Moller K.D. Optics. Learning by Computing, with Examples Using Mathcad, Matlab, Mathematica, and Maple
Подождите немного. Документ загружается.


7.2. BLACKBODY RADIATON 279
7.2.5 Files of Planck’s, Stefan–Boltzmann’s, and Wien’s
Laws. Radiance, Area, and Solid Angle
Using the radiance L
B
as discussed in Section 7.2.3, we can rewrite Planck’s
law in terms of the radiance per wavelength or per frequency interval as
dL
B
/dλ (C
1
/λ
5
)/(exp(C
2
/λT )) − 1)[W/{m
3
sr}] (7.20)
dL
B
/dν (C
3
ν
3
)/(exp(C
4
ν/T )) −1)[W/{(1/s)m
2
sr}], (7.21)
where
C
1
2hc
2
1.17610
−16
Wm
2
C
2
hc/k 1.43210
−2
mK (7.22)
C
3
2h/c
2
1.4710
−50
Ws
4
/m
2
C
4
h/k 4.7810
−11
sK.
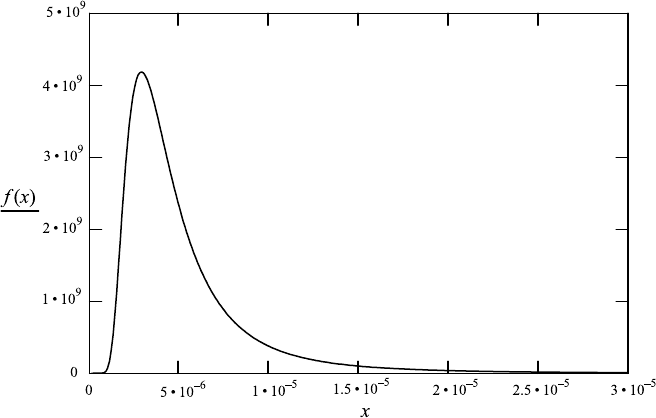
FileFig 7.2 (L2BBLS)
Graph of blackbody radiation depending on wavelength. The calculation of the
radiance of a particular wavelength range and calculation of the corresponding
radiant energy by multiplication with area times solid angle.
L2BBLS
1. Blackbody radiation. Graph of dL/dλ
c2: 1.43 · 10
−2
c1: 1.18 · 10
−16
T : 1000.
Planck’s law depending on wavelength is
x : 3 ·10
−5
, 2.99 · 10
−5
...10
−7
f (x):
c1
x
5
e
c2
x·T
− 1
x in meters.
280 7. BLACKBODY RADIATION, ATOMIC EMISSION, AND LASERS
2. Integration over the wavelength range from 3
·
10
−6
to 3·10
−5
meters to obtain
the radiance
R :
3·10
−5
3·10
−6
f (x)dx.
Radiance
R 1.316 ·10
4
.
3. Multiplication with area times solid angle to obtain the radiant energy
Area A, Solid angle SA: A : 1, SA : 4; radiant energy RR: RR : A ·
SA·R; RR 5.263·10
3
watts. RR has the same value as the corresponding
value when integrating over the frequency.
Application 7.2.
1. Calculate the radiance for different wavelength intervals.
2. Calculate the radiant energy and choose an area of 5 mm
2
and a solid angle
of π
4
.
3. Consider the wavelength range from 1 mm to 100 mm for comparison with
the Rayleigh–Jeans law. Calculate the corresponding frequencies and de-
rive from Planck’s law the corresponding energy density for this frequency
interval. Do the same calculation for the Rayleigh–Jeans law and give the
difference in the numerical values.
7.3. ATOMIC EMISSION 281
FileFig 7.3 (L3BBFS)
Graph of blackbody radiation depending on the frequency. The calculation of the
radiance of a particular frequency range and calculation of the corresponding
radiant energy by multiplication with area times solid angle.
L3BBFS is only on the CD.
Application 7.3.
1. Calculate the radiance for different frequency intervals.
2. Calculate the radiant energy, and choose the area times solid angle such that
the radiant energy is the same as you calculated in Application FF2.
3. Numerical calculation of the Stefan–Boltzmann law. Calculate, using the
same units, the integrated radiation from Planck’s law for a chosen
temperature T and compare with the Stefan–Boltzmann law (FileFig 7.2).
FileFig 7.4 (L4STEFS)
The Stefan–Boltzmann law is plotted using linear and logarithmic scales.
L4STEFS is only on the CD.
FileFig 7.5 (L5WIENS)
Wien’s law is plotted for two ranges of the temperature.
L5WIENS is only on the CD.
7.3 ATOMIC EMISSION
7.3.1 Introduction
The operation of a laser needs an “active medium” between the two mirrors
of the laser cavity. Energy is “pumped” from the outside of the cavity into this
medium and produces atoms or molecules in excited states. Here we discuss only
excited energy states of atoms in the gas phase and consider the hydrogen atom
and atoms with hydrogenlike spectra. The energy states are labeled by letters
with subscripts and superscripts and some of the notations have their origin
in the “old days” of spectroscopy. At that time, for example, one used for the
characterization of some spectral lines: s for “sharp,” and d for “diffuse.” The
282 7. BLACKBODY RADIATION, ATOMIC EMISSION, AND LASERS
letters s and d are still in use for the characterization of the angular momentum.
There is some truth to these notations, since we know that the s-state has less
degeneracy than the d-state.
7.3.2 Bohr’s Model and the One Electron Atom
In Bohr’s model, an electron with a negative charge circulates around the positive
charge in the center of its orbits, determined by the principal quantum number
n. The energy of such an orbit is given by
E (−2π
2
K
2
e
4
m)/(n
2
h
2
), (7.23)
where K is the constant of Coulombs’ law, e the electron charge, m the electron
mass, and h Planck’s constant. The principal quantum number n has integer
numbers 1, 2.3 .... Radiation is emitted when the electron changes its orbits and
the energy of the emitted photon is given as
ν
ni,nf
h [2π
2
K
2
e
4
m)/(h
2
)][1/n
2
i
− 1/n
2
f
], (7.24)
where n
i
is the quantum number of the initial orbit and n
f
is the quantum num-
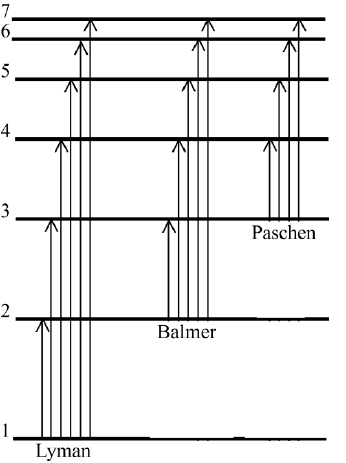
ber of the final orbit. In Figure 7.6 we show the series of lines originating from
the state n
f
1, called the Lyman series, from the state n
f
2, called the
Balmer series, and from the state n
f
3, called the Paschen series. The signif-
icant achievement of Bohr’s derivation was that he used fundamental physical
constants and could reproduce exactly the empirical constant of the expression
of the Balmer series.
7.3.3 Many Electron Atoms
7.3.3.1 Principal Quantum and Angular Momentum Quantum Numbers
The Schroedinger equation and the Pauli principle are needed to understand the
atomic energy schematics and transitions. As an example we look at an atom
with Z electrons and a nucleus with a positive charge of Ze.A list of some lower
energy states is shown in Figure 7.7. For such an atom, we have energy levels
labeled by the principal quantum number n and the angular momentum quantum
number l. The Schroedinger equation tells us that for each n there are n − 1
different possible states of the angular momentum.
7.3.3.2 Magnetic Quantum Number and Degeneracy
To each state labeled by the angular quantum number l, there are 2l+1 substrates.
They only have different energy values if the atom is in a magnetic field. The
corresponding quantum number is called the magnetic quantum number m.If
the magnetic field is zero, all states have the same energy, and therefore the state
is m fold degenerate.
7.3. ATOMIC EMISSION 283
FIGURE 7.6 Diagram of energy levels and transitions of Bohr’s model. The electrons change
from the state labeled n
i
to n
f
and emit light: Lyman series: n
f
1,n
i
2, 3, 4 ....; Balmer
series: n
f
2,n
i
3, 4, 5 ....; Paschen series: n
f
3,n
i
4, 5, 6. The energy difference
between n 1 and n ∞is the dissociation energy.
7.3.3.3 Spin States
Each electron has an angular momentum with respect to its own axis called the
spin, described by the spin quantum number s. In a magnetic field, the projection
has the values 1/2 and −1/2.
7.3.3.4 Pauli Principle and Occupation Rule
Each nondegenerate state of the atom has a different set of quantum numbers n,
l, m, s. For each n there are n − 1 values of l. For each l there are 2l + 1 values
of m and for each m there are two values of s (Figure 7.7).
7.3.3.5 Buildup Principle of Atoms and Labels for Energy Levels
The energy schematic of an atom with Z electrons may be obtained, in first
approximation, by using the “buildup principle.” There are special notations for
the principal and angular quantum numbers.
The principal quantum number n 1, 2, 3 ...is labeled K, L, M,....
The angular quantum number l 0, 1, 2, 3 ...is labeled s, p, d, f .
Following the buildup principle, the electrons first occupy the lowest n, then the
next, and so on. For each n, electrons fill all possible states labeled by l, m, and s.
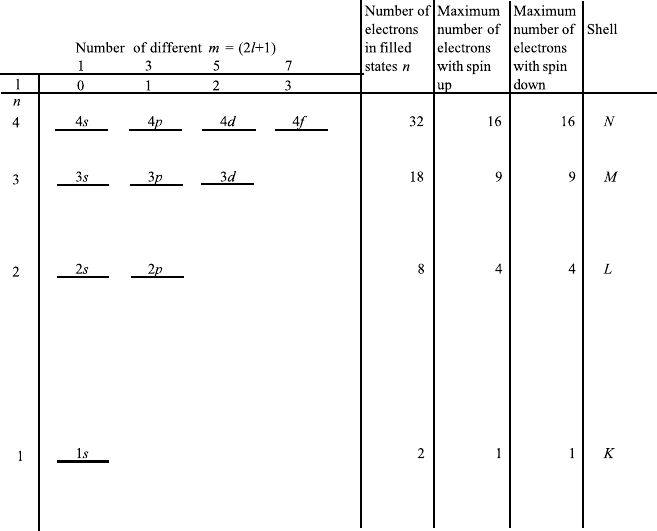
284 7. BLACKBODY RADIATION, ATOMIC EMISSION, AND LASERS
FIGURE 7.7 Energy levels and quantum numbers for a few states of the hydrogenlike atom. The
states are shown for each n and labeled by s, p, d, f . Spin states are also indicated.
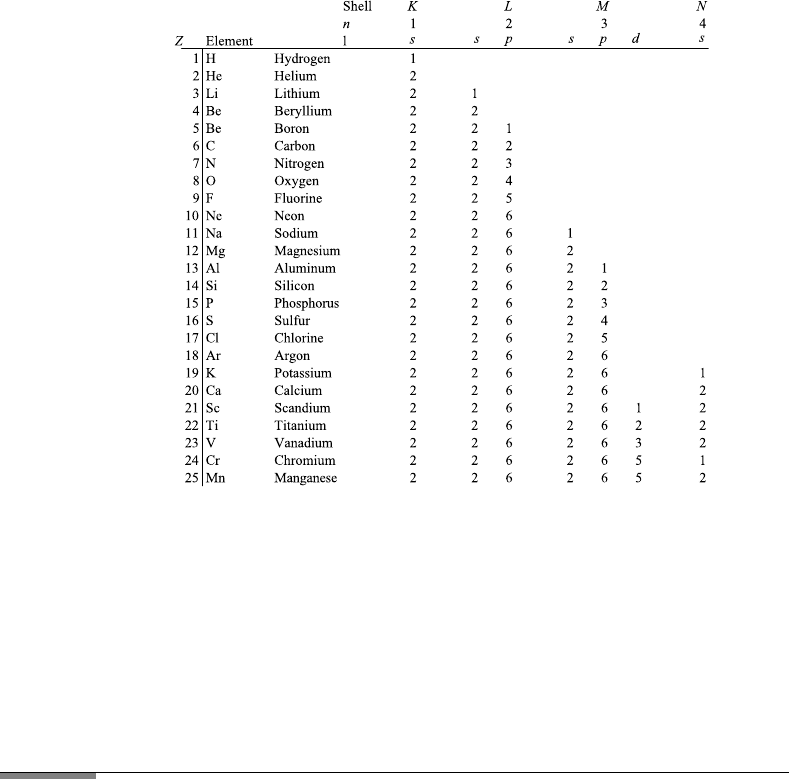
In Figure 7.8 the number of electrons occupying different states is listed for
the elements from Z 1toZ 25. There are irregularities, explained by
quantum mechanics. An example is Z 19, where the 4s state has lower energy
than the 3d state.
7.3.3.6 Transitions Between Energy States
Photons may be emitted when the atom changes from a higher energy state to a
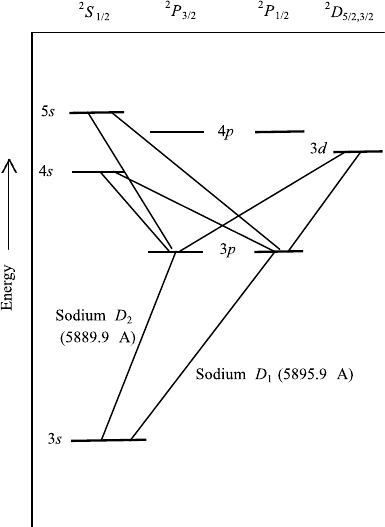
lower one. In an atom like the sodium atom, there is just one “outer” electron.
All other electrons are in “closed” shells. This outer most electron is an “s”
electron (Figure 7.9), and therefore the spectrum has a similarity to the spectrum
of the hydrogen atom. The columns in Figure 7.9 are labeled with capital letters,
referring to compound states of all electrons. Since the K and L shells are closed,
they do not contribute and the angular momentum of all electrons is the same as
the single electron in the M shell.
There are selection rules restricting the energy levels between which transi-
tions are possible. The general rule is that the angular momentum has to change
by plus or minus 1.Transitions are only allowedbetween the levelsof the columns
labeled by s, p, and d (Figure 7.9).
7.4. BANDWIDTH 285
FIGURE 7.8 Electronic states in atoms. Electrons having the same n value in “shells,” named K,
L, M. For each n we have n − 1 substates of angular momentum with quantum number l, named
s, p, d, f . There are 2l + 1 possible values of m, and two spin states. Therefore the maximum
occupations of K and L are K : n 1, 2[(2·0+1)] 2; L : n 2, 2[(2·0+1)]+2(2·1+1)] 8
(the M shell is more complicated).
7.4 BANDWIDTH
7.4.1 Introduction
The atom emits light when an electron makes a transition from a higher energy
state to a lower one. The emitted light is not monochromatic, since the emission
process last only for a short time. Therefore we have a wavetrain of limited
length. Such wavetrains are described by the superposition of a large number of
monochromatic waves having a certain frequency distribution (see Chapter 4).
The frequency spectrum shows a maximum at the transition frequency and the
bandwidth of the frequency distribution is related to the time of the emission
process.
We follow the book Lasers by F. K. Kneub
´
’uhl and M. W. Sigrist, B. G.
Teubner, Stuttgart, 1988, and use some of their numerical values in the examples.
286 7. BLACKBODY RADIATION, ATOMIC EMISSION, AND LASERS
FIGURE 7.9 Energy schematic for sodium. The energy level of 3s, that is, the ground state of the
outermost electron, has been chosen as 0. The two well-known “Sodium D” lines are indicated.
7.4.2 Classical Model, Lorentzian Line Shape, and
Homogeneous Broadening
Light is emitted from an atom when an electron leaves an excited state E
2
and
occupies a state with lower energy E
1
. One has for the energy of the process
E
2
− E
1
hν. (7.25)
Before the electron can make the transition, it has to be placed into the upper
state. This process is called population inversion (see Section 5.2). The electron
remains in the upper state for a limited time. On average the life-time of an
excited state is 10
−8
sec. However, longer lifetimes corresponding to metastable
states play an important role in laser action. The emitted light is described by
a wavetrain with decreasing amplitude (Figure 7.10), and may be described in
first approximation as
A A
0
e
−iω
0
t
e
−t/τ
. (7.26)
The lifetime τ is defined as the time in which the initial amplitude A
0
drops to
a value of A
0
/e A
0
/2.718. The frequency distribution of the wavetrain now

7.4. BANDWIDTH
287
FIGURE 7.10 Wavetrain decreasing over the time τ to the value A/e A/2.71.
discussed by application of a time-dependent Fourier transformation,
y(ω) (A
0
/
√
2π)
∞
∫
0
e
iω
0
t
e
−t/2τ
e
−iωt
dt. (7.27)
The integral may be calculated analytically and one gets
y(ω) (A
0
/
√
2π)[−1/{i(ω
0
− ω) − 1/2τ }]. (7.28)
The intensity is obtained as
I (ω) y(ω)y(ω)
∗
0
[1/{(ω
0
− ω)
2
+ (1/2τ )
2
}]. (7.29)
The constant ψ
0
is the total intensity and depends on the lifetime τ . From
Eq. (7.29) one defines the profile of the line or the Lorentzian line shape as
gl(ω) 2[(1/2τ )/{(ω
0
− ω)
2
+ (1/2τ )
2
}]. (7.30)
A graph of gl(ω) is shown in in Figure 7.11 with ω
0
at its center and a fixed value
of τ . The bandwidth
ω
(ω − ω
0
) at half-height is obtained from
(1/2)gl(ω ω
0
) gl(ω ω
0
+ ω/2 (7.31)
∆ω
ω
ο
FIGURE 7.11 Graph of the Lorentzian line shape gl(ω). The width at half-height ω is equal to
1/τ .

288 7. BLACKBODY RADIATION, ATOMIC EMISSION, AND LASERS
and one gets
ω 1/τ. (7.32)
Therefore, the bandwidth is related to the lifetime of the atomic emission pro-
cess, assuming that one has a waveform as given in Eq. (7.26). Introduction of
Eq. (7.32) into Eq. (7.30) results in
gl(ω) 2[(ω/2)/{(ω − ω
0
)
2
+ (ω/2)
2
}]. (7.33)
For an oscillator the quality factor is Q ω
0
/ω. This expression is similar to
the resolving power discussed for the Fabry–Perot in Chapter 2 and the grating
in Chapter 3. In FileFig 7.6 we show an example of the band shape of Eq. (7.33),
where the lifetime is chosen to be τ 1000 in order to show a graph in the
chosen frequency region.
FileFig 7.6 (L6BANDS)
Lorentzianline shape spectrum with angular resonancefrequencyω
0
and lifetime
τ .
L6BANDS
Lorentzian Line Shape
Frequency interval m : 11.
ω0:
49
(2
m
− 1)
ω : 1
1
(2
m
− 1)
,
2
(2
m
− 1)
···1.
To make a graph the lifetime is chosen such that the Lorentzian line shape can
be demonstrated.
τ : 1000
gl(ω): 2
1
(2·τ )
1
(2·τ )
2
+ (ω − ω0)
2
Q : τ · ω0
Q : 23.937.
