Martienssen W., Warlimont H. (Eds.). Handbook of Condensed Matter and Materials Data
Подождите немного. Документ загружается.

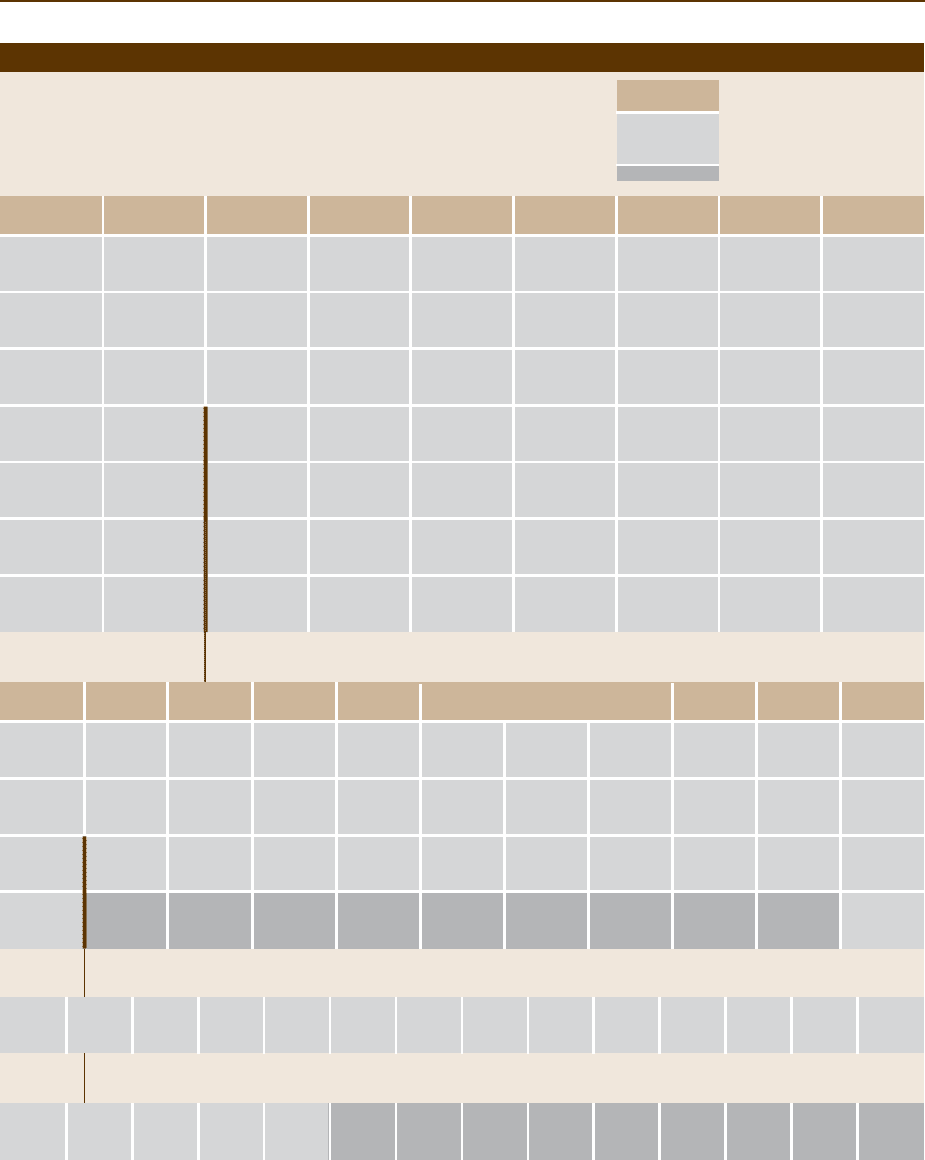
Atomic Number
VIIB
Periodic Table of the Elements
18
VIIIA
Main Groups
Subgroups
Lanthanides (Shells –N–O–P)
Unstable Nuclei
Element Symbol
IUPAC Notation
CAS Notation
2
He
1
H
3
Li
4
Be
5
B
6
C
7
N
8
O
9
F
10
Ne
K
K–L
K–L–M
–L–M–N
–M–N–O
–O–P–Q
–N–O–P
11
Na
12
Mg
13
Al
14
Si
15
P
16
S
17
Cl
18
Ar
19
K
20
Ca
31
Ga
32
Ge
33
As
34
Se
35
Br
36
Kr
37
Rb
38
Sr
49
In
50
Sn
51
Sb
52
Te
53
I
54
Xe
55
Cs
56
Ba
81
Tl
82
Pb
83
Bi
84
Po
85
At
86
Rn
87
Fr
88
Ra
21
Sc
22
Ti
23
V
24
Cr
25
Mn
26
Fe
27
Co
28
Ni
29
Cu
30
Zn
–L–M–N
39
Y
40
Zr
41
Nb
42
Mo
43
Tc
44
Ru
45
Rh
46
Pd
47
Ag
48
Cd
57
La
72
Hf
73
Ta
74
W
75
Re
76
Os
77
Ir
78
Pt
79
Au
80
Hg
89
Ac
104
Rf
105
Db
106
Sg
107
Bh
108
Hs
109
Mt
110
Ds
111
Rg
112
–M–N–O
–N–O–P
–O–P–Q
Shells
12
IIB
11
IB
10
VIII (3)
9
VIII (2)
6
VIB
5
VB
4
IVB
3
IIIB
87
58
Ce
59
Pr
60
Nd
61
Pm
62
Sm
63
Eu
64
Gd
65
Tb
66
Dy
67
Ho
68
Er
69
Tm
70
Yb
71
Lu
Actinides (Shells –O–P–Q)
90
Th
91
Pa
92
U
93
Np
94
Pu
95
Am
96
Cm
97
Bk
98
Cf
99
Es
100
Fm
101
Md
102
No
103
Lr
2
He
VIII (1)
1
IA
2
IIA
13
IIIA
14
IVA
15
VA
16
VIA
17
VIIA
18
VIIIA
Shells
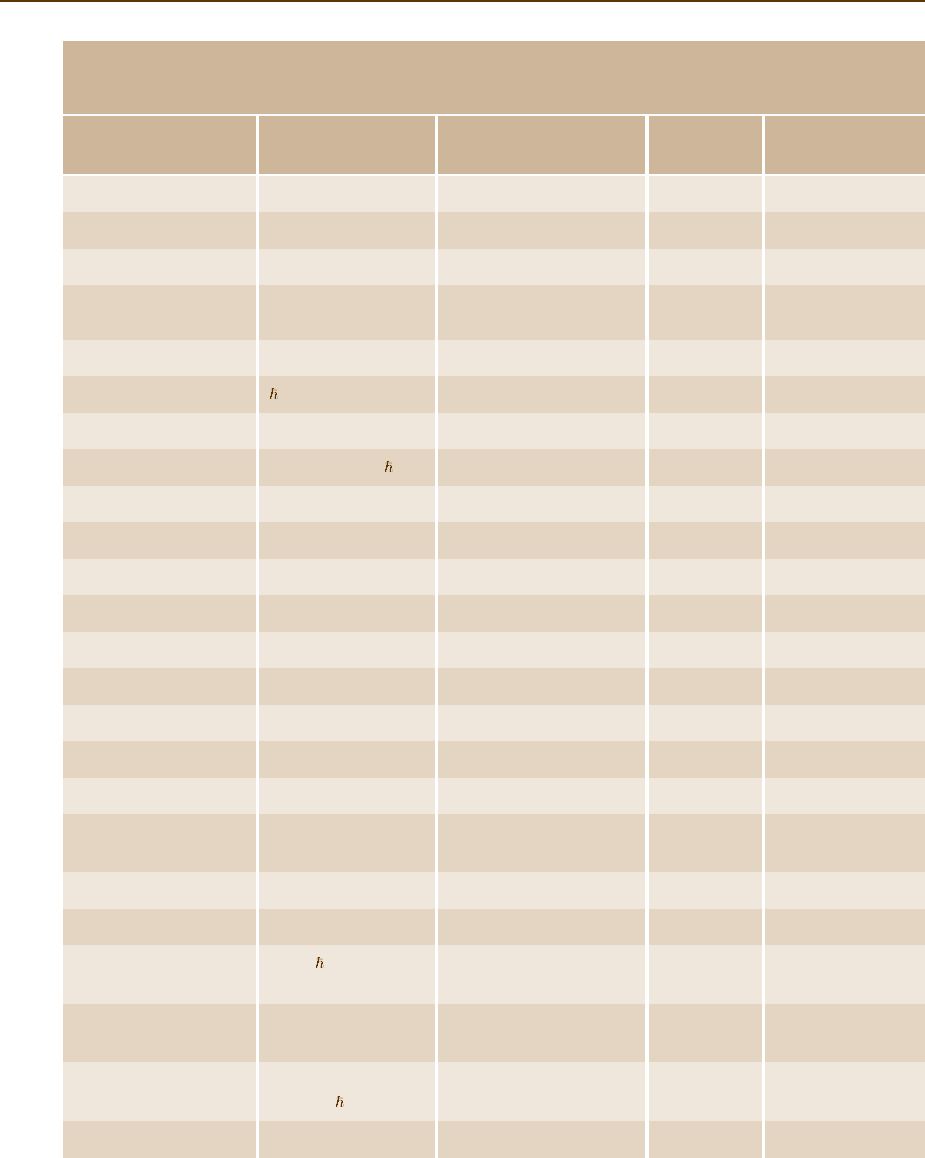
Most Frequently Used Fundamental Constants
CODATA Recommended Values of Fundamental Constants
Quantity Symbol and relation Numerical value Unit Relative standard
uncertainty
Speed of light in vacuum c 299 792 458 m/s Fixed by definition
Magnetic constant µ
0
= 4π ×10
−7
12.566370614 ...×10
−7
N/A
2
Fixed by definition
Electric constant ε
0
= 1/(µ
0
c
2
) 8.854187817 ...×10
−12
F/m Fixed by definition
Newtonian constant G 6.6742(10) ×10
−11
m
3
/(kg s
2
) 1.5×10
−4
of gravitation
Planck constant h 4.13566743(35) ×10
−15
eV s 8.5×10
−8
Reduced Planck constant = h/2π 6.58211915(56) ×10
−16
eV s 8.5×10
−8
Elementary charge e 1.60217653(14) ×10
−19
C 8.5×10
−8
Fine-structure constant α = (1/4πε
0
)(e
2
/ c) 7.297352568(24)×10
−3
3.3×10
−9
Magnetic flux quantum Φ
0
= h/2e 2.06783372(18) ×10
−15
Wb 8.5×10
−8
Conductance quantum G
0
= 2e
2
/h 7.748091733(26) ×10
−5
S 3.3×10
−9
Rydberg constant R
∞
= α
2
m
e
c/2h 10 973 731.568525(73) 1/m 6.6×10
−12
Electron mass m
e
9.1093826(16) ×10
−31
kg 1.7×10
−7
Proton mass m
p
1.67262171(29) ×10
−27
kg 1.7×10
−7
Proton–electron mass ratio m
p
/m
e
1836.15267261(85) 4.6×10
−10
Avogadro number N
A
, L 6.0221415(10) ×10
23
1.7×10
−7
Faraday constant F = N
A
e 96 485.3383(83) C 8.6×10
−8
Molar gas constant R 8.314472(15) J/K 1.7×10
−6
Boltzmann constant k = R/N
A
1.3806505(24) ×10
−23
J/K 1.8×10
−6
8.617343(15) ×10
−5
eV /K 1.8×10
−6
Josephson constant K
J
= 2e/h 483 597.879(41) ×10
9
Hz/V 8.5×10
−8
von Klitzing constant R
K
= h/e
2
= µ
0
c/2α 25 812.807449(86) Ω 3.3×10
−9
Bohr magneton µ
B
= e /2m
e
927.400949(80) ×10
−26
J/T 8.6×10
−8
5.788381804(39) ×10
−5
eV /T 6.7×10
−9
Atomic mass constant u = (1/12)m(
12
C) 1.66053886(28) ×10
−27
kg 1.7×10
−7
= (1/N
A
) ×10
−3
kg
Bohr radius a
0
= α/4πR
∞
0.5291772108(18) ×10
−10
m 3.3×10
−9
= 4πε
0
2
/m
e
e
2
Quantum of circulation h/2m
e
3.636947550(24) ×10
−4
m
2
/s 6.7×10
−9

Return or exchange
only possible when packaging unopened
System requirements:
· Windows 95/98/ME 32 MB RAM, Windows NT 4/2000/XP 64 MB RAM
· MAC OS 9 or higher, 64 MB RAM, 333 MHz recommended
· LINUX Pentium I /166 MHz, 64 MB RAM
