Middleton G.V. (Ed.) Encyclopedia of Sediments and Sedimentary Rocks
Подождите немного. Документ загружается.

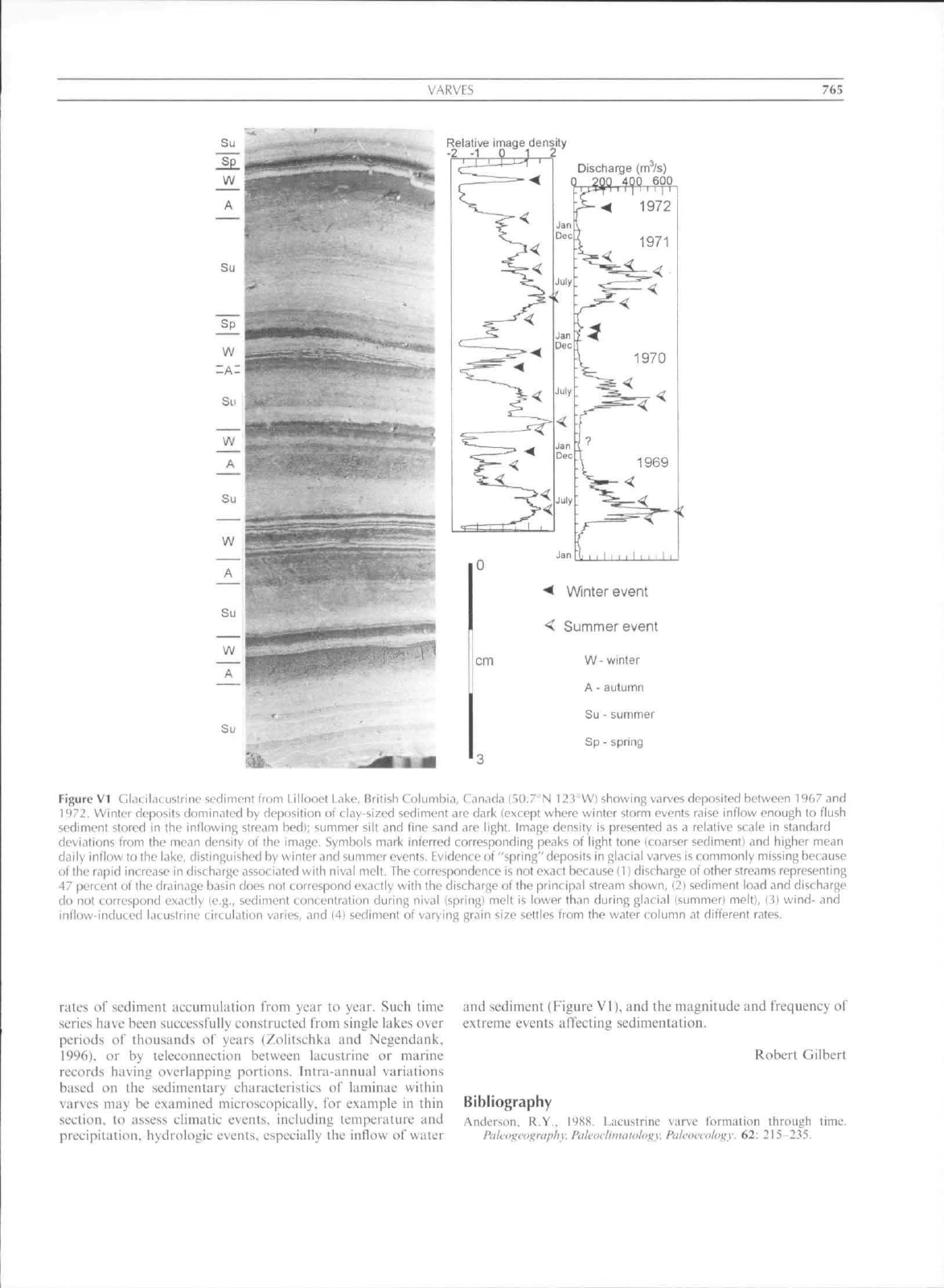
VARVBS
765
Relative image densiiy
-2
-1 0 1 2
Discharge (m /s)
0
200 400 600
Su
< Winter event
< Summer event
cm W-winter
A
-
autumn
Su
-
summer
Sp
-
spring
Figure
VI
Glatikuublrine sedimeni from Lillooei Lake, British Columbia, Canada (50.7
N 123
W) showing v^irves deposited between 1967 and
i*)7J. Winler deposits dominated
by
deposition
of
clay-si zed
sediment are dark (except where winter storm events raise inflow enough
to
flush
sedimeni stored
in the
inflowing stream
bedi;
summer silt and fine s.ind
arc
light. Image density
is
presented as a relative scale
in
standiird
deviations Irom
the
mean density
ot the
image. Symbols mark inferred corresponding peaks
of
light tone (coarser sediment) and higher mean
daily inflow to the lake, distinguished by winler and summer
events.
Evidence ot'"spring" deposits in glacial varves is commonly missing because
ofthe rapid increase in discharge associated with nival melt. The correspondence
is
not exact because
II)
discbargeof other streams representing
47 periLent
of
lhe drainage basin doe', no! correspond exactly with the discharge
of
the principal stream shown,
(21
sediment load and discharge
rIo
not
correspond exactly
(e.g,,
sediment concentration during nival (spring! melt
is
lower than during glacial (summer) melt),
i'i]
wind-
and
inflow-induced iac ustrine circulation varies,
and (4}
sediment
of
varying grain size settles from the water column
at
different rates.
rates of seditneni accLiiiiulation frotn ycur to year. Such time
series ha\e been successfully constructed frotii single lakes over
periods of thousands of years (Zolitschka and Ncgendank.
1996), or by teleconnection between lacustrine or marine
records having overlapping portions, Intra-annual variations
bused on the sedimentary characteristics of laminae within
varves may be examined microscopically, for example in thin
section, to assess climatic events, including temperature and
preeipitiition. hydfologic events, especially the inflow of water
and sediment (Figure VI), and the magnitude and frequency of
extreme events affecting sedimentation.
Robert Gilbert
Bibliography
Anderson.
R,Y,.
I98S, Lacustrine varvc formation through time.
Paleogeography,
Pateocliniatotogy,
Paleoeeology.
62:
215-235.
766
Dc Gcer.
G..
1912.
A
gcochronnlogy
of
the last 12.000 year^. Proceed-
ings of ihc Inlernatiinud Geologieal Compress. Stockholm (1910).
1:
241
253.
Fisher,
C.G-. 1990.
Bibliography
and
iiivenlory
of
holocenc varved
and latninatcd marinf sedimenls. Naiional Oceanic and
.Almaspheric
Adntinistralion (NOAA)
Paleoclinuiie
PuhlicaiionsSeries Report
No.
I.
lO7p.
Kemp. A.F.S. (cd.).
19%.
Palueoclintaiology
and
Palaeooceanogmphy
from LaminatedSedimetils. Geological Society. Speeiiil Publication,
116.
London. 2?Xp.
Ross.
J.. and
Gilbert.
R..
1998. Liiciistrini; sedimentation
in a
monsoon
environmeni, tlie record from Phewa
T;il,
Middle MoutUaiti region
of Nepal. Geomorphology.
27: 307 .123.
Stevens.
R., 1986.
Glacioniarine varves atid
the
character
of
deglaeiation. Savean valley. stmthueslL-rn Sweden. Boreas.
15:
289-299.
Stokes.
W.L., 1964.
Eolian varving
in the
Colorado Plateau. Jounuil
of Sedimentary
Petrology.
34: 429 432.
Zolitschka,
B.. and
Negcitdank, J.F.W., 1996. Sedimentology. dating,
and palaeoclimalic interpretation
of a
76.2
ka
record frotn Lago
Grande
de
Montiechio. southern Itiily. Quaternary Science
Reviews.
15: 101 112.
Cross-references
Climatic Control
of
Sedimentation
Colors
of
Sedimentary Roeks
Cyclic Sedimentation
Glacial Sediments: Processes, Environments,
and
Facies
Grain Size
and
Shape
Impregnation
Lacustrine SedimeiUiition
Upwelling
VERMICULITE
Introduction
"Vermiculite"
is
derived from
the
Latin word vcrmiculiis
{
=
small worm) which refers
to the
wormlikc projections this
mineral forms when subjected
to
high tetnperatures. Vermicu-
lite
is a
hydrous
2 : 1
phyllosilicate similar
in
structure
to
tiiica,
but has a
lower layer charge,
and
contains hydrated
exchangeable catiotis
iti the
interlayers versus "non-exchange-
able""
K' in the
interlayers
of
mica. Vermiculiics occur
in
macroscopic
and
clay-sized forms.
The
macroscopic vermicu-
litc
is
invariably trioetahedral
in
composition, whereas
the
clay-sized vermiculite
may be
either dioctahedrat
or
trioetahe-
dral
and is
frequently found
in
soil environments.
The
structure, crystal chemistry, mineralogy,
and
surface chemistry
of vermiculite
arc
responsible
for the
important roles they play
in natural environments
as
well
as
their useful environmental
and industrial applications.
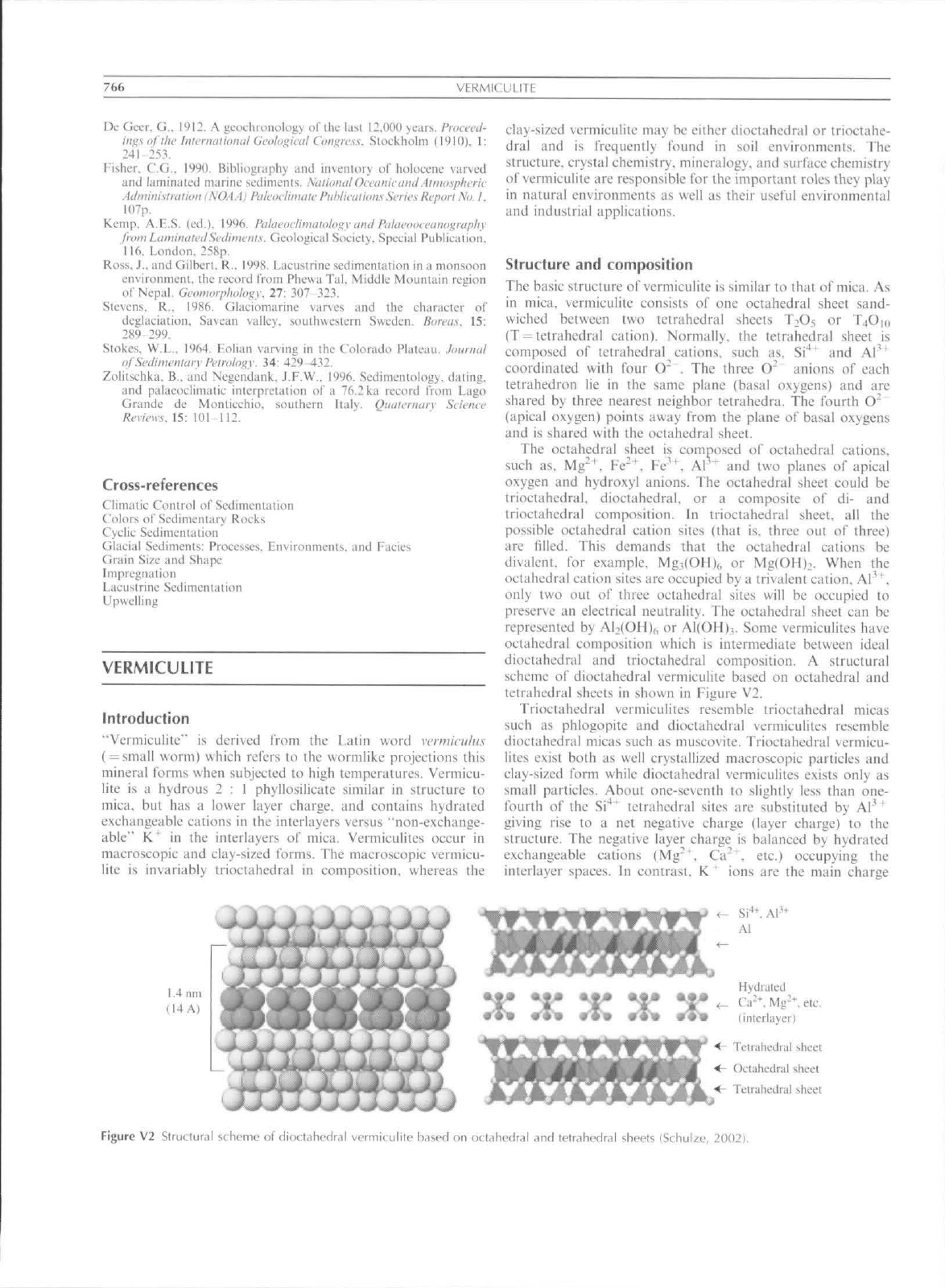
Structure and composition
The basic structure
of
vermiculite
is
similar
to
that
of
mica.
As
in mica, vermiculitc consists
of one
octahedral sheet sand-
wiched between
two
tetrahedral sheets TnO5
or
T4O10
(T-telrahedral cation). Normally,
the
tetrahedral sheet
is
composed
of
tetrahedral cations, such
as, Si*"^ and Al'^
coordinated with four
O" , The
three
O"
unions
of
each
tetrahedron
lic in the
same plane (basal o.xygens)
and are
shared
by
three nearest neighbor tetrahedra.
The
fourth
O"
(apical oxygen) points away from
the
plane
of
basal oxygens
and
is
shared with
the
octahedral sheet.
The octahedral sheet
is
composed
of
octahedral cations,
such
as, Mg"*.
Fe''', Fe''',
Al"^ and two
planes
of
apical
oxygen
and
hydroxyl anions.
The
octahedral sheet could
be
trioetahedral, dioctahedral,
or a
composite
of di- and
trioetahedral composition,
[n
trioetahedral sheet,
al! the
possible octahedral cation sites (that
is.
three
out of
three)
are filled. This demands that
the
octahedral cations
be
divalent,
for
example. Mg.,(OH),,
or
Mg(0H)2. When
the
octahedral cation sites
are
occupied
by
a trivaleiit cation. Al'^',
only
two out of
three octahedral sites will
be
occupied
to
preserve
an
electrical neutrality.
The
octahedral sheet
can be
represented
by
AU(OH),,
or
Al(OH)!. Some vermiculites have
octahedral composition which
is
intertnediale between ideal
dioctahedral
and
trioetahedral composition.
A
structural
scheme
of
dioetahedra! vermiculitc based
on
octahedral
and
tetrahedral sheets
in
shown
in
Figure
V2.
Trioetahedral vermiculitcs resemble trioetahedral micas
such
as
phlogopite
and
dioctahedral vermiculites resemble
dioctahedral micas such
as
muscovite. Trioctahedral vermicu-
lites exist both
as
well crystallized macroscopic particles
and
clay-sized form while dioctahedral vermiculites exists only
as
small particles. About one-seventh
to
slightly less than
one-
fourth
of the Si"*'
tetrahedral sites
are
substituted
by
Ai"*
"*
giving rise
to a net
negative charge (layer eharge)
to the
structtire.
The
negative layer charge
is
balanced
by
hydrated
exchangeable cations (Mg"', Ca'"^.
etc.)
occupying
the
interlayer spaees.
In
contrast.
K '
ions
are the
main charge
1.4 nm
(14 A)
Al
Hydraled
^ Ca-^ MJI'^ etc-
Itnterlayeri
•<-
Tetrahedral sheet
•<-
Octahedral sheet
•<-
Teirahedral sheet
Figure
V2
StruttuMl scheme
of
dioctahedral vermiculite h.ised
nii
oct.ihL'dral
and
tetrahedr,il sheets (Schulze. 2002).
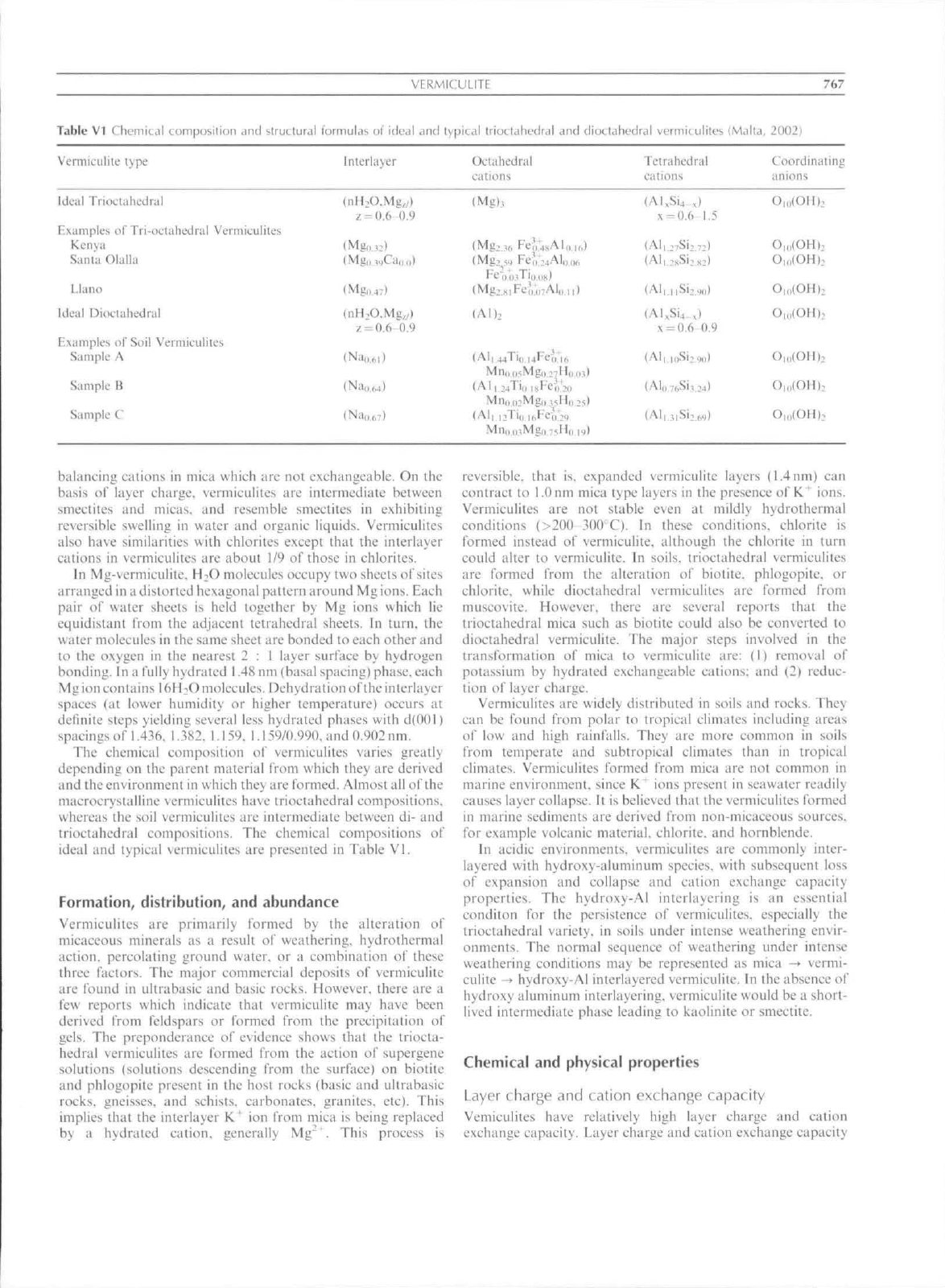
Table
VI Chemical
Vermietililc
lype
composition
and
structural
VERMICUEITE
formulas
of ideal and typical triodahedral
hUerlayer
Oclahedral
eations
and
dioctahedral
vermituliles
Icirahedral
cali Otis
(Mai
la,
767
20021
Coordinaiiiig
anions
Ideal IVioetahedral
Fxiimplcs of Tri-octaliednil Vermieulites
Kenya
Sania Olaila
Llano
Ideal
Dioetahedial
IZxamples of Soil Vermieulites
Sample A
Sample B
Sample C
z-0.6-0.9
=
0.6-0.9
(Mg),
(Alb
(All 44Ti(i uFcVifi
(All 24Ti(, uFe-0,'2,,
(All i:Ti,,,,,Feo;2<)
x-0.6
(Al|";.Si:
(All uSiz.
x
= 0.6'
(Alo7^Si,.
1.5
;;!
0.9
24)
O,,,(OHh
balancing cations in mica which are not exchangeable. On the
basis of layer charge, vermiculites are intermediate between
smectites and micas, and resemble smectites in exhibiting
reversible swelling in water and organic liquids. Vermiculites
iilso have similarities with chlorites except that [he interlayer
cations in vermiculites are iiboiit 1/'^ of those in chlorites.
In Mg-vermiculite, HiO molecules occupy two sheets of sites
iirranged in a distorted he.xagonal pattern around Mgions. Each
pair of water sheets is held together by Mg ions which lie
equidistant from the adjacent tetrahedral sheets. In turn, the
water tnoleeulcs in the same sheet are bonded to each other and
lo the o.\ygen itt ihe nearest 2 : I layer surface by hydrogen
bonding. In a fully hydrated \AS nm (basal spacing) phase, each
Mg ion contains
I
dH^O
molecules.
Dehydration of the interlayer
spaces (at lower humidity or higher temperature) occurs at
definite steps yielding several less hydrated phases with d(()()l)
spacingsof
1.436.
1.382.
1.159.
1.159/0.990, and 0.902nm.
The chemical composition o\' vermiculites varies greatly
depending on the parent material from which they are derived
and the cn\ironment in whieh they arc formed. Almost all of the
macrocrystalline \erttiiculites ha\e trioctahedral compositions,
whereas the soil vermiculites are intermediate bclwccn di- and
Irioctahedral compositions. The chemical compositions of
ideal and typical vermiculiles are presented in Table VI.
Formation, distribution, and abundance
Vermiculites are primarily formed by the alteration of
mieaceous minerals as a result of weathering, hydrothermal
action, percolating ground water, or a combination of these
three factors. The tiiajor comtnercial deposits of venniculite
are found in ultrabasic and basic roeks. However, [here are a
few reports which indicate thai vermiculite may have been
derived from feldspars or formed from the precipitation ol'
gels.
The preponderance of evidenee shows that ihe triocta-
hedral vertniculites are formed frotn the action of supergene
solutions (solutions descending from the surface) on biotite
and phlogopite present in the host roeks (basic and ultrabasic
rocks,
gneisses, and schists, carbonates, granites, etc). This
implies that the interlayer K ' ion from mica is being replaced
by a hydralcd cation, generally Mg~\ This process is
reversible, that is, expanded vermiculite layers (1.4nm) can
eontracl to I.Onm mica type layers in the presenee of K"^ ions.
Vermiculites are not stable even at mildly hydrothermal
conditions (>20O-3O0 C). In these conditions, chlorite is
tbrmed instead of vertiiiculite, althotigh the chlorite in [urn
could alter to vermiculite. In soils, trioclahedra! vermtculites
are formed from the alteration of biotite. phlogopite, or
chlorite, while dioctahedral vermiculites are formed from
museovite. However, there are several reports that the
trioctahedral mica such as biotite could also be converted to
dioctahedral vermiculite. The major steps involved in the
transformation of mica lo vertnicuHte are: (1) retnoval of
potassium by hydrated exchangeable cations; and (2) reduc-
tion oi layer charge.
Vernticulites arc widely distributed in soils and rocks. They
can be fotind from polar to tropical climates including areas
oi' low and high rainfalls. They are more eommon in soils
from temperate and subtropical climates than in tropical
climates. Vermiculites fortned from mica are not common in
tnarine environment, since K"^ ions present in seawater readily
causes layer collapse. It is believed that the vermiculites formed
in marine sediments are derived (rom non-micaceous sourees.
for example volcanic material, chlorite, and hornblende.
In acidic environments, vermiculites are commonly inter-
layered with hydroxy-aluminutn species, with subsequent loss
of expansion and collapse and cation exchange capacity
properties. The hydroxy-Al interlayering is an essential
conditon for the persistence of vermiculites, especially the
trioctahedra! variety, in soils under intense weathering envir-
onments. The normal sequence o\' weathering under intense
weathering conditions may be represented as mica -• vermi-
culite -* hydroxy-Al interlayered vermiculitc. In the absence of
hydroxy aluminum interlayering. vcrmieulitc would be a short-
lived intermediate phase leading to kaolinite or smectite.
Chemical and physical properties
Layer charge and cation exchange capacity
Vcmiculites have relatively high layer charge and cation
exchange capacity. Layer charge and eatioti exchange capacity

768
VERMICULITt
are cioseiy related. Layer charge refers to the net negative
charge of the 2 : 1 layer arising from the isomorphous
substitution of a higher valence cation by a lower valence
cation. The cation exchange capacity (CEC) refers to the
quantity of readily exchangeable cations neutralizing negative
charge. Both the permanent negative charge t>om isomor-
phous substitution and the negative charge (pH dependent)
from the broken bonds at the edges contribute to CHC. The
cation exchange capacity of vermiculite ranges from 130 to
2IOemol4;/kg'. The layer charge expressed per half unit cell
(OioOH.) range from' 0,6 to 0.9. The high CEC and layer
charge of vermiculites thus dominates the exchange properties
of sediments that contain it in significant amounts. Because of
its high CEC. vermieulite is suitable material lor removing
large quantity of several types of smaller heavy metal cations
such as Cu"", Pb''\ Cd"*, Zn"', Hr'. etc, IVom industrial
wastes. However, in most eases the sorption appears to be non-
selective.
Selective sorption and fixation of cations
The unique structure of vermiculite is also favorable for
selective sorplion and fixation of low hydration energy cations
such as NHj'. K' and Cs'. In selective sorption, certain
cations are favored for sorption compared to others. These
selectively sorbcd cations may or may not be held tightly. If the
cations are held tightly and resist replacement by other cations,
they are considered to have been tixed. Selective sorption and
fixation orNH4' and K' arc important for devising effective
soil management strategies, while that of Cs" is important for
radioactive waste disposal. Selective sorption and lixalion of
cations in vermictilite are influenced by several factors such as
cation size, cation valence, cation hydration energy, vermicu-
lite structural and crystal chemical parameters (layer charge,
charge location, composition of octahedral sheet) hydroxy
interlayermg. particle size, frayed edges or wedge zones, etc.
Organo-vermiculite
Vermiculites may also be modified using organic cations to
adsorb and trap varieties of nonionic and anionic organic
compounds that are detrimental to our environment. The
modified clays arc usually referred to as organo-elays (organo-
vermicutite). Potentially the organo-vermiculite can be used
for an in.^ilu treatment of contaminated sediments.
Osmotic swelling
Two types of swelling occur in vermiculites: normal swelling as
a result of uptake of as much as 2 molecular layers of water
(type 1), and osmotic swelling (type 2) which involves the
uptake oi' much larger volumes of water. The type I swelling,
also referred to as interlayer or erystailine swelling, is limited
to ^ 1.5nm with an inorganic cation, and l,94nm with an n-
butylammonium eation. The osmotic swelling in vermiculite is
characterized by a reversible large volume expansion leading to
the formation ofa cohereni gel. The layer expansion could be
as much as 9l,0tim with butylammonium ion. Although Li'
is the only inorganic cation that causes vermiculite to swell
osmotically several organic molecules, such as propyl and
butylammonium and certain amino acid cations are known to
exhibit osmotic swelling with vermiculite. The degree of
swelling increases as the concentration of the electrolyte
decreases. Osmotically swollen vermiculite can be delaminated
by mechanical agitation to produce high aspect ratio
vermiculite particles. The high aspect ratio vermiculites can
be used to produce non-burning paper, or fire proofing or
resistant films and coatings on combustible and noncombus-
tibie substrates.
Exfoliation
Macrocrystalline vermiculile expands (extbliates) to as much
as 8 12 times its original volume upon heating at high
temperature, >300 C and typically 870 1090^C. as the
interlayer and structural waler is converted to steam. The
expanded vermiculite forms light weight granules that have
the appearance of a large worm. The thermal expandability
and light weight characteristics of exfoliated vermiculite have
been exploited tor various industrial and agricultural applica-
tions,
including gaskets tor high temperature sealing, such as in
catalytic converters; insulation and fire relardants: various
construction products: potting soils: soil conditioners: carrier
for fertilizers: insecticides and herbicides: various livestock
applications: and ammonia filtering in aquaculture.
Identification
Vermiculite is routinely identified by X-ray diffraction based
on the 1,4 nm peak produced by a Mg-saturaied sample. The
Mg-saturated sample is further treaied with glycerol or
cthylcne glycol to differentiate it from smectite, which expands
with later treatments. The vermiculite peak collapses to I.Onm
upon K-satui-ation. Chlorite also has a peak at l.4nm bui it
neither expands with ethylene glycol/glycerol nor collapses
with K treatment. Smectites peaks tend to be broader than
vermiculite peaks, Vermiculite is more likely to occur in the
coarse fraction and smectite in the fine clay fVaction (<0.2
[.im).
The hydroxyAI-interlayered vermiculite will resist both col-
lapse and expansion. K-saturated hydroxy-AI interlayered
vermiculite will not collapse to ^l.Onm. but to a broad
1.1 l.3nm peak. It may be necessary to heat the sample at
300 C or higher temperature to collapse the layers lo I.Onm
depending upon the polymeric nature of the hydroxyAI-
interlaycr species.
Vermiculite can also be identified by determining its layer
charge using the alkylammonium ion exchange technique. This
method essentially involves the treatment of each sample with
n-a!kylatnmonium cations, with n ranging from 6 to 18 cabon
atoms folloued by XRD measurements. In addition to the
total charge, the alkylammonium method also provides the
information on charge heterogeneity or distribution. This
method when combined with the Green-Kelly test (Hofniann
and Klemen effect) also allows one to estimate the magnitude
of both octahedral and tetrahedral charges of dioetahedral
vermiculite.
Prakash B, Malla
Bibliography
CalL-. C. Dc La, iind .Suquct. H.. !9HK. VL-rmk-ulite- In Bailey. S,W.
(ed.),
llydniti.s Phyllo.silivates (E.\rlusi\'L' of Mica). Reviews
in Mineralogy 19. Mincralogical Society of Americii, pp.
455-496.

VERMICUI.ITF 769
Douglas. L.A.. I9K9. VL'rmiculites. I[\ Dixon. J.B.. and Weed. S.B.
(cds.).
Miiii'itil.s
in Soil tnviniiiinctiii, 2nd cdn. Soil Science Society
ol"
.America, pp. 635-674.
Deer. W.A,. Howie. R.A., and Zussman. J.. 1992. AnhilnnhiclioiiU'lhi-
Ri)ck-Fiiriniitg
Mittcful.s.
Longman.
Malia. P.B.. 2(102. Vermiculites chemistry, mineralogy, and applica-
lions.
In Di^on. J.B.. and Scliiilze. D.G. (eds.). SoilMiitcralogywiih
Ettviitinmcntul
.•\ppliciition.s.
SSSA Scries
No.
7. Soil Science Society
of America, pp. 501 529.
Schiil/e. D.Ci.. 2fH)2. An introduction to soil mineralogy. In Dixon.
.i.H.
and Scliul/_e. D.G. (eds.). Soil
Mim-raldi-y
Kith Em
iroiuiieiiiu!
Appitccitions. SSSA Series No. 7. Soil Scienee Society of Americii.
pp.
1-35.
Cross-references
Clay Mineralogy
Mixed-Layer Clays
Weaihcrinii. Soils and Paleosols

w
WEATHERING, SOILS, AND PALEOSOLS
Soils are fundamental to life on this planet. The mineral
nutrients provided by weathering within soil and the degree of
drainage of soil eontrol what kind of life can (hrive in a
particular plaee. On the other hand, living creatures with their
roots.
Jaws, and other means of acquiring nutrients do much to
determine the nature of soil. Soils include billions of bacteria,
millions of nematodes and a few plants in just about every
square eentimeter. Soil's diverse microbes and internal
absorptive surfaces of elay neutralize poisons and purify
water. By fueling photosynthesis, soil regulates the composi-
tion ofthe atmosphere. Through the engine of soil, life has far-
reaehing effeets on land, water, and air. The intimate
interrelationship between soil, life, and surfaee environments
also has a long fossil record in the form of fossil soils, or
paleosols. These remains of soils ofthe past are now known as
old as 3.5(10 million years on Earth. Even more aneient soils
and paleosols are now known on the Moon and Mars, and
certain kinds of meteorites may be fragments of paleosols as
old as 4.600 million years.
Weathering
Weathering is the relentless alteration of sediments and roeks
by a variety of chemical, biological, and physical agents at the
surfaee of planetary bodies. In humid forested environments of
good soil fertility, as in the oak forests of northern Europe and
eastern North America, the general weathering regime is a soil-
forming proeess ealled /ww/n/^^c (Eignrc WI). Chetnically,
lessivage is destruetion of primary minerals, such as feldspars,
by weak acids, such as carbonic acid, which liberate into soil
solution cationie nutrients sueh as calcium (Ca""''). magnesium
(Mg^"'"),
sodium (Na') and potassium (K."^). This ehemical
reaction, known as hydralysi.s, is not simple dissolution of
minerals, but incongruent dissolution whieh leaves a residue of
silica (Si) and sesquioxides (Al "''. Ee"^) in clays, such as
smectite
((/.
v.),
and oxide and hydroxide minerals
(</.
r.). such as
goethite. The proeess of lessivage thus creates soil with fewer
feldspar grains and more reddish clay. Biologically, hydrolysis
is important because base cations are fundamental plant
nutrients: calcium for cell walls, magnesium as a critical
component of ehlorophyll and potassium and sodium as cell
electrolytes. Plants promote hydrolysis in many ways. Their
root respiration of carbon dioxide (CO2) enriches soil water in
carbonic acid (H2CO1). Other acids and chelates produced by
plants and their assoeiated microbes accelerate release of
nutrients by weathering. Physically, plants hold the soil
together with their roots, and regulate water loss by means
of ground cover. Earthworms, squirrels, and gophers physi-
cally churn the soil.
A variety of weathering regimes is found in different parts of
the world (Eigure WI). In humid regions of high water table,
such as swamps, the principal soil building process is
accumulation of plant debris in peat, within which deeay is
suppressed by lack of oxygen and acidic leaehates from plants.
Chemically reducing soil below the peat can be leached of iron
in its ferrous (Ee""''') ionic form which is green to gray in color
and more soluble than red, oxidized (Ee" ^) iron. The process
by which reddish brown soils are turned gray by leaching of
redueed iron is called glchation. In humid regions of conifer
forests on sediments and rocks rich in quartz, plants produce
unusual amounts of acid, with the result that even clay is
destroyed in the soil, whieh builds a subsurfaee horizon
reddened by sesquio.xidcs and humus in a proeess called pod-
zolizatian. Humid tropical rain forests grow in soils of low
fertility because of long and deep weathering of nutrient
eations promoted by abundant moisture and warmth. They
have thick soils rich in oxide minerals, sueh as hematite, and
elay minerals, such as kaolinite
{qA\).
Their chemical enrich-
ment in iron (Ee ') and aluminum (Al"*"^) is a soil forming
process called Jerallltization. The deep and thorough weath-
ering of tropical soils ean enrich sesquioxides (Ee'' and AP )
and silicon (Si) elements to the grade of economically valuable
ores of iron (laterite.
(/.
r.),
aluminum (bauxite, t/. v.) and silica
(silerete.
q.v.).
In deserts ((/.v.) there is limited water for
hydrolysis and mobilization of nutrient eations. Calcium, and
also some magnesium, hydrolytieally released from feldspars
and other minerals, is not washed from the profile or taken up
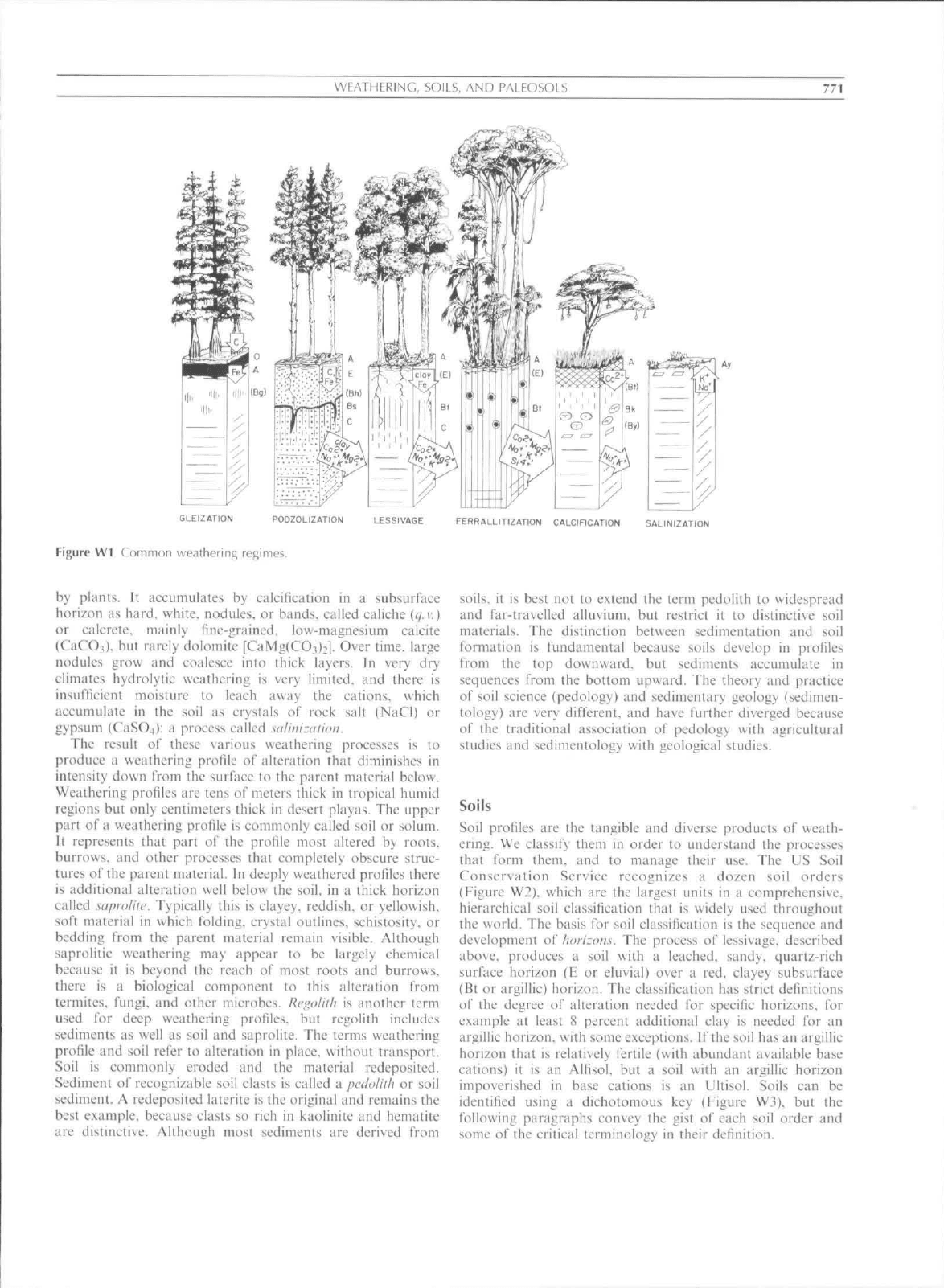
WFATMKKING, SOILS, AND PALEOSOLS
771
GLErZATlON PODZOLIZATION t^SSIVAGE FERRALLITIZATION CALCIRCATION SALINIZATION
Figure Wl Common weatherinj; regimes.
by plants. It accumuliites by calcification In a subsurface
horizon as hard, white, nodules, or bands, called caliche
((/.
r.)
or calcretc. mainly fine-grained, low-magnesium calcile
(C aCOi). but rarely dolomite [CaMglCO,):]. Over time, large
nodules grow and coalesce into thick layers. In very dry
climates hydrolytic weathering is very limited, and there is
insuflicient moisture to leach away the cations, which
accutnulatc in the soil as crystals of rock salt (NaCl) or
gypsutn (CaSO4): a process called salinizalion.
The result of these \arious weathering processes is to
produce a weathering profile of alteration that diminishes iti
intensity doun from the surface to the parent materia! below.
Weathering profiles are tens of meters thick in tropical humid
regions but only centimeters thick in desert playas. The upper
part ofa weathering profile is commonly called soil or soluni.
It represents ihat part of the profile most altered by roots,
burrows, and other processes that completely obscure struc-
tures of the parent material. In deeply weathered profiles there
i.s additional alteration well belov\ the soil, in a thick horizon
called
.•iapriiliic.
Typically this is clayey, reddish, or yellowish,
sott material in which folding, crystal outlines, schistosity. or
bedding from the parent material remain visible. Although
saproliiic weathering may appear to be largely chemical
beeause it is beyond the reach of most roots and burrows,
there is a biological component to this alteration from
termites, fungi, and other tnicrobes, Rci^olitli is another tertn
used for deep weathering profiles, but regolith includes
sediments as well as soil and saprolite. The terms weathering
profile and soil refer to alteration in place, without transport.
Soil is comtnonly eroded and the material redeposited.
Sediment of recognizable soil clasts is called a palolitli or soil
sediment. A redeposited laterite is the original and remains the
best example, because clasts so rich in kaolinite and hematite
are distinctive. Although tnost sediments are derived from
soils,
it is best not to extend the term pedolith to widespread
and far-tra\el!ed alluvium, but restrict it to distinctive soil
materials. The distinction between .sedimentation and soil
formation is fundamental because soils develop in profiles
from the top downward, but sediments accumulate in
sequences from the bottom upward. The theory and practice
of soil science (pedology) and sedimentary geology (sedimen-
tology) are very different, and have further di\erged because
of the traditional association of pedology with agricultural
studies and sedimentology with geological studies.
Soils
Soil profiles are the tangible and diverse products of weath-
ering. We classify them in order to understatid the processes
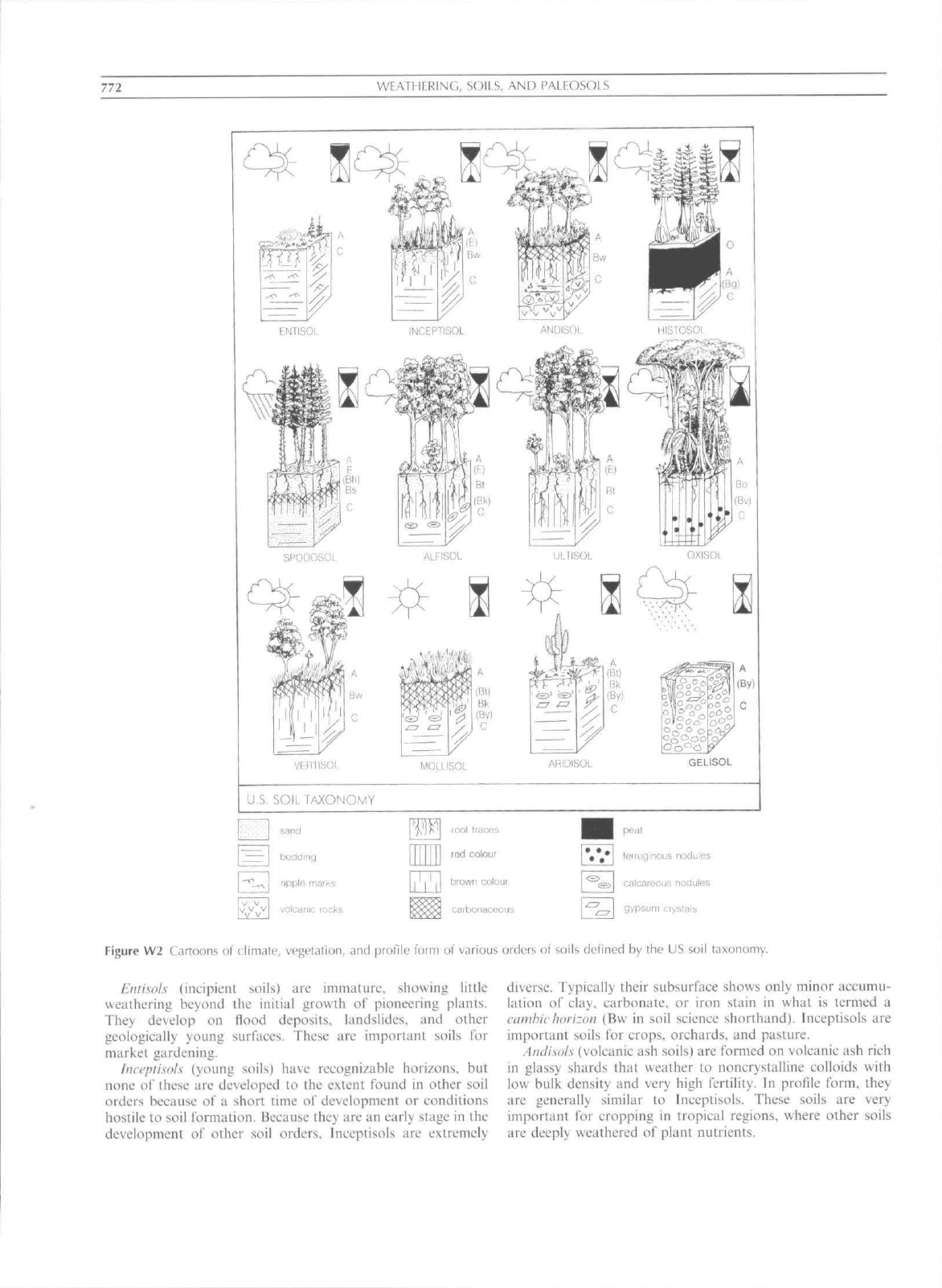
that form them, and to manage their use. The US Soil
Conservation Service recognizes a dozen soil orders
(Figure W2). which are the largest units in a comprehensive,
hierarehical soil classification that is widely used throughout
the world. The basis for soil classification is the sequence and
development of horizons. The process of lessivage. described
above, produces a soil with a leached, sandy, quartz-rich
surface horizon (E or eluvial) over a red. clayey subsurface
(Bt or argillic) horizon. The classification has strict definitions
of the degree of alleration needed for specific horizons, tor
example at least 8 percent additional clay is needed tor an
argillic horizon, with sotne e.xceptions. If the soil has an argillic
horizon that is relatively fertile (with abundant available base
cations) it is an Alfisol, but a soil with an argillic horizon
impoverished in base cations is an Ultisol. Soils can be
identified using a dichotomous key (Figure W3). but the
following paragraphs convey the gist of each soil order and
some of the critical tcrtniiiology in their definition.
772
VVFATHERING, Sdll.S. AND PALEOSOLS
MOLLISOL
ARIDISOL
GELISOL
U.S. SOIL TAXONOMY
sand
bedding
tipple marks
volcanic rocks
red colour
I' I' I brown colour
Sco<x^ carbonaceous
[•
•
.1
, , ,
B ^* iermginous nodules
(2> calcareous nodules
K^_, gypsuFTi cryslals
Figure W2 Cartoons oi climate, vegetation, and profile form of various orders <if soils defined by the US soil taxonomy.
Entisols (incipient soils) are immtitiire. showing little
weathering beyond the initial growth of pioneering plants.
They develop on flood deposits, landslides, and other
geologically young surfaces. These arc important soils for
market gardening.
Imepiisols (young soils) have recognizable horizons, but
none of these are developed to the extent found in other soil
orders because of a short time of development or conditions
hostile to soil formation. Because they arc an early stage in !hc
development of other soil orders. Inceptisols are extremely
diverse. Typically their subsurface shows only minor accumu-
lation of clay, carbonate, or iron stain in what is termed a
ciinihic
horizon (Bvv in soil science shorthand). Inceptisols are
important soils for crops, orchards, and pasture.
.Andisols (volcanic ash soils) are formed on volcanic ash rich
in glassy shards that weather to noncrystalline colloids with
low bulk density and very high fertility, ln profile form, they
arc generally similar to Inceptisols. These soils are very
important for cropping in tropical regions, where other soils
are deeply weathered of plant nutrients.
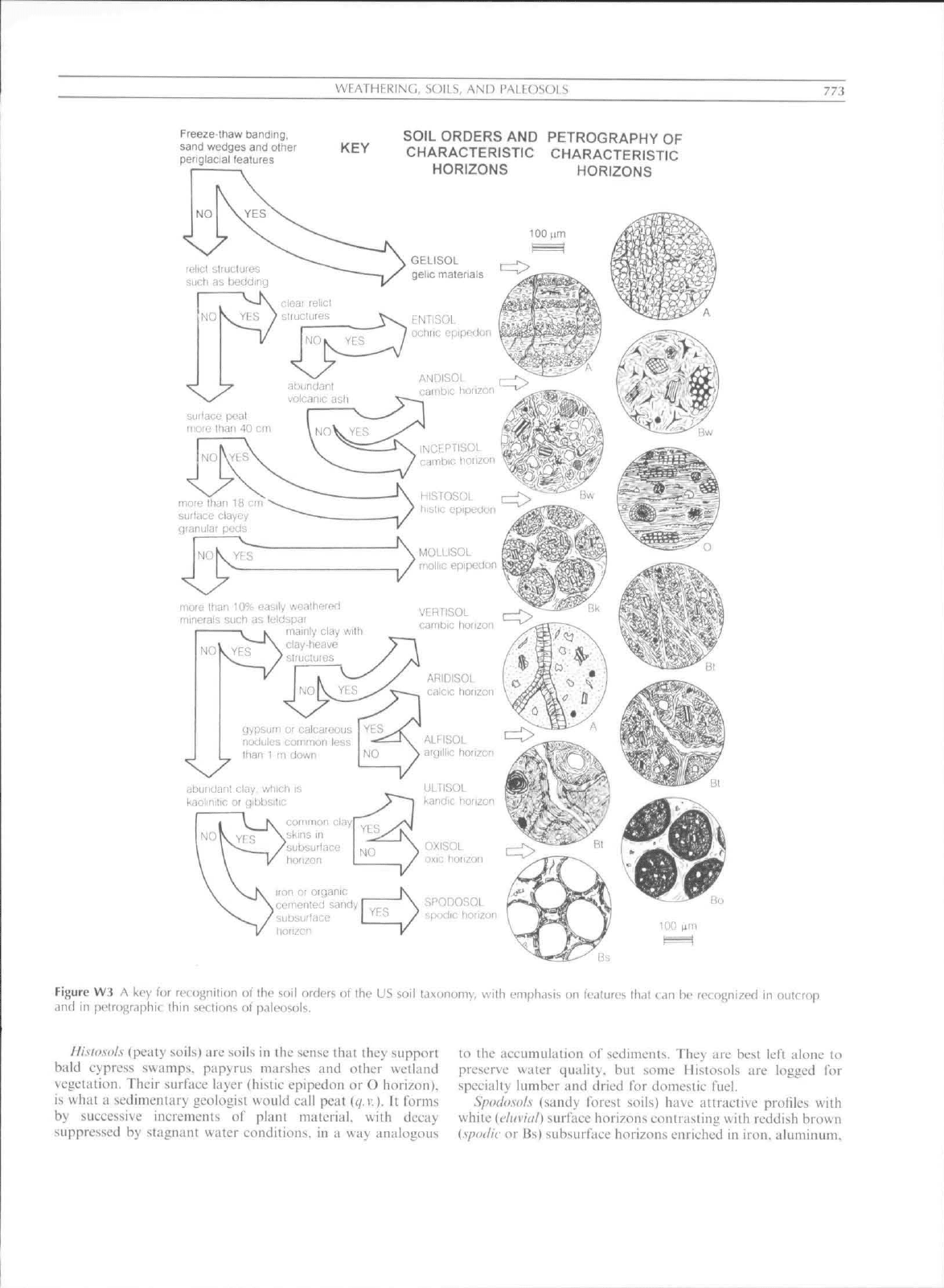
WEATHERINC;, SOILS, AND PALEOSOI
773
Freeze-thaw banding,
sand wedges and other
periglacial features
SOIL ORDERS AND PETROGRAPHY OF
KEY CHARACTERISTIC CHARACTERISTIC
HORIZONS
HORIZONS
reiicl slruclures
such as bedding
clear relict
YFS } slrucUires
EINTISOL
ocliric epipedoi
ANDISOL
carnbic honzc
surface peal
more Ihan 40 cm
INCEPTISOL
cambic horizon
lilSTOSOL
hislic epipedun
more Ihan la err
surface clayey
qranular peds
MOLLISOL
Kioliic epipedoi
more Ihan 10% easily wealhered
minerals such as leldspar
mainly clay with
VERTISOL
cambic horizon
gypsum or calcareous
nodules corTirnon less
than 1 m down
abundant clay, which is
kaolinitic or gibbsilic
iron or otganic
cemenled sandy
subsurface
horizon
SPODOSOL
spodic horizon
Figure W3 A key for rcoignifion of the soil orders of the US soil laxon(,)my, with emphasis on tealures that can be recognized in outcrop
,incl in petrogrdphit thin sections of paleosols.
Hl.sio.stil.s
(peaty soils) arc soils in the sense that Ihey support
b;ild cypress swamps, papyrus marshes and other wetland
vegetation. Their surface layer (histic epipedon or O horizon).
Is what a sedimentary geologist would call peat
{q.v,).
It forms
by successive increments of plant material, with decay
suppressed by stagnant water eonditions. in a way analogous
to the accumulation of sediments. They are best left alone to
preserve water quality, but some Histosols are logged for
specialty lumber and dried for domestic fuel.
Sptidosols (sandy forest soils) have attractive protllcs with
white (i'luvial) surface horizons contrasting with reddish brown
{spotllc or Bs) subsurfaee horizons enriched in iron, aluminum.

774
WEATHEKINC, SOIES, AND PALEObOLS
and organic matter. They are quartz-rich, clay-poor,
and infertile. They support conifer forests and heath that can
tolerate such low fcrlility, and arc used mainly for softwood
lumber production and water quality preservation.
.Atfisots (fertile forest soils) have a subsurface horizon
enriched in clay (ar^ittic or Bt horizon) thai is rich in nutrient
cations. Such clays are typically smectite and illite. These soils
support broadleaf forest vegetation, but are widely cleared for
erops and grazing.
Utiisot.s (base-poor forest soils) have a clayey subsurface
horizon thai is poor in nutrient cations and usually dominated
by kaolinitic clay [tcaiKtic and Bt horizon). Other than this
mineral and chemieal ditlerencc. they appear generally similar
to Allisois, Mixed conifer-broadleaf forest is typical. Once
cleared these soils are fertile enough for cattle grazing,
orehards, and vineyards, but are best left forested for lumber
and water quality preservation.
O.\i.s()t.s
(deeply weathered tropical soils) are thiek, nutrient
poor, and highly aluminous and ferruginous (oxic or Bo
horizon). A eommon micromorphology is sand-sized clods of
hematite and quart/. These soils support tropical rain forest,
but are used for sugar cane, as well as tropical tree crops such
as cocoa, mango, and papaya,
Verttsots
(swelling clay soils) are rich in smeetite clays, whieh
have the physical property of swelling when wet and then
cracking as they shrink and dry. They form in climates with
pronounced seasonality of rainfall. The most distinctive
physical feature of these soils is a pattern of troughs or pits
a few to se\eral decimeters deep (gitgui mieroreliel") between
the pressure ridges around the deep cracks. At depth, the
criss-crossing sliekensided planes and deformed pressure ridges
create a characteristic thinning and thickening of soil horizons
{mukkara structure). Vertisols support mainly grassland and
wooded grassland, and are used primarily for grazing. Their
physieal instability is very destructive of roads, fences, and
buildings,
Mottisot.s
(grassland soils) have a thick, dark, clayey surfaee
of high fertility [motlkepipcdtui or A horizon). This consists of
smail rounded clayey crumbs enriehed and bound with finely
decayed organie matter. Mollisols are widely used for grazing,
as well as herbaeeous erops such as corn and wheat.
AridLsots (desert soils) are little weathered, elay-poor. and
have nodules or layers of ealcitc or dolomite (calcic or Bk
horizon) within a meter of the surface. Salts such as gypsum
may also be found at depth. Some Aridisols are irrigated for
crops,
but water flushes salts to the surface with disastrous
results. Others are used for grazing at low stocking densities,
but most Aridisols remain unused.
Gettsots (permafrost soils) have ground ice or other
permafrost features (gelie materials) within a meter of the
surface. Ice wedges, slone stripes and other deformation
features are characteristie. Some Gelisols support taiga forest
at high latitudes and krummholz at high altitudes. Other
Gelisols support tundra and alpine fellfield. A short growing
season limits agricultural use of Gelisols. although forestry,
reindeer herding and caribou hunting does support some
human activity.
Another way of looking at soils useful in understanding how
they form such diversity is analyzing the factors that create
them:
climate, organisms, topographic relief, parent material
and time for formation. The diversity of soils can be
considered a vast natural laboratory of concurrent experiments
in soil formation. If we wish to study one ofthe faetors in soil
formation such as time, then we should find a group of soils of
comparable climates, vegetation, geomorphic setting and
parent materials but varied time for formation. Such a suite
of soils is called a ctirtmoscquviuc. and mathematical relation-
ships between soil properties and time derived from sueh a
group of soils is called a ctitviiojufu'lion. Examples include soils
of
a
flight of alluvial terraces excavated by a river cutting lower
into its valley during upliit. or soils of moraines left behind by
retreating glaciers. Chronofunctions may quantify the aeeu-
mulation of clay in argillic horizons, of carbonate in calcic
horizons and of peat in histic cpipedons with the progress of
time.
Soils of the chronosequence may have been dated by
radiocarbon, human artifacts or fossils, but the chronofunc-
tions derived from them ean be used to assess the age of soils
nearby that laek dateabic materials. Landscape histories
reconstructed in this way are important for siting permanent
facilities sueh as bridges, dams, and nuclear power plants. In
addition to chonofunctions, comparable approaches can be
used to quantify the role of elimate (dinmfiinction.s). organisms
{tvofunctioiis). topographic relief
(topo/unctinn.s)
and parent
material (lithojuiulkms). In this way it is possible to investigate
the process
O'L
soil formation with rigor.
Paleosols
Soils have
a
fossil reeord
as
paleosols. Most
of
these
are
fossilized
by
burial
in
flood deposits
or
voleanics (Figure
W4),
but some
are
still
at the
surface, either
by
exhumation
or by
Figure
W4 A
mudeni gr.issUind
siiil.
and
Ivvu Lomparjhle
cill Mollisols, buried
in
volcanic sandstones
al the
middk' Miocene
(MM.ii sile
of
Eoft Ternan, Kenya. Hammer
tor
scale
has a
handle
25cm
long.
These
are the
earliest known well-drained grassland soils
in Africa.
