Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

measurements concluded in the formation of Cr-enriched passive films. Mechanistic
aspects of the process have been investigated in detail by Kirchheim et al.
[178,189,190] and several other groups [101,174,191].
Reaction Mechanism of the Anodic Dissolution of Fe-Cr
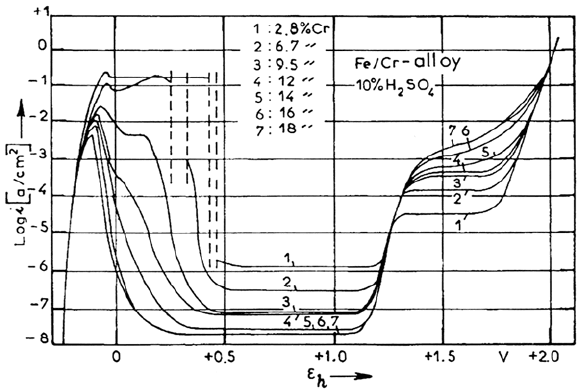
The anodic behavior of Fe-Cr alloys was investigated by using steady-state
polarization curves and sampling by a large number of impedance measurements
over the concentration domain (0–22% Cr) in H
2
SO
4
-Na
2
SO
4
media (0 < pH < 3)
[105,106]. The OH
–
and Cr contents are found to play very similar roles in the
reaction mechanism. The more salient characteristics of the role of Cr are visible
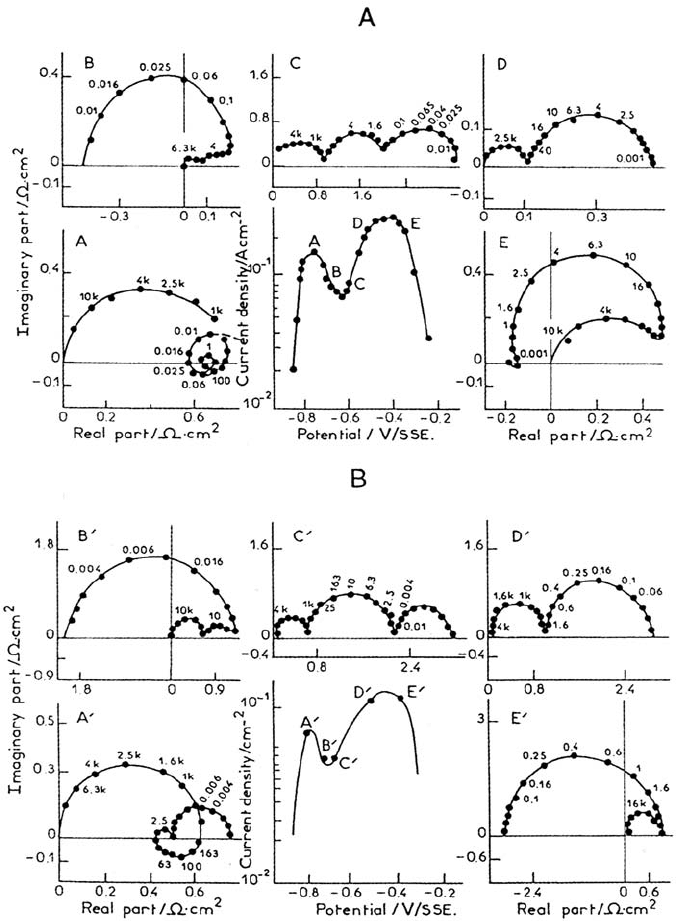
at 7% Cr in pH 0 solution, as shown in Figure 24A, which exhibits a two-peak
polarization curve and two perfectly separated passivation loops.
The passivating feature attributed to Cr at point D (capacitive loop of
characteristic frequency 4 Hz) is therefore already visible at the beginning of the
active domain (point A). Simulations of the current-voltage curves and of the
impedance diagrams were performed on the basis of a mechanistic description
derived from the reaction pattern previously elaborated for pure iron [61,62].
Therefore, coverages by five surface species were introduced in the derivation. The
whole body of data led to taking into consideration, at the same time, the three
types of interaction between Fe and Cr listed earlier namely
1. Alloying effects
2. Surface composition of the alloy distinct from the bulk one
3. Interaction of surface species with the neighbor atoms of either of the two
alloy components
The last interaction was found to be the determining one for interpreting the
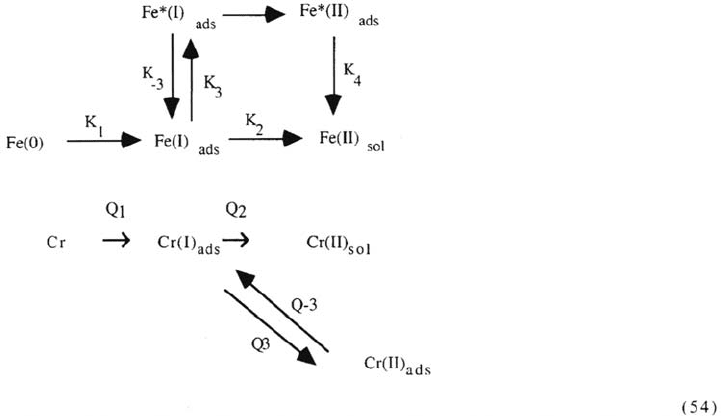
impedance data and the steep onset of passivity. The mechanism is shown below. A
146 Keddam
Figure 23. Current-potential curves of iron-chromium alloys as a function of Cr
content. From ref [163].
Copyright © 2002 Marcel Dekker, Inc.
Anodic Dissolution 147
Figure 24. Experimental (A) and calculated (B) current-potential curves and impedance
diagrams for a Fe-7Cr alloy (IRSID, France). 1M H
2
SO
4
, 25°C. Impedance diagrams at
the corresponding polarization points on the I(E) curve. Frequency in Hertz. Simulations
according to the reaction mechanism (54). From ref [105].
Copyright © 2002 Marcel Dekker, Inc.
specific nonlinear interaction with the chromium passivating species Cr(II)
ads
was
supposed to affect the rate of iron dissolution. Figure 24B shows the result of
simulations of the polarization curves and impedance diagrams. The sharp decay
of the iron dissolution at increasing coverage θ
Cr(III)
by Cr(II)
ads
was expressed by
introducing the factor (1 – θ
x
Cr(II)
) with x ≅ 0.5. A similar interaction function is
adopted in the description of poisoning effects by foreign atoms in binary alloy
catalysts. As emphasized for the first time in Ref. 106, this power law suggests that
the threshold behavior arises from surface percolation of passivated chromium sites.
According to this interpretation, passivation would occur on an incompletely
covered surface via long-range interactions between bare iron atoms and passive
chromium connected in large clusters. A computer simulation later demonstrated
the validity of this idea [184,192]. In the same paper [106], attention was also drawn
to the atomistic description of the catalytic mechanism of dissolution (see earlier)
in the case of a binary alloy. Therefore, purely macroscopic modeling of the
kinetics of dissolution is no longer possible without consideration of the alloy
microstructure, distribution of first and second nearest neighbors, and statistical
composition at the atomic level. Concepts such as the size of clusters of atoms A
and B and percolation phenomena [193] appear as determining and actually have
been extensively worked out (see later).
Analytical and Mass Balance Approaches to Dissolution
and Passivation of Fe-Cr Alloys
An important contribution is due to Japanese authors who developed rotating
double-ring and split-ring disk electrode devices [191] and introduced channel flow
arrangements [85] and data processing in the transient regime [101]. The respective
behavior of Fe and Cr in the presence of Cl
–
was investigated [191] for an Fe-30Cr
alloy. It was concluded that chloride enters the soluble ferro-complex species while
it behaves as an inhibiting species with respect to Cr. By employing time-resolved
148 Keddam
Copyright © 2002 Marcel Dekker, Inc.
measurements on CFDE, previously introduced for iron studies [85], it was then
shown [101] that in a sulfate medium the amounts of Fe and Cr dissolving at
steady state are proportional to their concentrations in the alloy.
Active and Active-Passive Transition; Impedance and Frequency-Resolved
RRDE Measurements The association of impedance and frequency-resolved
RRDE techniques initially introduced for iron [38,117,197] has been extensively
applied to the prepassive and passivation ranges of Fe-Cr alloys with and without
chloride added [174,194,195]. Of course, the interpretation is more intricate than
for pure metals (even with multiple dissolution valences). For kinetic reasons, Cr
species are not detectable on the ring. In order to draw unambiguous conclusions,
reasonable assumptions had to be made concerning simultaneous alloy dissolution
at steady state (see thermodynamics and rate constant approach earlier) and
dissolution valences of Cr.
Completely different behaviors were found depending on whether or not
chloride is present. The salient features are
Emission efficiency for Fe(II) greater than 1 (for Fe-12Cr), an apparent paradox for
a pure metal because that violates the principle of electrical charge conservation
A positive imaginary part of N
d
(passivating charge) in the absence of Cl
–
A negative imaginary part of N
d
(dissolution intermediate) in the presence of Cl
–
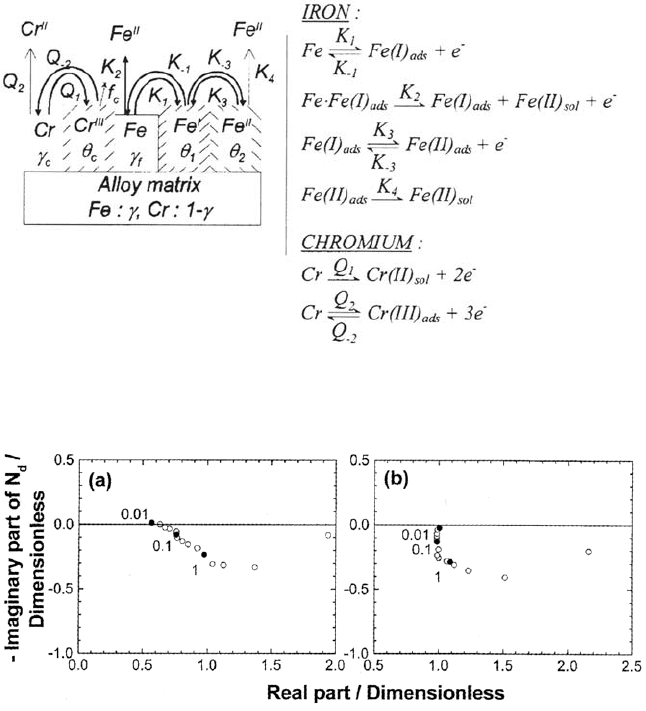
A model depicting the surface processes in terms of reaction paths and topographic
interaction has been elaborated. It incorporates the key points previously introduced
in the model based on impedances [37]. In particular:
Modification of the iron rate constants by chromium
Sharp passivation of the alloy represented by a nonlinear dependence of the
blocking on the Cr species coverage
The reaction model and the corresponding surface picture are shown in
Figure 25. It accounts for the nature and position of the first-order nearest neighbor
for making explicit the interaction and mass balance of the surface species. This
kind of approach was introduced in pioneering work [107] and then worked out in
the framework of percolation models of passivation [180,192]. In view of the process
complexity, an accurate fit is hopeless, but the main features could be semi-
quantitatively simulated, including the amazing N
d
> 1. A comparison of the
experimental and computed N
d
relative to Fe(II) is displayed in Figure 26.
According to the model, this extra emission of Fe(II) with respect to the
electrical current across the electrode can be understood in terms of the contribution
of chemical dissolution of an Fe(II) species by a step such as K
4
.
Later on, the same model was considered for interpreting the results in
chloride-containing media. The main modification was to enhance considerably
the rate of dissolution (catalytic) via the Fe(III)
ads
, whereas in Cl
–
-free media most
of the iron dissolves from the Fe(II)
ads
(step K
2
). The resulting change in the sign
of N
d
arises from the role of the dissolution intermediate of Fe(II)
ads
. These results
supported to some extent those of Ref. 191.
Dissolution in the Passive State In a series of papers by Kirchheim et al.
[173,189,190], the dissolution rates of Fe and Cr in Fe-Cr alloys were investigated
Anodic Dissolution 149
Copyright © 2002 Marcel Dekker, Inc.
within their passive range in H
2
SO
4
solution. Time-resolved chemical analysis of
the solution was performed by atomic absorption spectroscopy of samples of
electrolyte. Selective dissolution of iron during the transient passivation stages
was exploited in terms of Cr enrichment in the passive layer, and once the steady
state was reached, simultaneous dissolution was accurately verified.
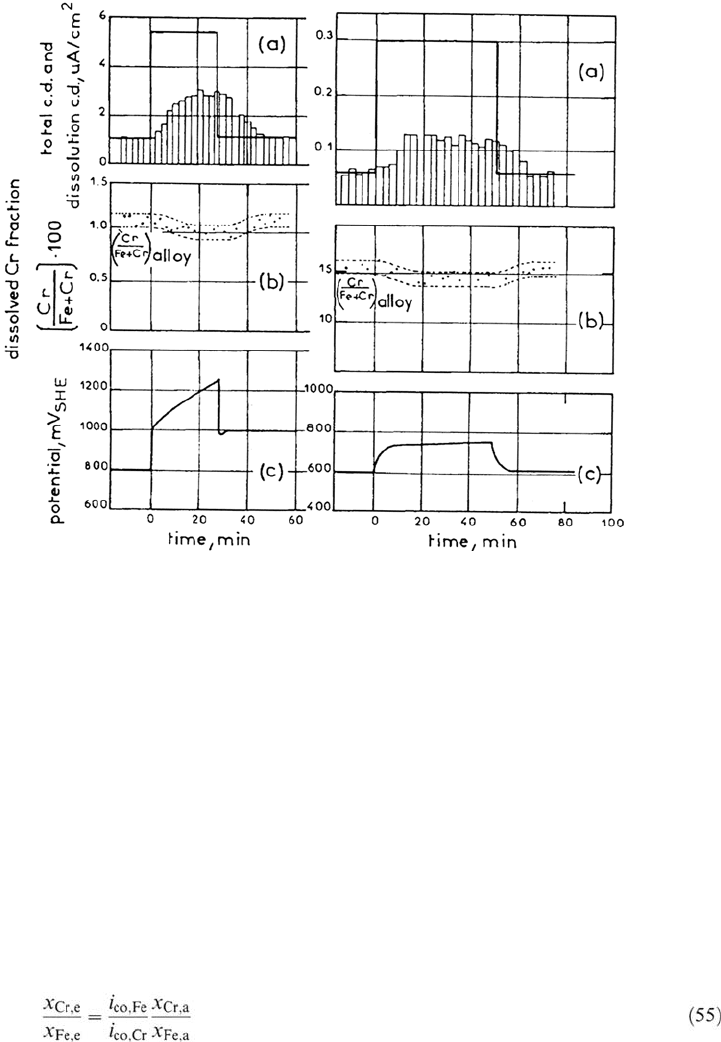
Figure 27 is an illustration of the time-resolved monitoring of the percentage
of Cr in the dissolution products during a phase of film growth triggered by a
galvanostatic square pulse [173]. In-depth concentration profiles of Fe and Cr in
the film were concomitantly measured by XPS, and the significant enrichment in
Cr for corrosion in the passive state was attributed to the outer first layer of the
film. Equation (53) expressing simultaneous dissolution is applied to both the
alloy phases (a) and the film outer layer (e):
i = i
Fe
+ i
Cr
150 Keddam
Figure 25. Schematic representation of the reaction mechanism and of the topographical
interaction of reaction step and surface coverages. From ref [195].
Figure 26. Emission efficiency of Fe(II) for Fe-l2Cr alloy in 0.5 M H
2
SO
4
. In the active
dissolution (“a”: E = 0.07V/SSE) and passivation (“b”: E = 0.l8V/SSE) ranges. From [195].
Copyright © 2002 Marcel Dekker, Inc.
and for the same dissolution valences of both components (3+).
i
Fe
= ix
Fe,a
i
Cr
= ix
Cr,a
where the x’s refer to the molar fractions in the alloy. At the outer side of the film,
the same partial currents are supported by the molar fractions of cations x
Fe,e
and
x
Cr,e
. The ratio of the individual dissolution rates of each of the elements from the
metal (a) and the first layer of the passive film (e) is reflected in the dissolution
currents of the pure metal components in the same passive conditions, i
co,Fe
and
i
co,Cr
, so that
Anodic Dissolution 151
Figure 27. Galvanostatic transients for the dissolution (bars in a), the Cr fraction in the
dissolution products (b) and the potential (c) for two different alloys (left: 1 at.%Cr, right:
14 at.%Cr) after attainment of steady-state. From ref [173].
Chromium enrichment in the film, according to Eq. (53), is directly correlated
with the lower dissolution rate of pure Cr with respect to pure iron. This is consistent
with a lower diffusivity of this element in the film and tentatively explained by the
high-field migration model. However, this is done at the expense of introducing,
nonclassically, the diffusivity in the preexponential factor of Eq. (42) [188]. After
Copyright © 2002 Marcel Dekker, Inc.
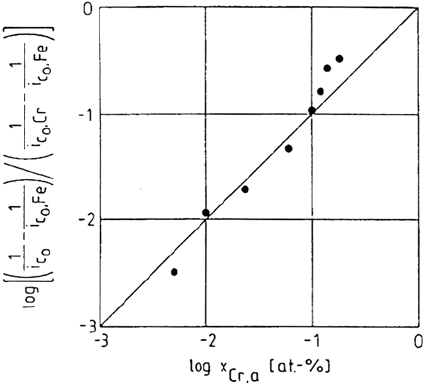
simple transformation, Eq. (55) can be written as a reciprocal dependence of the
passive current of the alloy with respect to its Cr content.
Figure 28 shows an example of the good agreement reported in Ref. 179 and
considered as supporting the model. The model validity has been also discussed in
the case of Fe-Mo and Fe-Al alloys [189]. Subsequently [190], precipitation from
supersatured anolyte in the initial stage of active dissolution was proposed as a
determining step of the passive film growth on alloys with a low Cr content at low
pH. This hypothesis can be traced back to the 1930s [11].
Selective Dissolution
Conditions for simultaneous dissolution generally involve the formation of a 2D
or 3D surface layer in which the relative dissolution rates of the elements are equal
to their alloy fraction. Even in cases where simultaneous dissolution is obeyed, the
process must begin by an initial period of selective dissolution at least at a 2D
level [191]. As briefly mentioned earlier, many mechanisms have been invoked to
explain how, for a nonpassive alloy, the dissolution of the more reactive element
can continue across the dealloyed structure [32,1194]. Surface changes have been
attributed to surface diffusion and recrystallization, redeposition, short-range atomic
rearrangement, and a roughening transition by capillary effects.
Electrochemical measurements coupled with solution analysis were progres-
sively backed up by analysis of the surface and in-depth profiling of the alloy
composition in the dealloyed layer [198]. Current-voltage, current-time, and
potential-time relationships, as a function of the alloy composition and of the degree
of selective dissolution, are the techniques employed extensively. Contributions to
the field using this kind of approach can be found in Ref. 173, 196, 199.
152 Keddam
Figure 28. Relation between the passive current densities, i
co
, and the concentration of
chromium in Fe-Cr alloy, x
Cr.a
according to the model relating the dissolution of the alloy
to the Cr/Fe proportion at the outer side of the film. From ref [179].
Copyright © 2002 Marcel Dekker, Inc.

Anodic Dissolution of Binary Alloys Studied by Electrochemistry,
Solution, and Surface Analysis Techniques
These investigations have dealt with alloys such as AgPd, CuPd, NiPd, and AgAu
under conditions of no passivation. Alloy-electrolyte (LiCl) combinations offer the
possibility of observing both selective and simultaneous dissolution behaviors
depending on the concentration of the noble metal. Two quantities are defined for
describing the amount and in-depth property of the dealloyed surface:
e
B
= surface excess of the more noble metal B:
Anodic Dissolution 153
where X
B
(z) is the mole fraction of B at distance z from the surface (z) and X
B,b
in
the bulk alloy.
S
B
= selectivity coefficient (dimensionless):
where Q
B
is the charge corresponding to the amount of B dissolved (from solution
analysis) and Q
B,th
its amount calculated for simultaneous dissolution by the
same charge (S
B
= 1 corresponds to complete selective dissolution of A, S
B
= 0
to simultaneous dissolution).
The value of e
B
can be estimated from potential-time transients or AES analysis,
and the value of S
B
is determined by weight loss and solution analysis. The potential
increases with the dissolving charge passed at the interface in the selective
dissolution stage, then reaches a critical value E
c
and exhibits a plateau where
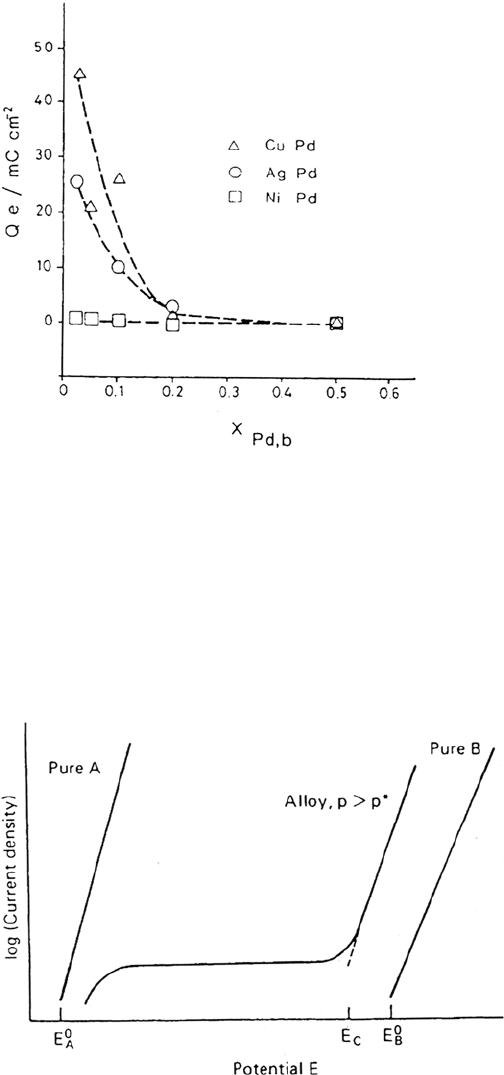
either both components dissolve in stoichiometric proportion or only one keeps
dissolving with formation of surface roughness. On Ag-Pd alloys the e
Pd
values at
the critical potential, estimated by electrochemistry and AES, are in reasonably good
agreement; they show a steady decay when the Pd content in the alloy is increased
and become negligible above 50%. Figure 29 shows an example of this dependence
for three alloys [199]. A threshold of Pd concentration is observed around
0.2, beyond which no Pd enrichment takes place. This can be understood in the
framework of percolation theory (see the next section).
Some mechanistic data on surface enrichment can be inferred. Surface
enrichment is essentially constant after a minimal amount of dissolution (5 mC cm
–2
),
independent of the dissolution rate. Redeposition and surface diffusion are ruled out
in favor of short-range rearrangements of surface atoms. A reciprocal shift of binding
energy between Ag and Pd atoms is assumed to participate to some degree in
simultaneous dissolution of Pd at potentials lower than from the pure metal [156].
Quite identical conclusions are reached for Cu-Pd, Ni-Pd, and Ag-Au alloys [180].
Investigation of the anodic dissolution below the critical potential is likely to
provide more relevant information on the mechanism of selective dissolution [178].
The emphasis is generally put on the current-time relationships, which are supposed
to reflect the mechanism of transport within the surface layer. Dynamic polarization
curves and current-time decays have been investigated for Cu-Au and Ag-Pd in LiCl
Copyright © 2002 Marcel Dekker, Inc.
and acidic sulfate solutions. In all cases, current-voltage profiles display a plateau
followed by a steep increase at the same critical potential E
c
evidenced on the
potential-time transient. The shape of these curve is shown schematically in Figure 30.
154 Keddam
Figure 29. Measured charge corresponding to Pd enrichment as a function of Pd bulk
mole fraction. From ref [199].
Figure 30. Schematic illustration of the anodic polarization curves for a binary A-B
alloy with respect to the curves for the individual elements A and B. From ref [32].
Copyright © 2002 Marcel Dekker, Inc.

In agreement with previous work, the current-time transients recorded at
various potentials below the critical potential are correctly represented by a
reciprocal power law:
Anodic Dissolution 155
regardless of the alloy composition. The interpretation suggested in Ref. 196 is
derived from the model of divacancy diffusion [182]. Diffusivity of the vacancies
is supposed to depend on concentration and potential. The resulting nonlinear
diffusion is most probably responsible for a t
–m
current dependence, as indicated
by numerical simulations.
Actually, even the sophisticated studies combining electrochemistry and surface
analysis seem unable to yield any further decisive information on the detailed
mechanism of selective dissolution. Atomic arrangements in which dissolution of
atoms A can proceed throughout a rough electrode structure enriched in B type
have been recognized as amenable to percolation theory [193]. The same theory
was also applied to the passivation of binary Fe-Cr alloys in which, as suggested
in Ref. 105, 106, passivation of the more soluble element, Fe, is enhanced by a
connected surface lattice of passive Cr atoms. The main power of percolation theory
is to provide diagnostic criteria for computer simulations in terms of concentration
thresholds.
Atomistic Modeling of Selective Dissolution and Related Passivation
by Percolation Theory
The now popular concept of percolation has proved quite successful in many
fields in which a macroscopic property depends on the existence of a connected
path within a two-phase discrete medium, most often a regular 2D or 3D arrangement
of sites (site lattice percolation). Typical features related to percolation are the
existence of a critical phenomenon, for instance, a threshold concentration of
conducting sites when conduction is considered, and of a power law dependence
with respect to the critical quantity in the close vicinity of the critical point. Both
the site percolation thresholds and the power exponents have well-established
theoretical values for any given lattice geometry and connection rules [193].
In spite of its extensive use in the description of heterogeneous systems,
including electrolytic crystal growth, it seems that the percolation concept was
considered relatively recently in the field of electrode processes for explaining
sharply varying properties of alloys. The characteristic feature of a critical concentration
of Zn in α-brass and Al in Al-Cu alloy was correlated with the percolation threshold
on an fcc lattice [175].
Computer Simulations of Selective Dissolution An extensive contribution
was reported [32] addressing the problems raised by experimental dealloying
thresholds p* at variance with theoretical site percolation thresholds p
c
.
Possible interpretations are listed:
1. Dissolution from low-coordination sites (kinks and ledges) faster than from
terraces
2. Not randomly distributed atoms in the alloy
3. Surface diffusion of B (noble atoms)
Copyright © 2002 Marcel Dekker, Inc.
