Middleton G.V. (Ed.) Encyclopedia of Sediments and Sedimentary Rocks
Подождите немного. Документ загружается.

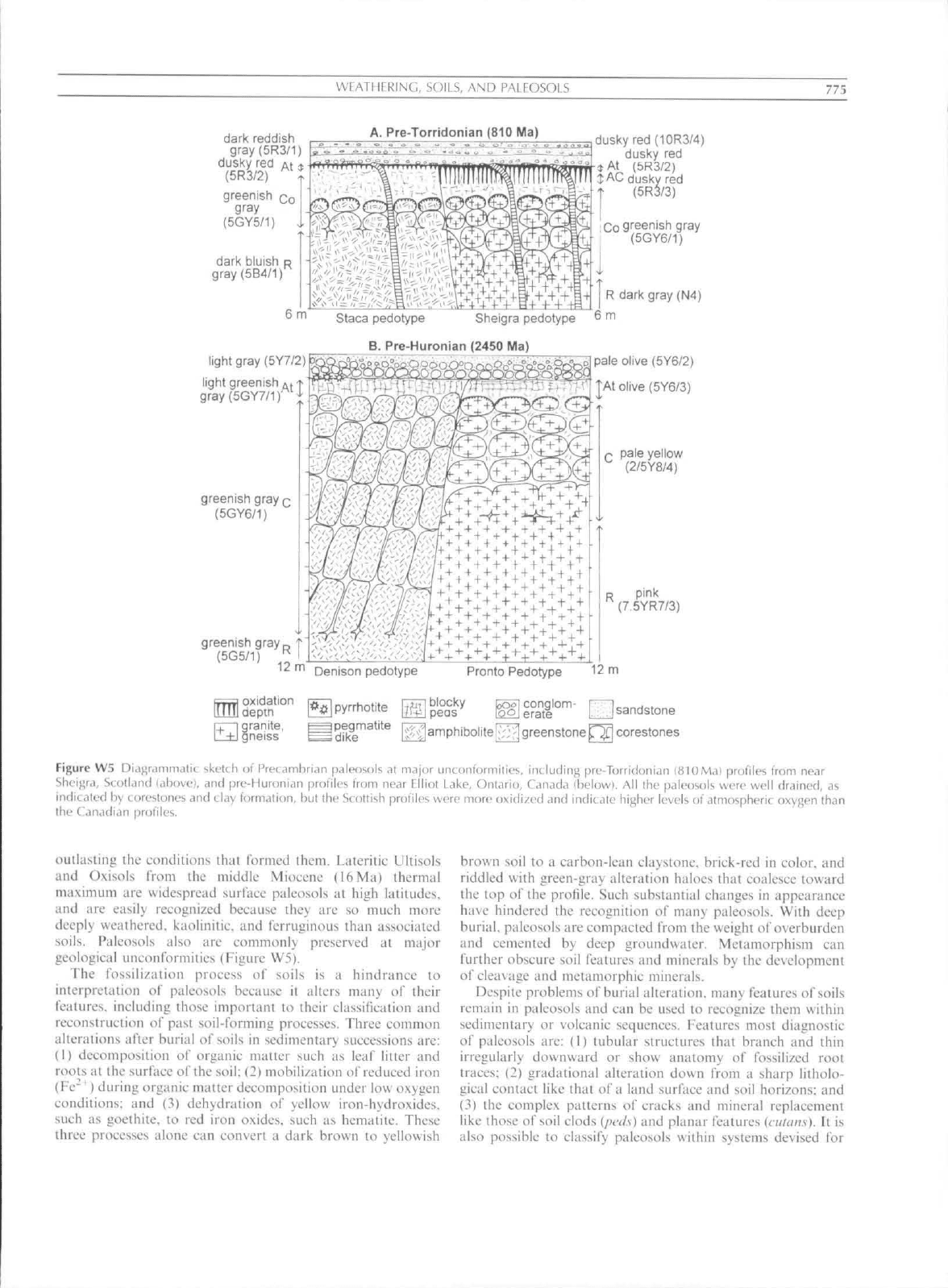
WFATHrRINCi, SOILS, AND PAI FOSOl.S
775
dark reddish
gray(5R3/1)
dusky red at t
(5R'3/2)
^'*
A. Pre-Torridonian (810 Ma)
greenish
gray
(5GY5/1)
dark bluish R
gray(5B4/1)
6m
K"^^'^^^
'^^<l^;_
dusky red (10R3M}
dusky red
* At (bR3/2)
jAC dusky red
{5R3/3)
'Co3''eenish gray
(5GY6/1)
R dark gray (N4)
light gray (5Y7/2)
light greenish A. t
gray(5GY7/1) J-
Staca pedotype Sheigra pedotype 6 m
B. Pre-Huronian (2450 Ma)
greenish
{5GY6/1)
greenish
(5G5/1)
pale olive (5Y6/2)
TAt olive (5Y6/3)
pale yellow
(2/5Y8/4)
pink
(7.5YR7/3)
Denison pedotype
Pronto Pedotype 12 m
oxidation
deptn
granite,
Qneiss
1
pyrrhotite
pegmatite
dik
rrrn blocky
7i+'
peas
ilS°^-
Qsandstone
^Oamphibolite kjHgreenstoneOdcorestones
Figure W5 Diagrammatic sketch ot' Precambrian paleosols at major unconformities, including pre-Torridanian miOMa} profiles from near
iilieij^ra,
Scolkind (aliove], and pre-Huronian profiles from near Elliot Lake, Ontario, Canada (below). All the paleosols were well drained, as
indic.iled hy t orestones and clay formation, but the Scottish profiles were more oxidized and indicate higher levels of atmospheric oxygen than
the Canadian profiles.
outlasting the conditions that Ibrnied them. Latcritic Ultisols
;ind Oxisols from the iiiiddlc Miocene (16 Ma) thermal
maxinnitn are widespread surtaee paleosols at high latitudes,
and are easily reeognized because they are so much more
deeply weathered, kaolinitic. and ferruginous than associated
soils.
Paleosois also are commonly preserved al major
geological unconformities (Figure W5).
The fossilization process of soils is a hindrance to
interpretation of paleosols because it allers many of their
features, including those important to their ektssification and
reconstruction of pasl soil-forminjz processes. Three common
alterations after burial of soils in sedimentary successions are:
(!) decomposition of organic matter such as leaf Htler and
roots at the surfaee of ihe soil; (2) mobilization of reduced iron
(Fe'') during organic matter decomposition undei' low oxygen
conditions: and (3) dehydration of yellow iron-hydroxides,
sueh as goethite. to red iron oxides, such as hematite. These
three processes alone can convert a dark brown to yellowish
brtiwn soil to a carbon-lean claystone. brick-red in color, and
riddled with green-gray alteration haloes that coalesce toward
the top of the profile. Such substantial changes in appearance
have hindered the recognition of many paleosols. With deep
burial, paleosols are eompacted from the weight of overburden
and cemented by deep groundwater. Metamorphism can
further obscure soil features and minerals by the development
of eleavage and metamorphie minerals-
Despite problems of burial alteration, many features of soils
remain in paleosols and can be used to recognize them within
sedimentary or volcanie sequences. Features most diagnostic
of paleosols are: (1) tubular structures that braneh and thin
irregularly downward or show anatomy of fossilized root
traces;
(2) gradational alteration down from a sharp litholo-
gical contact like that ofa land surt~ace and soil horizons: and
(3) the eomplex patterns of cracks and mineral replacement
like those of soil clods
[pciis]
and planar features (cutans). It Is
aiso possible to classify paleosols within systems devised
\'OT
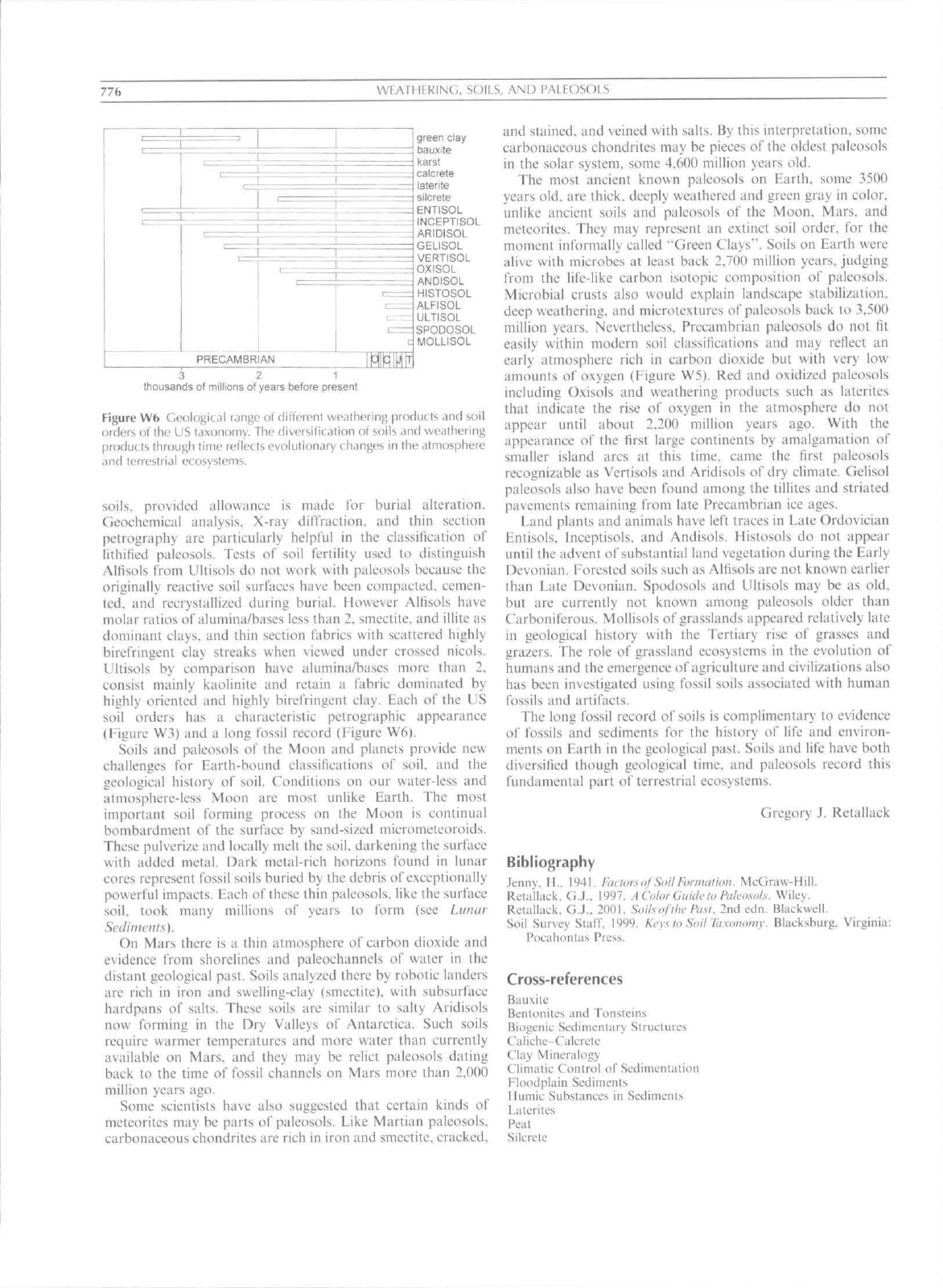
776
VVEATHEKINO, SOILS, AND PALbOSOI.S
1
n
—
PRECAMBRIAN
IPHHIPIIT
green clay
bauxite
karst
calcrete
laterite
silcrete
ENTISOL
INCEPTISOL
ARIDISOL
GELISOL
VERTISOL
OXtSOL
ANDISOL
HISTOSOL
Ai-FISOL
ULTISOL
SPODOSOL
MOLLISOL
3 2 1
thousands of millions of years before present
Figure Wb Geological riinge of different weathering fjroducts and soil
orders ol the LJS taxonomy. The diversification of soils and weathering
products through time reflects evolutionary changes in the atmosphere
and terrestrial ecosystems.
soils,
provided allowance is made for burial alteration.
Geochemical analysis. X-ray diffraction, and thin section
petrography are particularly helpful in the classificalion of
iithified palcosols. Tests of soil fertility used lo distinguish
Alfisols from Ultisols do not work with paleosols because the
originally reaciive soil surfaces have been compacted, cetnen-
ted, and rccrystalli/ed during burial. However Altisols have
molar ratios of alumina/bases less than 2. smectite, and illite as
dominant clays, and thin section fabrics with scattered highly
birefringent clay streaks when viewed under crossed nicols.
Ultisols by comparison have alumina/bases more than 2,
consist mainly kaolinite and retain a fabric dominated by
highly oriented and highly birefringent clay. Each of the US
soil orders has a characteristic petrographic appearance
{Figure W3) and a long fossil record (Figure Wfi).
Soils and paleosols of the Moon and planets provide new-
challenges for Earth-bound classifications of soil, and the
geological history of soil. Conditions on our water-less and
atmospherc-lcss Moon arc most unlike Earth. The most
itiiportaiit soil fonning process on the Moon is continual
bombardment of the surface by sand-sized niicrometeoroids.
These pulveri7e and locally melt the soil, darkening the surface
with added metal. Dark metal-rich horizons found in lunar
cores represent fossil soils buried by the debris of exceptionally
powerful impacts. Each of these thin paleosols, like the surface
soil, took many millions of years to form (see Lunar
Sediments).
On Mars there is a thin atmosphere of carbon dioxide and
evidence from shorelines and paleochanncis of water in the
distant geological past. Soils analy-^ed there by robotic landers
arc rich in iron and swelling-clay (smectite), with subsurface
hardpans of salts. These soils are similar to salty Aridisols
now forming in the Dry Valleys of Antarctica. Such soils
require warmer temperatures and more water than currently
available on Mars, and they may be relict paleosols dating
back to the time of fossil channels on Mars more than 2.01)0
million years ago.
Some scientists have also suggested that certain kinds of
meteorites may be parts of paleosols. Eike Martian paleosols,
carbonaceous chondrites are rich in iron and smectite, cracked.
and stained, and veined with salts. By this ititerpretation. some
carbonaceous chondrites may be pieces of the oldest paleosols
in the solar system, some 4.600 million years old.
The most ancicnl known paleosols on Earth, some 3500
years old, are thick, deeply weathered and green gray in color,
unlike ancient soils and paleosols of the Moon, Mars, and
meteorites. They may represent an extinct soil order, for the
moment informally called "Green Clays". Soils on Earth were
alive with microbes at least back 2,700 million years, judging
from the life-like carbon isotopic composition of paleosols.
Microbial crusts also would explain landscape stabilization,
deep weathering, and mierotexturcs of paleosols back to 3,500
million years. Nevertheless. Precambrian paleosols do not iit
easily within modern soil classifications and may reflect an
early atmosphere rich in carbon dioxide but with very low
amounts of oxygen (Figure W5). Red and oxidized paleosols
including Oxisols and weathering products such as laterites
that indicate the rise of oxygen in the atmosphere do not
appear until about 2,200 million years ago. With the
appearance of the first large continents by amalgamation of
smaller island arcs at this time, came the first paleosols
recognizable as Vertisols and Aridisols of dry climate. Geliso!
paleosols also have been found among the tillites and striated
pavements remaining fi'om late Precambrian ice ages.
Land plants and animals have left traces in Eate Ordovician
Entisols. Inceptisols, and Andisols, Histosols do not appear
until the advent of substantial land vegetation during the Early
Devonian. Forested soils such as .Mfisols are not known earlier
than Late Devonian. Spodosols and Ultisols may be as old,
but arc currently not known among paleosols older than
Carboniferous. Mollisols of grasslands appeared relatively late
in geological history with the Tertiary rise of grasses and
grazers. The role of grassland ecosystems in the evolution of
humans and the emergence of agriculture and civilizations also
has been investigated using fossil soils associated with human
fossils and artifacts.
The long fossil record of soils is complimentary to evidence
of fossils and sediments for the history of life and environ-
ments on Earth in the geological past. Soils and life have both
diversified though geological time, and paleosots record this
fundamental part of terrestrial ecosystems.
Gregory J. Retallack
Bibliography
.fenny. II.. 1941. Faclorsof Soil Formation. McGnnv-Hill.
Retallack. G.J., 1997.
.4
ColorGuuk-to
Paleosols.
Wiley.
Retallack, Ci.J..
2(101.
Soilsoflhc
Pu.sl.
2tid edn. Blackwell.
Soil Survey
Stalf,
1999. Keys to Soil
Ta-xonomy.
Blacksburg, Virginia:
Pocaliontas Press.
Cross-references
Biiuxite
Bentonitos and Tonsteins
Biogenic Sedimentary Siriictures
Caliclic-Caicrete
Ciay Minciiilogy
Climatic Control of Sedimentation
Floodplain Sediments
Humic Substances in Sediments
Laterites
Peat
Silcrete
X
X-RAY RADIOGRAPHY
X-ray radiography (commonly shorlened to radiography) is a
non-dcstruclivc tcchniqtic based on differeiilial passage o\'
X-rays through a sample onto a specific
fihii.
The dilTercnces in
density ill the sample, caused by variations in composition,
resuit in dilTerences in attenuation.
This method was introduced into sedimentology by
Hamblin (1962). Basically it does not differ from X-rays used
in medicine, biology, and paleontology, except that an
industrial quality tilm is used. X-ray radiography can
significantly enhanee large and small variations in lithology
and often brings out sedimentary structures that were not
detected by the naked eye. ln addition to those observations,
radiography reveals mierofaults and folds, presence of and
disturbances by fauna and tlora, and the presence of nodules
and other postdepositional chemical inelusions (Krinitzski,
1970).
The technique is not limited to the shape or thickness of a
sample. It is advised, however, to work with a thin plane-
parallel slice. A core is too thick in the center to distinguish
phenomena because those are projected on top of each other.
The sides are too thin and thus will be overexposed. Imbedding
the core, or irregular sample, in a plexiglas box with fine sand
can reduce those thickness variations. Because the pores ofthe
loose sand are not
filled,
the core has a higher density than the
sand,
and the thickness differences are only partly eliminated.
If
one
deals with a lot of indurated or non-indurated cores, it is
advised to make a mold of fine sand impregnated with a plastic
(Bouma. 1969).
Indurated samples should be cut to obtain a plane-parallel
slice about I cm thiek. Sofi sediments should also be sliced.
For those it is advised to construct a plexiglas tray a Htlle
narrower and shorter than the core width and film length,
respectively. Glue or tape 1-cm high sides onto the base of lhe
tray. Cut the sampling tube lengthwise and remove the upper
half of the tube. Cut sutTicient ofthe upper part off the eorc to
lil the tray. Place the tray over the upper part. Next remove the
core segment and the tray from the lower half of the sampling
tube and remove all sediment that protrudes the tray (for
details see BoutTia. 1969). A nice, thin slice of sediment results.
Plexiglas and most plasties are transparent to X-rays. Saran
wrap is transparent also but the wrinkles show up on lhe
film.
Grains of eoarse sediment are visible on the lilm (Bouma,
1969).
There are severai X-ray units on the market. Commonly
used are small units with a small foeal spot. The smaller the
focal spot the sharper the picture, A focal distance of
90-100
cm
is common. The X-ray beam has to
be
perpendicular
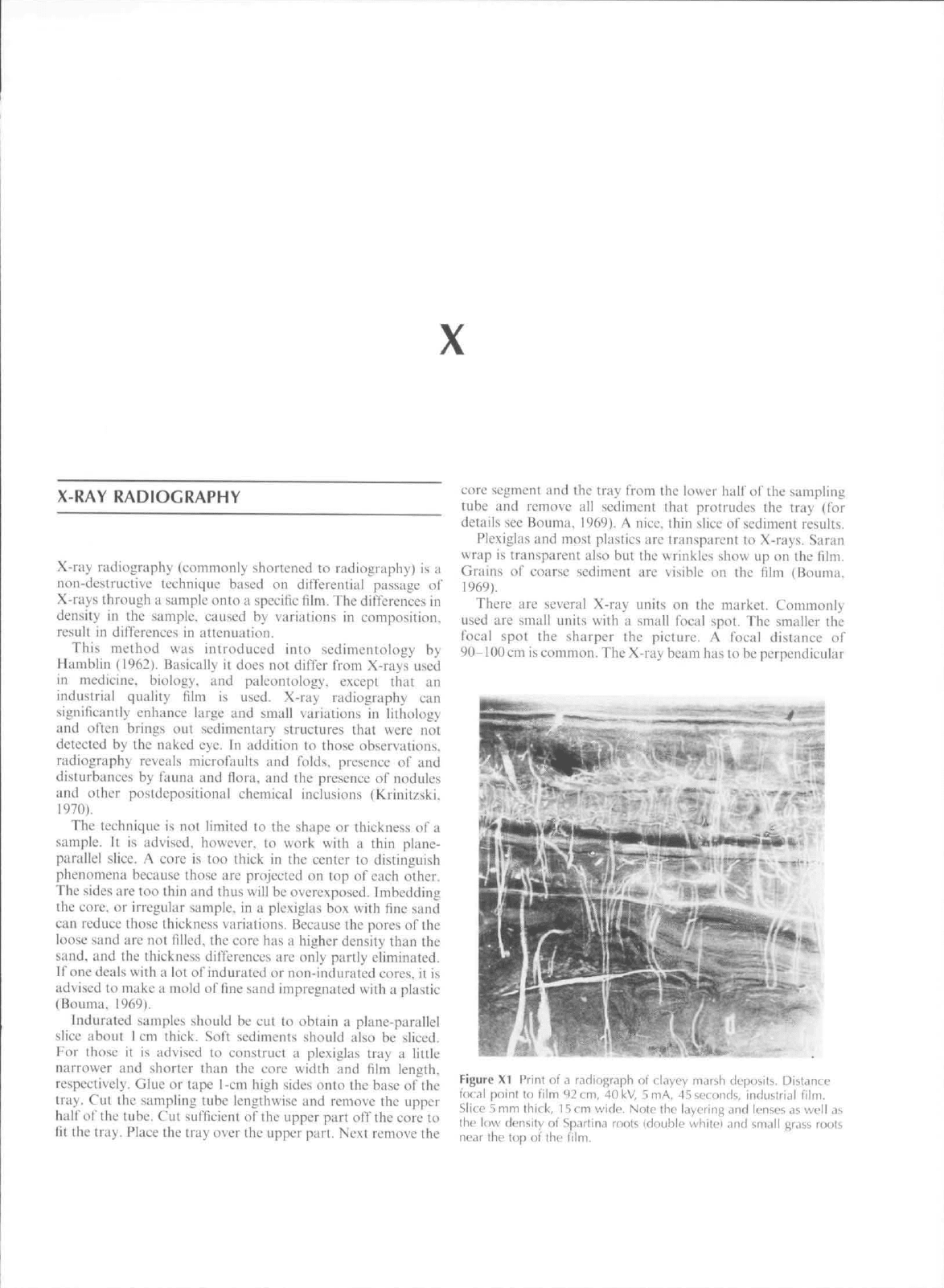
Figure XI Print of
d
radiograph oi clayey marsh clepostts. Distance
i(K.ii point lo film
92
cm,
40
kV,
5
mA,
45
seconds,
industrial
film.
Slice
5 mm
thick,
15 cm
wide. Nole ihc layering and lenses
as
well as
the luw density of Spartina roots {double white} and small grass roots
near lhe top of the
film.

778
X-KAY RADIOGKAHHY
to the film. A tnitximum tube current of 100 140 kV is
sufficient for samples up lo
9
cm thick. An increase in ttibe
current results in ii change of intensity o\' Ihc emitted
rLtdiation. The intensity is approximately proportional to the
milliamperagc. An increase in voltage adds shorter wave-
lengths to the beam which results in a decrease of the picture
contrast. A lowering of the kV incre:tses the scattering.
One has to gel used to inlcrpretitig radiographs (Figure XI).
The developed film shows dark for low-density tnatcrial. and
light for dense material (sand). Making a print of the
radiograph reverses the dark and light presentations. Bouma
(1969) shows a large nutnber of photographs and radiographs
of different rock types, making it clear that trial exposures are
needed to reach the best results. In general the muds and
mudstones give the best radiographs because present sandy
lamitiae and plant fragmenls stand out, Pnre sands and many
carbonates lack sufficient densily contrast.
Arnold ll. Bouma
Bibliography
Bouma. A.H,. 14M. Mcllitnhfbr the Study of
Si-dinictUuyy
Srrmlures.
New York: Wiley-intcrscience. (Can be obtained from Krieger
Publishing Co,. Hiintingtoii. New York. 197').)
Hamblin, W.K.. 1962. X-Ray radiography in the study of structures in
homogeneous sediments, .louriuil uf Sediiuciilurv Pclnilai^v. 32;
201 210.
Krinitzsky. E.L.. 1970. Rudiography in the Earth Scii'iiccs and Soil
Mechiinks. New York; Plenum Press.
Cross-references
Bedding and liU;:rnal Structures
Cormg Methods. Cores
Impregnation
Relief Peels

ZEOLITES
IN
SEDIMENTARY ROCKS
Introduction
Zeolites
are
among
the
most common authigenic silicates
in
sedimentary rocks. They occur
in a
wide variety
of
rocks types
and
are
especially common
in
altered vitric tuffs. Zeolites
are
tectosilicates
and
have
a
3-dimensional anion framework with
an atomic ratio
of
(A1
+Si):0
=
2.0.
The
charge
of the
framework
is
balanced
by
cations, principally
Ca, Na, and
K. Zeolites
can be
viewed
as
hydrated equivalents
of the
feldspars
in
which
the
Si: Al ratio varies from about
1.5-5.
The
structural framework
is
relatively porous
and
contains
interconnected cavities
in
which cations
and
water molecules
reside.
The
cations
and H2O
molecules
are
bound loosely,
which gives properties
of
cation exchange
and
reversible
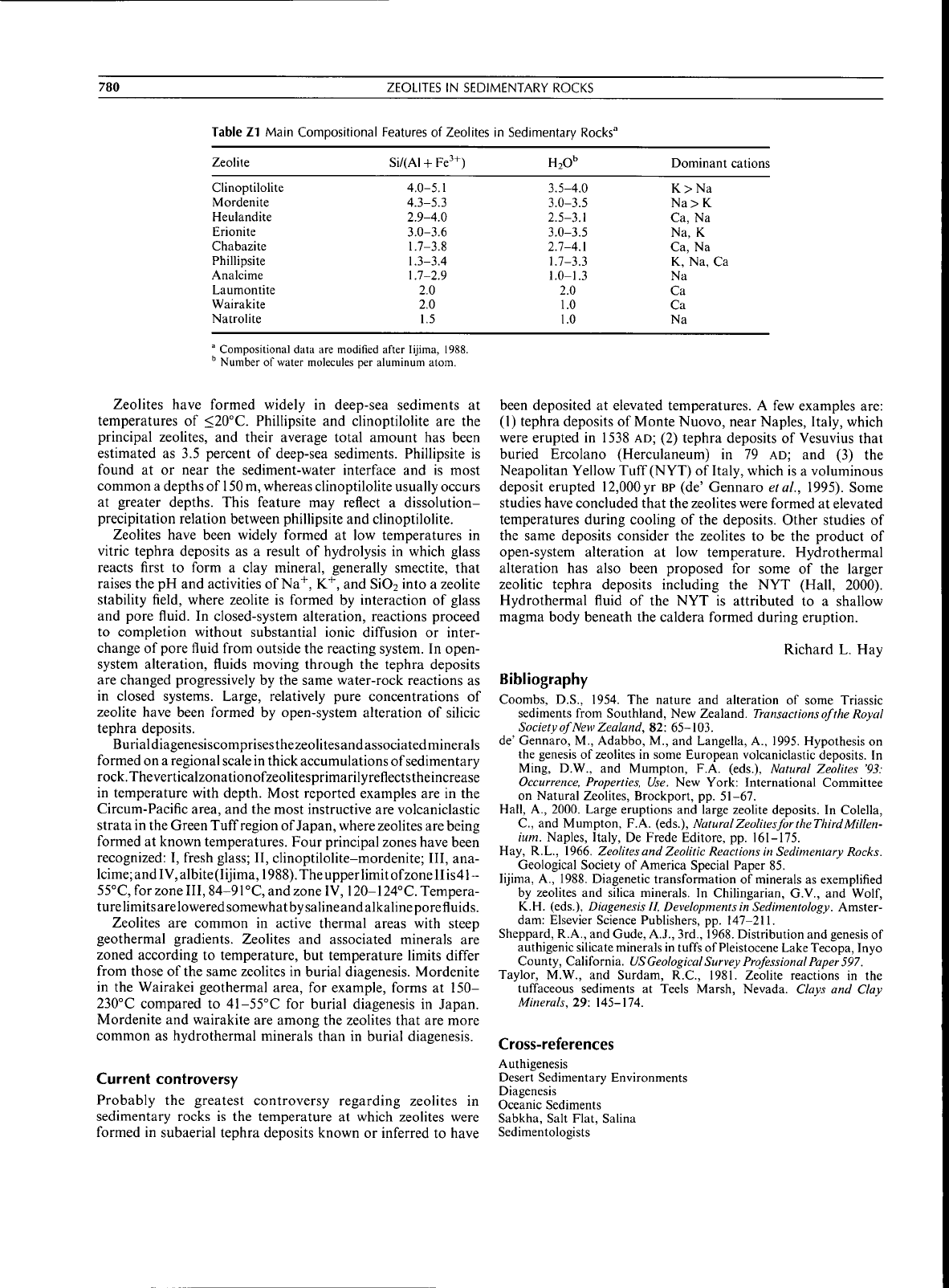
dehydration. More than 35 different zeolite minerals have been
identified
and the
seven most common
in
sedimentary rocks
are analcime, phillipsite, natrolite, laumontite, heulandite,
clinoptilolite, erionite,
and
mordenite (Table
Zl).
The report
of
Murray
and
Renard
in 1891 was the
first
to
document
a
zeolite
as
widespread
in
sedimentary deposits. This
zeolite
was
phillipsite,
a
significant constituent
of
deep-sea
sediments sampled
by the
Challenger expedition. Ross
and
Bradley
in 1928
reported
the
occurrence
of
analcime
as the
major alteration product
in
tuffs deposited
in
saline, alkaline
lakes.
The
common silicic zeolite clinoptilolite
was
first
reported
in 1933 as an
alteration product
in
marine silicic
tuffs
by
Bramlette
and
Posnjak. Research
on the
zeolites
of
sedimentary rocks greatly increased
in the
1950s, due
to the use
of X-ray diffraction
for
mineral identification
and to
interest
in
natural zeolites
for
adsorption
and as
molecular sieves.
Coombs (1954) recognized
a
vertical zoning
of
zeolite types
in
a
10,000 m sequence
of
clastic sediments, which
led to
considering zeolite mineral assemblages
in
terms
of
burial
depth
and
pressure-temperature stability fields. Studies
of
saline-lake deposits (Hay, 1966) showed that variations
in pH,
salinity,
and
cation content
of
lacustrine pore fluids resulted
in
mineralogic effects comparable
to
those caused
by
different
burial depths
(see Hay,
1966,
for
these early references).
Essential concepts
Zeolites occur
in a
wide variety
of
sedimentary rocks. They
are
postdepositional minerals
and
form from varied types
of
materials including volcanic glass, feldspar, feldspathoids,
smectite,
and
kaolinite. Zeolites
and
some clay minerals
can
form from
the
same aluminosilicate materials,
and the
most
critical requirement
for
zeolites
is a
high activity ratio
of
Na"*"
+
K"*"
+
Ca^"*'/H'*'. Thus zeolites
are
generally forrned
in
alkaline environments.
The
nature
of
cations
is
important
in
determining which cationic type
is
formed. Zeolites
are
subdivided into three groups based
on the
silica activity
in
pore fluid
at the
time
of
origin. Silica-poor zeolites (e.g.,
natrolite)
are
favored
by
environments undersaturated with
respect
to
quartz; some other zeolites co-exist with quartz (e.g.,
heulandite);
and
silicic zeolites
may
only
be
stable
in
solutions
supersaturated with respect
to
quartz (e.g., clinoptilolite).
Zeolites
may
alter
to
other zeolites under changed physio-
chemical conditions. Temperature
is an
important control
on
both
the
rate
of
reaction
in
forming zeolites
and the
species
of
zeolite, with higher temperatures
and
pressures favoring
the
less hydrous zeolites.
Types
of
geologic occurrence
Most zeolite occurrences
in
sedimentary rocks
can be
grouped
into several types
of
geologic environment
or
hydrogeologic
system. These are: (1) saline
and
highly alkaline lakes; (2) deep-
sea sediments;
(3)
low-temperature open
to
closed tephra
systems;
(4)
burial diagenesis;
and (5)
hydrothermal alteration.
These occurrences generally exhibit characteristic patterns
of
zeolite zoning
in
tephra deposits
(Hay,
1977).
Zeolites
are
common
in
deposits
of
saline, highly alkaline
lakes (pH
=
9.5-10). Vitric tephra
is
readily altered
to
zeolites,
and
the
largest relatively pure concentrations
of
zeolites
are
found here. Early-formed zeolites
may
alter
to
analcime
and or
authigenic K-feldspar. Small amounts
of
zeolite, most
commonly analcime,
are
commonly formed
by
reaction
of
smectite
in
lacustrine clays. Zoning
in ash
layers
is
chiefly
lateral
and
reflects salinity gradients
in the
original lake water
(Sheppard
and
Gude, 1968). Vitric
ash may
alter
to
zeolites
in
less than
1,000
years (Taylor
and
Surdam, 1981).
780
ZEOLITES IN SEDIMENTARY ROCKS
Table Z1 Main Compositional Eeatures of Zeolites in Sedimentary Rocks"
Zeolite
HjO"
Dominant cations
Clinoptilolite
Mordenite
Heulandite
Erionite
Chabazite
Phillipsite
Analcime
Laumontite
Wairakite
Natrolite
4.0-5.1
4.3-5.3
2.9-4.0
3.0-3.6
1.7-3.8
1.3-3.4
1.7-2.9
2.0
2.0
1.5
3.5-4.0
3.0-3.5
2.5-3.1
3.0-3.5
2.7-4.1
1.7-3.3
1.0-1.3
2.0
1.0
1.0
K>Na
Na>K
Ca, Na
Na, K
Ca, Na
K, Na, Ca
Na
Ca
Ca
Na
° Compositional data are modified after lijima, 1988.
Number of water molecules per aluminum atom.
Zeolites have formed widely in deep-sea sediments at
temperatures of <20°C. Phillipsite and clinoptilolite are the
principal zeolites, and their average total amount has been
estimated as 3.5 percent of deep-sea sediments. Phillipsite is
found at or near the sediment-water interface and is most
common a depths of
150
m, whereas clinoptilolite usually occurs
at greater depths. This feature may reflect a dissolution-
precipitation relation between phillipsite and clinoptilolite.
Zeolites have been widely formed at low temperatures in
vitric tephra deposits as a result of hydrolysis in which glass
reacts first to form a clay mineral, generally smectite, that
raises the pH and activities of Na*, K'*', and SiO2 into a zeolite
stability field, where zeolite is formed by interaction of glass
and pore fluid. In closed-system alteration, reactions proceed
to completion without substantial ionic diffusion or inter-
change of pore fluid from outside the reacting system. In open-
system alteration, fluids moving through the tephra deposits
are changed progressively by the same water-rock reactions as
in closed systems. Large, relatively pure concentrations of
zeolite have been formed by open-system alteration of silicic
tephra deposits.
Burialdiagenesiscomprisesthezeolitesandassociated minerals
formed on a regional scale in thick accumulations of sedimentary
rock.Theverticalzonationofzeolitesprimarilyreflectstheincrease
in temperature with depth. Most reported examples are in the
Circum-Pacific area, and the most instructive are voicaniciastic
strata in the Green Tuff region of Japan, where zeolites are being
formed at known temperatures. Four principal zones have been
recognized: I, fresh glass; II, clinoptilolite-mordenite; III, ana-
lcime;
and
IV,
albite(Iijima,
1988).
The upper limit ofzone II
is41-
55°C,
for zone III, 84-91°C, and zone IV, 120-124°C. Tempera-
turelimitsareloweredsomewhatbysalineandalkalineporefluids.
Zeolites are common in active thermal areas with steep
geothermal gradients. Zeolites and associated minerals are
zoned according to temperature, but temperature limits differ
from those of the same zeolites in burial diagenesis. Mordenite
in the Wairakei geothermal area, for example, forms at 150-
230°C compared to 41-55°C for burial diagenesis in Japan.
Mordenite and wairakite are among the zeolites that are more
common as hydrothermal minerals than in burial diagenesis.
Current controversy
Probably the greatest controversy regarding zeolites in
sedimentary rocks is the temperature at which zeolites were
formed in subaerial tephra deposits known or inferred to have
been deposited at elevated temperatures. A few examples are:
(1) tephra deposits of Monte Nuovo, near Naples, Italy, which
were erupted in 1538
AD;
(2) tephra deposits of Vesuvius that
buried Ercolano (Herculaneum) in 79 AD; and (3) the
Neapolitan Yellow Tuff (NYT) of Italy, which is a voluminous
deposit erupted 12,000yr
BP
(de' Gennaro
etal.,
1995). Some
studies have concluded that the zeolites were formed at elevated
temperatures during cooling of the deposits. Other studies of
the same deposits consider the zeolites to be the product of
open-system alteration at low temperature. Hydrothermal
alteration has also been proposed for some of" the larger
zeolitic tephra deposits including the NYT (Hall, 2000).
Hydrothermal fluid of the NYT is attributed to a shallow
magma body beneath the caldera formed during eruption.
Richard L. Hay
Bibliography
Coombs, D.S., 1954. The nature and alteration of some Triassic
sediments from Southland, New Zealand.
Transactions
ofthe Royal
Society of New
Zealand,
82: 65-103.
de'
Gennaro, M., Adabbo, M., and Langella, A., 1995. Hypothesis on
the genesis of zeolites in some European voicaniciastic deposits. In
Ming, D.W., and Mumpton, F.A. (eds.). Natural Zeolites '93:
Occurrence, Properties, Use. New York: International Committee
on Natural Zeolites, Brockport, pp. 51-67.
Hall, A., 2000. Large eruptions and large zeolite deposits. In Colella,
C, and Mumpton, F.A. (eds.). Natural Zeolites for the Third Millen-
ium.
Naples, Italy, De Frede Editore, pp. 161-175.
Hay, R.L., 1966. Zeolites and Zeolitic Reactions in Sedimentary Rocks.
Geological Society of America Special Paper 85.
lijima. A., 1988. Diagenetic transformation of minerals as exemplified
by zeolites and silica minerals. In Chilingarian, G.V., and
Wolf,
K.H. (eds.), Diagenesis II, Developments in Sedimentology. Amster-
dam: Elsevier Science Publishers, pp.
147-211.
Sheppard, R.A., and Gude, A.J., 3rd., 1968. Distribution and genesis of
authigenic silicate minerals in tuffs of Pleistocene Lake Tecopa, Inyo
County, California. US Geologieal Survey Professional
Paper
597.
Taylor, M.W., and Surdam, R.C, 1981. Zeolite reactions in the
tuffaceous sediments at Teels Marsh, Nevada. Clays and Ctay
Minerats, 29: 145-174.
Cross-references
Authigenesis
Desert Sedimentary Environments
Diagenesis
Oceanic Sediments
Sabkha, Salt Flat, Salina
Sedimentologists

Index
of
Authors Cited
Aagaard,
P. 29, 66
Aagaard,
T. 625
Abbott,
P.L. 190
Abdel-Wahab, A.A. 224
Abed,
A.M. 526
Abel, W.A. 27
Abrajano, T. 292
Abt, S.R. 294
Acrivos, A. 360
Adabbo, M. 780
Adam,
D.P. 424
Adams, A.E. 69
Adams, J. 25
Adams, R.D. 446
AEUB
502
Agassiz, L. 633
Aggarwal, P.K. 394
Agrawal, Y.C. 285
Agricola,
G. 249
Agterberg,
F.P. 685
Aguilar,
C. 384
Aharon,
P. 526
Ahlbrandt,
T.S. 253
Ahn, J.H. 65, 126
Ahnert,
F. 605
Ahr, W.M. 397
Aigner,
T. 354
Aiken,
G.R. 362
Ainsworth,
P. 371
Ainsworth,
R.B. 202
Aissaoui, D.M. 395
Aitken,
J.D. 446
Aksu,
A.E. 292, 498, 499, 672, 673
Al Farraj, A. 7
Al-Salem,
A.A. 626
Albritton,
C.C. 292
Aleinikoff,
J.N. 548
Aleva,
G.J.J.
145,411,534
Alex, CM. 673
Alexander,
CR. 605, 606, 736
Alexander,
H.S. 354
Alexander,
J. 433, 760
Alexandersson,
E.T. 438
Algeo, T.J. 183
Ali,
Y.A. 18
Allan,
G.L. 397
Allan,
J. 502
Allen,
E.A. 587
Allen,
G.P. 498, 583
Allen,
H.E. 751
Allen,
J.R.L.
16, 40, 42, 59, 61, 137, 168,
172,
183, 195,213,214,231,267,271,
287,
296, 335, 356, 413, 414, 433, 498,
514,
530, 536, 551, 567, 582, 596, 618,
633,
650, 668, 711, 728, 733, 736, 748
Allen,
P.A. 183, 354, 358, 364, 609, 733, 736
Aller,
R.C. 438
Alley,
R.B. 331, 746
Allison,
K.R. 287
Allison,
P.A. 458, 728
Allmendinger,
R.W. 733
Allouc, J. 71
Almbaydin,
F. 526
Almon,
W.R. 126
Alonso-Zarza,
A.M. 7, 9
Alvarez, L.W. 656
Alvarez, W. 656
AmSrk,
M. 137
American Geological Institute
(AGI) 746
American Society
of
Civil Engineers
618
Amery,
G.B. 674
Amit,
R. 8
Amodio, S. 183
Anderson,
E.J. 184
Among, H.L. 744
Amorosi, A. 333
Amos, CL.
498,687,711
Amthor,
J.E.
241,242
An, Z.S. 443
Anbar,
A.D. 384
Andersen,
H.V. 414
Andersen,
M.A. 120
Andersen,
T. 299
Anderson,
J.B. 330, 498, 499, 746
Anderson,
K.B. 565
Anderson,
L.C. 498
Anderson,
R.F. 396, 492, 605, 763
Anderson,
R.S.
253,711
Anderson,
R.Y. 765
Anderson,
S.P. 395
Anderson,
T.F. 394
Anderton,
R. 271
Andresen,
A. 436
Andrews, J.E. 754
Andrews, J.T. 424, 605, 606
Andrews, L.M. 223, 692
Angel, M.V. 763
Anguy,
Y. 519
Anketell, J.M 551
Ankley,
G.T. 751
Anonymous
223, 633
Anselmetti, F.S. 314
Antia,
E.E. 157
Aomine, S. 4
Apelt,
CJ. 480
Aplin,
A.C 167
Appelo,
C.A.J.
542
Appleby,
P.G. 753
Appleman,
D.E. 139
Arakel, A.V. 91
Aravena,
R. 516
Arbey,
F. 63
Archer,
A.W. 736, 743
Archer,
J. 137
Archie, G.E.
267,314
Arikan,
F. 554
Armi, L. 464
Armijo, R. 734
Armstrong,
R.L. 599
Arnott,
R.W. 364, 567, 687, 711
Aronson,
J.L. 29
Arora,
V.K. 344
Arp,
G. 3
Arrhenius, G. 379, 341
Arribas, J. 548, 549
Arthur,
M.A. 85, 384, 394, 526, 702
Arvidson,
R.S. 99, 100, 241, 526
Asako, Y. 711
Asaro, F. 656
Asgaard,
U. 82, 700
Ashi, J. 673
Ashley,
G.M. 711
Ashmore, P.E. 87, 582
Asian,
A. 35, 287
Asmerom,
Y. 394
782
INDEX
OF
AUTHORS CITED
Asserto, R. 234, 681
Astin,
T.M. 720
Astin,
T.R. 183, 223, 659
ASTM (American Society
for
Testing
and
Materials) 344,751
Atchley,
S.C. 446
Athy,
L.F. 167
Attalla,
M.N. 502
Attia,
K-.M. 526
Atwater,
B.F. 183, 756
Aubrey,
D.G. 609
Aubry,
M.-P. 443
Auffret,
J.P. 498
Augustinus, P.G.E.F. 157, 736
Auman,
H.J. 751
Ausich,
W.I. 249, 728
Austen,
I, 285
Austin,
W.C. 682
Autin,
W.J. 287
Auton,
T.R. 229
Awramik,
S.M. 3, 690
Awwiller,
D.N. 394
Ayalon,
A. 394, 396
Aydin,
A. 733
Azam,
F. 491
Azevedo, T.M. 91
Baag, C. 557
Baas,
J.H. 567, 568
Baas,
M. 594
Baba,
J. 338
Bachtel, S.L. 447
Bachu,
S. 502
Back,
W. 190, 241, 542
Badiozamani,
K. 241
Bagnold,
R.A. 31, 172, 229, 253, 335, 352,
528,
529, 618, 625, 638, 672, 711, 759
Bahlburg, H. 548
Bailard,
J.A. 625
Bailey,
E.B. 335, 628
Bailey,
E.M. 403
Bailey,
S.D. 246
Bailey,
S.W. 65, 126, 400, 722
Bain,
D.C 126, 167
Baird,
G.C. 446, 728
Baker,
A.J. 394
Baker,
C.L. 651
Baker,
J.C. 53
Baker,
P.A. 134, 241, 242
Baker,
V.R. 292,
292-293
Bakken,
B. 668
Baldauf,
J. 763
Baldauf,
J.G. 492
Bale,
A.J. 285
Bailantyne, C.K. 7
Bailard,
R.R.B. 224
Balling, R.C 123
Balme, B.E. 148
Baltzer,
F. 241
Banfield,
J.F. 219
Bangs, N.L. 673
Banks, N.L. 195
Banner,
J.L. 394
Bao,
H, 368, 395
Baozhen,
Z 299
Bar-Matthews, M. 396
Barbarossa,
N.L. 294, 480
Barber,
D.J. 100
Barber,
R 491
Barbieri, M. 507
Bardaji, T. 274
Bardossy,
G.
145,411
Bargar,
E. 592
Barkan,
E. 396
Barmawidjaja,
D.M. 594
Barnaby,
R.J. 105
Barnes, H.L. 703
Barnes, M.A. 403, 428
Barnes, N.F. 491
Barnes, P.M. 672
Barnes, P.W. 157
Barnes, S.S. 378
Barnes, W.C 403, 428
Barnett,
A. 757
Barnhisel, R.I. 126, 449
Barr,
J.L. 563
Barrell, J.
172,639
Barrett,
P.J. 330
Barrett,
T.J. 384
Barrie, J.V.
561,682
Barron,
E.J, 146, 491
Barson,
D. 502
Bartholoma,
A. 27, 736
Barwis, J. 741
Baryosef,
B. 4
Bascom,
W.N. 22, 27
Bassler,
R.S. 308
Basu,
A.
416,430,548,549,554
Basu,
A.R. 394
Batchelor,
G.K. 530
Bateman,
R.M. 148, 314
Bates,
R.L. 224, 283, 534, 736
Bates,
T.F. 646
Bathurst,
R.G.C. 69, 93, 134, 219, 224, 438,
462,
474, 505, 633, 640, 688
Battjes, J.A. 718
Baturin,
G.N. 525, 526
Bauer,
A. 371
Bauer,
B.O. 246, 625
Bauer,
R.A. 35
Bauld,
J. 39
Baum,
G.R. 333, 526
Baum,
R.L. 248
Baumann,
P.C 751
Baumiller,
T. 728
Baumiller,
T.K. 728
Bayliss, P. 126
Bayram,
A. 625
Bazykin,
D.A. 183
Bazylinski, D.A. 424, 549
Beard,
B.L. 384
Beauchamp, B. 682
Beaufort,
D. 450
Beaufort,
L. 594, 763
Bebout,
D.G. 19, 242, 446, 475
Beck,
J.W. 394, 395
Beck,
V.H. 91
Beckinsale, R.P. 633
Becq-Giraudon,
J.F. 403, 428
Bedard,
D.C 751
Befring, S. 668
Behl, RJ. 665
Behling, P.J. 146
Behr,
H.J. 418
Behrens, E.W. 506, 674
Behrensmeyer,
A.K. 287, 728
Behrensmeyer,
K. 728
Behringer,
R.P. 353
Beier,
J.A. 594
Bein,
A. 234
Belderson,
R.H. 498, 499, 609
Belknap, D.F. 587, 588
Bender,
M.L. 526
Benitez-Latorre,
J. 516
Benito, M.I. 394
Benn,
D.I. 292, 331
Bennett,
J.G. 344
Bennett,
J.L. 751
Bennett,
M.R. 331
Bennett,
R.H. 206
Bennett,
S.J.
301,480,536,618,759
Benson,
L. 754
Benson,
W.H. 751
Bentley,
CR. 746
Bentor,
Y.K. 134, 525
Berelson,
W. 492
Berendsen,
H.J.A.
36
Berezniuk,
T. 502
Berg, R.C 746
Berg, R.R. 518
Bergan,
M. 281
Berger,
A. 443
Berger,
A.L. 183
Berger,
W.H. 89, 120, 491, 492, 594, 673,
763
Berggren,
W.A. 443
Bergman,
H.L. 751
Bergman,
K.M. 498
Berhane, H. 502
Berne, S. 498, 499
Bernard,
H.A. 47, 271
Berner,
K.E. 599
Berner,
R.A. 85, 145, 160, 219, 226, 408,
451,
542, 557, 594, 599, 633, 702, 728
Bernoulli, D. 507, 508
Berry,
W.J. 751
Berryhill,
H.L.J.
499
Berthois, L. 338
Bertling, M. 71
Bertram,
M.A. 99
Bertrand,
P. 548, 763
Bertsch,
P.M. 126, 449
Besly,
B.M. 549
Best,
J.L. 301, 530, 531, 536, 537, 567, 618
Bettess, R. 582
Beukes, N.J. 384, 531
Beutner,
E. 436
Beveridge, T.J. 38, 39
Bevins, R. 137
Bhattacharya,
J.B. 203
Bhattacharya,
J.P. 202, 203
Bhattacharyya,
D.P. 65, 385
Bice,
K.L. 396
Biddinger,
G.R. 751
Bideau,
D. 267
Bierkens, M.F.P. 287
Bierman,
P.R. 394
Biggs, D.L. 665
Biggs, R.B. 202, 609
Bildgen,
P. 394
Billings, L.G. 756
Bilodeau,
G. 673
Biot,
M.A. 314
Bird,
D.K. 29
Bird,
M.I. 123, 394, 395, 397
Birkemeir,
W. 625
Biscaye, P.E. 464, 672, 673, 763
Bischof,
S.A. 438

INDEX
OE
AUTHORS CITED
783
Biscboff,
A. 278
Bischoff,
W.D, 99, 100
Bish,
D.L. 400
Bishnoi, PR. 138
Bishop, CA. 751
Bishop, F.C 99, 100
Bishop, J.K.B. 763
Bissell, H.J. 249
Bittencourt,
ACSP
202
Bjorkum,
P.-A, 118, 167, 168
Bj0rlykke, K. 29, 118, 167
Blachere, J.R. 241
Black,
J.M.W.
716
Black,
K.P. 625
Black,
K.S. 618
Black,
M. 440
Blackwell,
D.D. 316
Blair,
T.C 7, 374, 582
Blais,
J.M. 408
Blake, W. 753
Blanc, P. 105, 549
Blanford,
G. 416
Blankenship, D.D. 746
Blasco, S.M. 673
Blatt,
H. 60, 118, 458, 554, 592, 599, 633
Blissenbach,
E, 7
Blockley,
J.G. 384
Bloom,
A.L. 183, 395
Bloos, G. 354
Blount,
A.M. 400, 722
Bluck,
B.J. 22, 27
Blum,
J.D. 395
Blum,
M.D. 582
Boardman,
M.R. 242
Bobrowsky,
P.T. 756
Bock,
W.D. 135
Bodine, M.W. 722
Boersma,
A.
507-508
Boersma,
J.R, 59
Boggild,
O.B. 69
Bogli, J. 15
Boggs, S.Jr. 184, 430, 549
Boguchwal,
L.A.
301,711
Bohacs, K.M. 85, 458
Bohn,
H.L. 108
Bohor,
B.F. 63, 264, 656
Boichard,
R. 241
Boiron,
M.C 105
Boles,
J.R. 20, 29
Bolt,
G.H. 108
Bonatti, E. 507,
507-508
Bond,
G.C 733
Bondevik,
S. 756
Bone, Y. 474
Bonnecaze, R.T. 352
Bonney,
T.G, 633
Bookin,
A.S, 400
Boon,
J.D, 609
Boothroyd,
J.C 741
Bora,
J.A. 185
Borchert, H.
263
Bordeaux,
Y.L.
728
Borer, J.M.
446
Bornhold,
B.D, 31, 668
Borre, M 120
Bory,
A. 548
Bosak,
P. 678
Bosellini, A. 183
Bosence, D.W. 102, 688, 728
Boswell,
T.G.H.
633
Botrell, S.H. 702
Bottcher,
M.E. 594
Bottjer,
D. 440
Botz, R. 722
Boudreau,
B.P.
226,451,606
Boudzoumou,
F. 722
Bouloubassi,
I. 403, 763
Boulton,
G.S. 193, 331, 746
Boulvain,
F. 688
Bouma,
A.H. 59, 168, 170, 271, 375, 491,
499,
563, 633, 672, 698, 759, 778
Bourbonniere, R. 681
Bourgeois, J. 59, 364, 647, 673
Bourillet,
J.F. 498
Bouroz, A. 63
Bourque, P.-A.
101,474,688
Boutakoff,
N. 308
Bouysse, P. 498
Bowen,
A.J.
31,625,626,711
Bowen,
R. 395
Bowman,
D. 7
Bowman,
J.R. 224, 395
Bowman,
M. 698
Bowyer,
D.A. 599
Boyce, F.M, 408
Boyd,
R, 47, 202, 582
Br0rs,
B. 31
Bradley,
J.B. 638
Bradley,
W.C 25
Bradley,
W.H. 594
Brady,
L.L. 356, 531
Brady,
P.V. 225, 242
Braekman,
J.-C 683
Braitsch,
O. 263, 722
Brakenridge, G.R. 287
Bramlett,
M.N. 89, 665
Brancaccio, L. 754
Brandon,
M.T. 436
Brandtstadter,
H. 224, 242
Branney,
M.J, 759
Brassell, S.C 85
Bray,
CJ. 599
Bray,
D.I, 294, 344, 434
Bray,
J.W. 425
Brayshaw,
A.C
531,618
Brazier,
V. 7
Breed,
CS. 711
Brencbley,
P.J. 364, 728
Brennan,
S.T. 263, 599
Brenner,
R.L. 364, 743
Brett,
CA. 458
Brett,
CE. 206, 446, 728, 729
Bretz, J..H. 292
Bricker,
O.P. 53, 118
Bridge, J.S. 35, 60, 87, 202, 287, 433, 480,
536,
537, 618
Bridges, E.M. 4
Bridges, P.H. 102, 688
Brien,
D.L. 34
Brierley,
G.J. 433, 582
Briggs,
D.E.G.
702, 728
Brindley,
G.W. 65, 142, 400, 633
Brinkhuis, H. 594
Brinkmann,
R. 633
Bristow,
CS. 246, 498
Brodie, I. 763
Brodzikowski,
K, 746
Broecker,
W.S.
183,396,491
Bromley,
R.E. 120, 700
Bromley,
R.G. 71, 82, 120, 700
Brook,
G.A. 681
Brookfield,
M.E. 60, 253
Brooks,
J.R.V.
206
Brooks, N.H. 301
Brors,
B. 31
Brosse, E. 167
Brotherton,
D.I. 582
Broussard,
M.L. 202
Brown,
G.C. 142, 599
Brown,
L.F., Jr. 183
Brown,
P.G. 599
Brown,
P.J. 561
Brown,
R.G. 234
Brown,
R.J.E.
8
Brown,
S.M. 109
Brozovie, N. 733
Bruder,
K.F. 674
Bruhn,
CH.L. 698
Brumer,
CA. 293
Brumfield,
D.S. 519
Brumsaek,
H.-J. 594
Brune, G.M. 408
Brunn,
P. 157
Brunnacker,
K, 755
Brunner,
CA. 760
Brunsden,
D. 668
Brunsdon,
CF. 344
Brush,
L.M. Jr. 529
Bryan,
W.B. 757
Bryant,
E.A. 292, 756
Bryant,
I.D. 202
Bryant,
W.R. 170, 206, 274
Brzezinski, M.A. 395, 492
BSI
(British Standards Institution)
344
Buat-Menard,
P. 763
Buatois, L.A. 82
Bubela,
B. 505
Buccino, G. 754
Bucher,
W.H. 567
Bucholtz
ten
Brink,
M.R. 665
Buck,
S.G. 385
Buczynski, C 462, 505
Budai, J.M. 190
Budd,
D.A.
241,446
Buddemeier,
R.W. 606
Buffington,
E.C 31
Buffington,
J.M.
294,618,633
Buffler,
R. 139
Bugge, T. 668
Buie,
B.F. 412
Bull, P.A. 716
Bull, W.B. 7, 8, 145, 7-8
Bullard,
T.F, 430
Bullen,
S.B. 234
Bullen,
T.D, 395
Burban,
P.-Y. 609
Burbank,
D. 733
Burchette, T.P. 474, 475
Burden,
J. 362
Burge, L.M. 433
Burke,
E.A.J.
299
Burke, K. 733, 734
Burke, R.B. 560
Burke, S.K. 763
Burke, W.H.
118,395
Burland,
J.B. 121
Burley,
S.D. 20, 105, 106
Burmeister,
E.G. 516
Burnett,
L. 751
Burnett,
W.C 525, 526
784
INDEX
OF
AUTHORS CITED
Burnham,
A.
Burns, L.E.
Burns, R.G.
Burns, S.J.
Burns, V.M.
Burr,
G.S. :
Burrell, D.G.
Burruss, R.C
Burst,
J.F.
Burstyn,
H.L
Burt,
D.M.
Burt,
N.T.
Burt,
R. 4
Burton,
E.A.
Burton,
G.A,
Burton,
J.H.
Busby,
C.J.
Busby,
J.F.
Busch,
D.A.
Buschkuehle,
Buseck,
P.R.
Busenberg,
E
Bustillo, M.
Bustin,
R.M.
Butler,
G.P.
Butler,
J.B.
Butler,
R.F.
Butler,
R.M.
Butt,
T. 62^
Butterfield,
C
Butterfield,
^
Buxton,
T.M
Buynevich,
1.
Byerly,
G.R.
Byers,
G.W.
Byrne, J.V.
Byrne, R.J.
K. 306
599
378
21,
118,
134,241,396
378
395
606
. 299
333,
720
,. 647
100
285
106,
118,438
, Jr. 751
65,
126
733
190
202
B.E. 242
127,
424
. 99
660
85,
403, 428
19,
585
344
423
502
J.R. 253
i.J. 394
. 692
V. 47, 741
264
446,
672
19
609
Byrne, T. 436
Gabrerra,
J.G. 248
Cabrol, P. 681
Cacchione, D.A. 498, 625, 672, 687
Gadee, G.G. 633
Gai,
J. 743
Gaincross, B. 583
Cairns-Smith,
A.G. 677
Galabrese, E.A. 606
Galder,
E.S. 31
Caldwell, M. 592
Galdwell,
W.B.E.
397
Gallander,
R.A. 434
Galle, G. 768
Galon,
T.J. 672
Calvache, M. 8
Galvert,
S. 491
Galvert,
S.E. 66, 378, 379, 384, 594, 672, 763
Galvet,
F. 446
Cameron,
A.R. 403
Gaminade, J. 756
Gampbell, G.S. 33, 336
Campbell, C.V. 59, 60, 364
Gampbell, LH. 599
Gampbell, L.M. 751
Gampbell, S. 716
Campion,
K..M. 185, 203
Gande, S.G. 443
Ganfield,
D.E. 395, 599, 702, 728
Gannon,
J.R. 18
Gannon,
R.L. 519
Gannon,
W.F. 633
Gant,
D.J. 434
Gao,
Z. 618
Gapo,
R.G. 241
Garey,
S. 292
Garling, P.A. 292
Carlson,
G. 508
Garlson,
G.P. 42
Garlson,
J. 698
Carlson,
P.R. 498
Garlson,
W.D. 462
Carmona,
M.C. 682
Carnes, M. 673
Garozzi, A.V. 633, 665
Carpenter,
A.
B.
241,542
Garpenter,
G. 672
Carpenter,
S.J. 395
Garpenter,
J.R. 185
Garr,
S.J. 375
Carr,
T.R. 183
Carrier,
G.F. 718
Garrigy,
M.A. 16
Garroll, A.R. 85, 506
Carson,
M.A. 33, 256
Garstens, H. 224
Garter,
A. 548
Gartwright,
J.A. 720
Garver,
R.E. 358, 618
Gas,
R.A.F. 296, 335
Gasas, E. 299
Gasey,
M. 167
Gasey,
R.E. 763
Gassells,
J.B.G.
294
Gastaing, P. 498
Gastens-Seidell,
B. 585
Castillo, M.M. 138
Castro, L.N. 526
Gathelineau,
M. 105
Gaumette, P. 39, 440
Gavazza,
W. 592
Cavell, P.A. 242
Gawood,
P.A. 358
Gayeux,
L. 505, 640,
640-641
Gegia,
J. 551
Gembranos, M.L. 4
Gentineo, M.G. 333
Ceding, T.E. 91, 395
Cermak,
V. 315
Ghadwick,
CA. 91, 241
Ghafetz, H.S. 53, 395, 505, 754
Ghaloncr,
W.G. 123
Ghamberlain,
G.P. 397
Ghamberlin,
T.G. 643
Ghamley,
H. 127, 134, 142, 458, 498, 677
Chan,
L.H. 599
Chan,
M.A. 224
Ghandler,
F.W. 554
Ghandler,
J.H. 344
Ghandler,
R.J. 248
Ghang, H.H. 529, 618
Ghannell,
J.E.T.
424
Ghanner,
D.M.DeR.
599
Ghapelle, F.H. 542
Chapman,
G.R. 278
Chapman,
D.L. 109
Chapman,
P. 668
Chapman,
P.M. 751
Ghapman,
R.E. 267
Ghapman,
S. 229
Ghapman,
V.J. 736
Ghappell,
J.M.A.
395
Ghappie, D.J. 751
Gharaklis, W.G. 39
Charlie, D. 756
Chase, E.B. 605
Ghaudhuri, S. 395
Ghazot,
G. 395
Gheel, R.J. 356, 364, 537, 687
Chen,
C.L. 352
Chen,
J. 681
Chen,
W. 224
Chen,
Y. 4
Cheng,
D.C.-H.
360
Cherry,
J.A. 268
Ghesnut,
D.R. 148
Ghi,
G. 508
Childs, C.W. 395
Ghilingar,
G.V. 249
Ghillingarian,
G.V. 168
Ghivas, A.R. 358, 395, 397
Choi, K.S. 743
Ghoi,D.R.
446
Ghoppin,
G.R. 438
Ghoquette, P.W. 15, 118, 267, 678, 684
Ghorley,
R.J. 34, 633
Ghough,
S.K. 274
Ghoung, K.-S. 687
Chow,
N. 446
Ghowns, T.M. 224, 308, 665
Christ,
G.L. 63, 634
Christiansen,
E.A. 678
Christie, J.M. 554
Ghristie, P.A.F. 167
Ghristman,
R.F. 362
Ghrzastowski,
M.J. 35
Chuber,
S. 183
Chuhan,
F.A. 167
Church,
M. 8, 11, 22, 25, 345, 434, 480, 531,
582,
618
Church,
M.A. 344
Ghyba,
G.F. 599
Gialone, M.A. 43
Cicero, A.D. 599
Girac, P. 498
Gisne, J.L. 183
Gita,
M.B. 594
Glague, D.A. 733
Glague, J.J. 33, 605, 756
Glark,
I.D. 395
Glark,
J.B. 751
Glark,
J.D. 698
Glark,
J.S. 123
Glarke,
J.D.A.
474
Clarke, T.L. 561
Clauer,
N.
333,371,395,450
Glaxton,
B.L. 300
Glaypool, G.E. 306, 395
Glayton,
G.J. 306
Clayton,
J.L. 605
Glayton,
R.H. 385
Glayton,
R.N. 395, 396
Clayton,
W. 137
Clements, R.G. 83
Glemett,
S.J. 549
Glemmensen,
L. 137
Glenell, M.B. 167
Gleveringa,
J. 625
Glifford,
G.H. 594
Glift,
R. 229
