solution:
Alternatively, the partial derivative (0s0v)
T
can be estimated using numerical methods and computer-generated data. The
following IT code illustrates one way the partial derivative, denoted dsdv, can be estimated:
v = 0.4646 // m
3
/kg
T = 240 // C
v2 = v + dv
v1 = v – dv
dv = 0.2
v2 = v_PT(”Water/Steam”, p2, T)
v1 = v_PT(“Water/Steam”, p1, T)
s2 = s_PT(“Water/Steam”, p2, T)
s1 = s_PT(“Water/Steam”, p1, T)
dsdv = (s2 – s1) / (v2 – v1)
11.3 Developing Property Relations 503
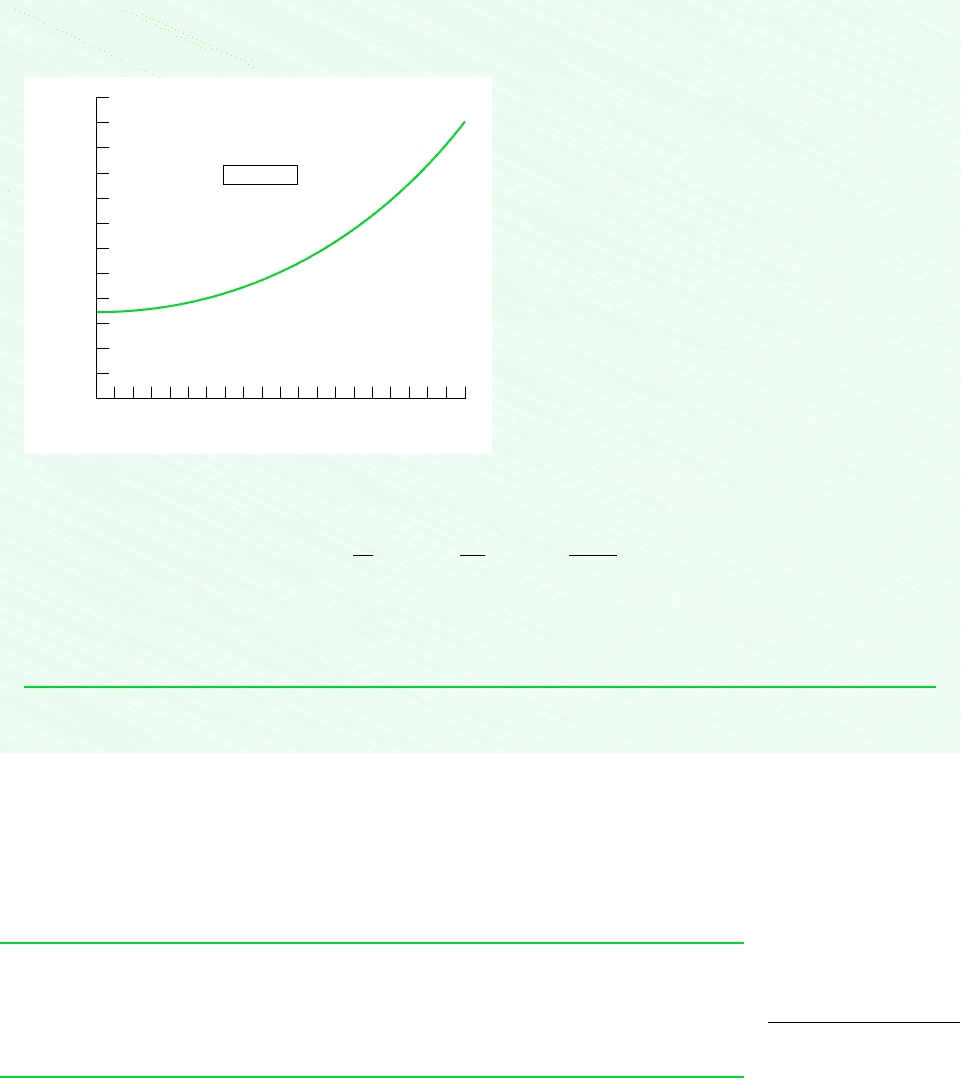
Using the Explore button, sweep dv from 0.0001 to 0.2 in steps of 0.001. Then, using the Graph button, the following graph
can be constructed:
1.12
1.10
1.08
1.06
1.04
1.02
1.00
0.00 0.05 0.10
dv
dsdv
0.15 0.20
T = 240°C
Figure E11.3b
From the computer data, the y-intercept of the graph is
This answer is an estimate because it relies on a numerical approximation of the partial derivative based on the equation of
state that underlies the steam tables. The values obtained using the Redlich–Kwong equation of state and the graphical method
agree with this result.
It is left as an exercise to show that, in accordance with Eq. 11.34, the value of (0p0T )
v
estimated by a procedure like the
one used for (0s0v)
T
would agree with the value obtained here.
a
0s
0v
b
T
lim
¢v S0
a
¢s
¢v
b
T
1.033
kJ
m
3
#
K
❶
❶
11.3.3 Fundamental Thermodynamic Functions
A fundamental thermodynamic function provides a complete description of the thermody-
namic state. In the case of a pure substance with two independent properties, the fundamental
thermodynamic function can take one of the following four forms:
(11.37)
Of the four fundamental functions listed in Eqs. 11.37, the Helmholtz function and the
Gibbs function g have the greatest importance for subsequent discussions (see Sec. 11.6.2).
Accordingly, let us discuss the fundamental function concept with reference to and g.
In principle, all properties of interest can be determined from a fundamental thermody-
namic function by differentiation and combination.
for example. . . consider a fun-
damental function of the form (T, v). The properties v and T, being the independent
g g1T, p2
c c1T, v2
h h1s, p2
u u1s, v2
fundamental function
504 Chapter 11 Thermodynamic Relations
variables, are specified to fix the state. The pressure p at this state can be determined from
Eq. 11.28 by differentiation of (T, v). Similarly, the specific entropy s at the state can be
found from Eq. 11.29 by differentiation. By definition, u Ts, so the specific internal
energy is obtained as
With u, p, and v known, the specific enthalpy can be found from the definition h u pv.
Similarly, the specific Gibbs function is found from the definition, g h Ts. The specific
heat c
v
can be determined by further differentiation, c
v
( u T )
v
. Other properties can be
calculated with similar operations.
for example. . .
consider a fundamental function of the form g(T, p). The proper-
ties T and p are specified to fix the state. The specific volume and specific entropy at this
state can be determined by differentiation from Eqs. 11.30 and 11.31, respectively. By defi-
nition, g h Ts, so the specific enthalpy is obtained as
With h, p, and v known, the specific internal energy can be found from u h pv. The
specific heat c
p
can be determined by further differentiation, c
p
( h T)
p
. Other proper-
ties can be calculated with similar operations.
Like considerations apply for functions of the form u(s, v) and h(s, p), as can readily be
verified. Note that a Mollier diagram provides a graphical representation of the fundamen-
tal function h(s, p).
00
h g Ts
00
u c Ts
11.4 Evaluating Changes in Entropy, Internal
Energy, and Enthalpy
With the introduction of the Maxwell relations, we are in a position to develop thermody-
namic relations that allow changes in entropy, internal energy, and enthalpy to be evaluated
from measured property data. The presentation begins by considering relations applicable to
phase changes and then turns to relations for use in single-phase regions.
11.4.1 Considering Phase Change
The object of this section is to develop relations for evaluating the changes in specific en-
tropy, internal energy, and enthalpy accompanying a change of phase at fixed temperature
and pressure. A principal role is played by the Clapeyron equation, which allows the change
in enthalpy during vaporization, sublimation, or melting at a constant temperature to be eval-
uated from pressure-specific volume–temperature data pertaining to the phase change. Thus,
the present discussion provides important examples of how p–v–T measurements can lead to
the determination of other property changes, namely s, u, and h for a change of phase.
Consider a change in phase from saturated liquid to saturated vapor at fixed temperature.
For an isothermal phase change, pressure also remains constant, so Eq. 11.19 reduces to
Integration of this expression gives
(11.38)s
g
s
f
h
g
h
f
T
dh T ds
11.4 Evaluating Changes in Entropy, Internal Energy, and Enthalpy 505
Hence, the change in specific entropy accompanying a phase change from saturated liquid
to saturated vapor at temperature T can be determined from the temperature and the change
in specific enthalpy.
The change in specific internal energy during the phase change can be determined using
the definition h u pv.
(11.39)
Thus, the change in specific internal energy accompanying a phase change at temperature T
can be determined from the temperature and the changes in specific volume and enthalpy.
CLAPEYRON EQUATION. The change in specific enthalpy required by Eqs. 11.38 and 11.39
can be obtained using the Clapeyron equation. To derive the Clapeyron equation, begin with
the Maxwell relation
(11.34)
During a phase change at fixed temperature, the pressure is independent of specific volume
and is determined by temperature alone. Thus, the quantity ( p T )
v
is determined by the
temperature and can be represented as
where “sat” indicates that the derivative is the slope of the saturation pressure–temperature
curve at the point determined by the temperature held constant during the phase change
(Sec. 11.2). Combining the last two equations gives
Since the right side of this equation is fixed when the temperature is specified, the equa-
tion can be integrated to give
Introducing Eq. 11.38 into this expression results in the Clapeyron equation
(11.40)
Equation 11.40 allows (h
g
h
f
) to be evaluated using only p–v–T data pertaining to the phase
change. In instances when the enthalpy change is also measured, the Clapeyron equation can
be used to check the consistency of the data. Once the specific enthalpy change is deter-
mined, the corresponding changes in specific entropy and specific internal energy can be
found from Eqs. 11.38 and 11.39, respectively.
Equations 11.38, 11.39, and 11.40 also can be written for sublimation or melting occur-
ring at constant temperature and pressure. In particular, the Clapeyron equation would take
the form
(11.41)
a
dp
dT
b
sat
h– h¿
T 1v– v¿ 2
a
dp
dT
b
sat
h
g
h
f
T 1v
g
v
f
2
s
g
s
f
a
dp
dT
b
sat
1v
g
v
f
2
a
0s
0v
b
T
a
dp
dT
b
sat
a
0p
0T
b
v
a
dp
dT
b
sat
00
a
0s
0v
b
T
a
0p
0T
b
v
u
g
u
f
h
g
h
f
p 1v
g
v
f
2
Clapeyron equation
