Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

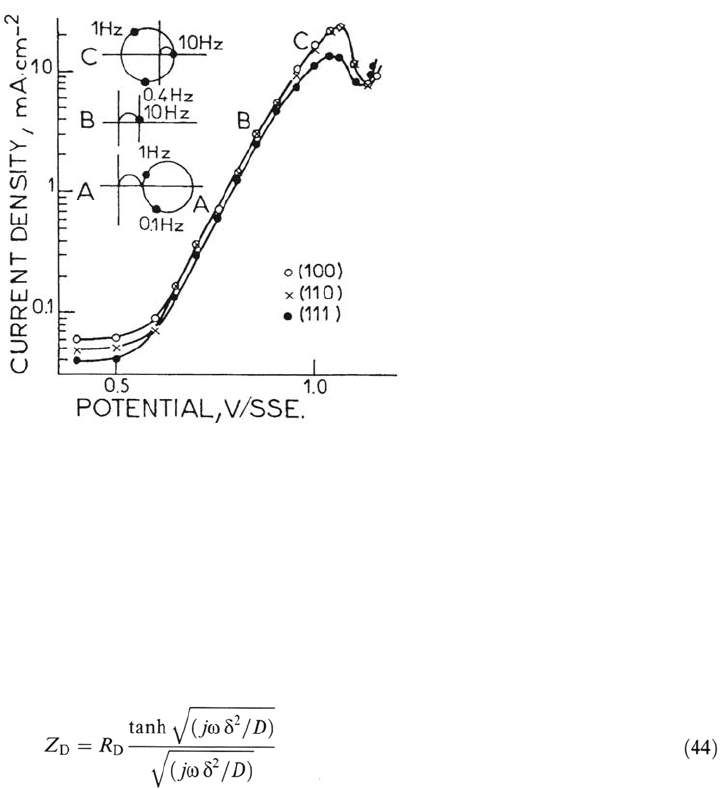
At the minimal c.d., Bp, typical diffusion control is observed consistent with
the local plateau of the current-voltage curve. A diffusion term is associated with a
transport limitation across the passive layer, whereas the inductive loop at the low-
frequency end visualizes the contribution of a decaying film protection at increasing
potentials. Application of a diffusion impedance equation to a finite-thickness layer
(
δ
= 3 nm) given by
136 Keddam
Figure 18. Current-potential curves and schematic impedance diagrams of transpassive
Ni, 3 different single crystal orientations. 1 M H
2
SO
4
. From ref [148].
led to a diffusivity D of the order of 10
–l6
cm
2
s
–1
, This value is in agreement with
solid-state diffusion at room temperature, and a decrease of the film thickness by
a factor of 3 takes place between C
p
and E
p
.
An early paper [145] claimed on the basis of linear Tafel plots that the
dissolution in the transpassive range is controlled by a single charge transfer reaction.
It was then suggested that the electrochemical impedance behaves as a resonant
circuit, i.e., at least two relaxation times [146]. This prediction was directly verified
shortly after Ref. 147. Finally, a thorough investigation by impedance analysis at
various pH values and anion concentrations was at the origin of a more elaborate
model.
Figure 18 shows the current-voltage characteristics measured on three different
crystal plan orientations. As reported in Ref. 145, a Tafel law is observed over more
than two decades, but the impedance shapes display surprisingly intricate kinetics. A
very characteristic impedance feature is observed at point B (but not on the polar-
ization curves), where the impedance behaves like a perfect wave-trap circuit (the
impedance becomes infinite at a resonant frequency ≅ 0.4 Hz). Above this transition
Copyright © 2002 Marcel Dekker, Inc.
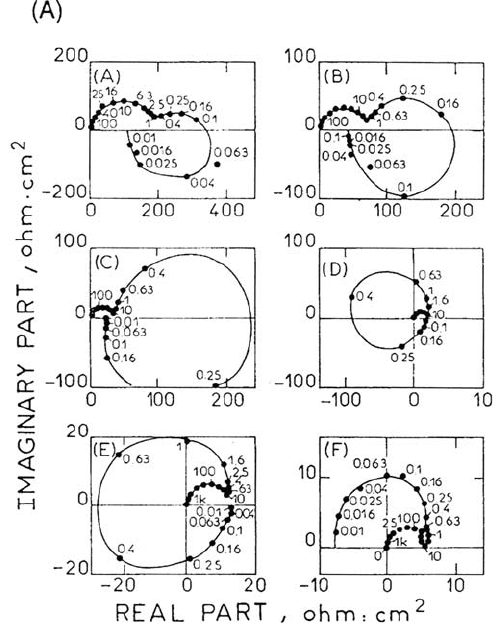
point, e.g., C, the diagram is totally consistent with the electrochemical resonator
described in Ref. 146.
Figure 19(1) shows a detailed evolution of the impedance. The impedance
diagram turning counterclockwise when the frequency is decreased was related to the
reaction pattern shown below elaborated and compared with experiments by
numerical simulations [148].
Here Ni(II) stands for Ni cations pertaining to the lattice of the passive oxide
film and Ni
*
(II) is a cation in the film solubilized by chemical bonding with anions.
The likelihood of this species is also supported by the evidence of Ni=SO
4
band structures in the in situ Raman spectra of the Ni-H
2
SO
4
interface in this
particular potential region. At higher potentials all the film cations are converted into
Ni(III) and Ni enters the secondary passivity region. Numerical simulations are
presented in Figure 19(2) and reproduce with a fair accuracy the main features of
experimental data, particularly the typical change of impedance trajectory between
C′ and D′.
Anodic Dissolution 137
Figure 19. Experimental and calculated impedance diagrams of transpassive nickel
(111) single crystal. 1M H
2
SO
4
, 25°C. (1) Detailed evolution, (2) numerical simulations.
Measurement points are: A: 0.75 V/SSE; B: 0.8 V/SSE; C:0.8 V/SSE; D: 0.9 V/SSE; E: 0.95
V/SSE ; F: 1.1 V/SSE From ref [148].
Copyright © 2002 Marcel Dekker, Inc.
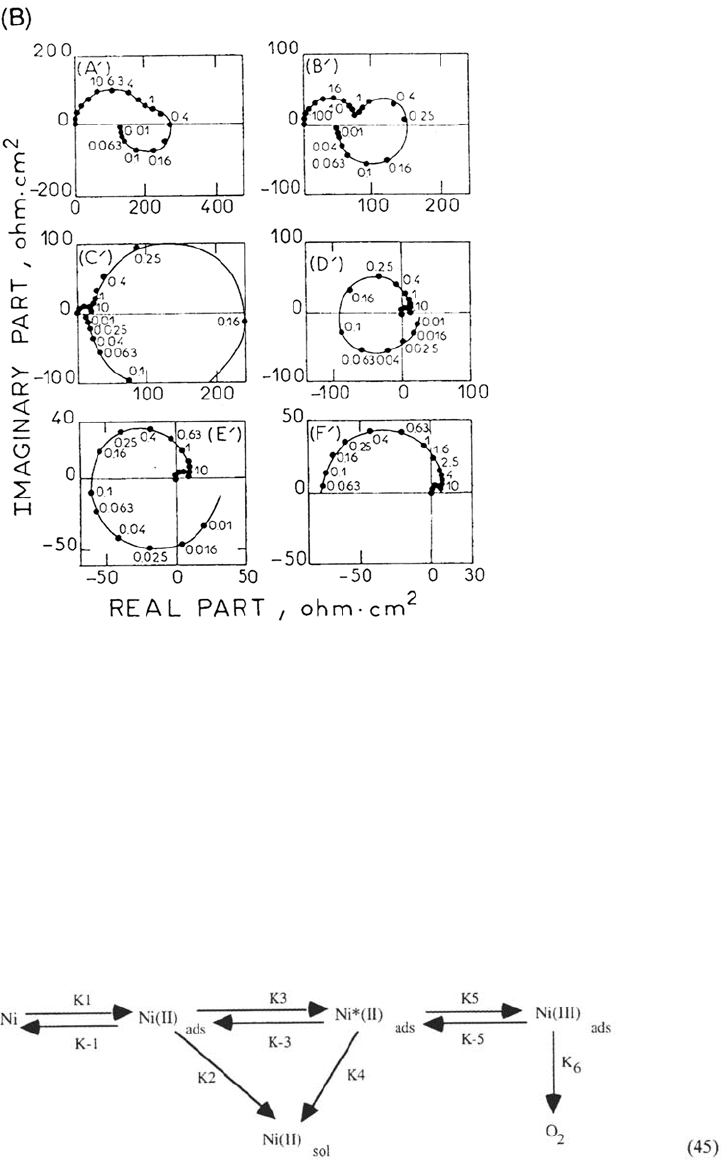
Anodic dissolution of chromium in the transpassive range as well shows a
behavior generally attributed to the oxidation of the Cr(III) of the passive film to
soluble Cr(Vl) oxyanions [43,149,150].
Transpassive dissolution of chromium, molybdenum, and alloys containing
these elements has been extensively investigated by Bojinov et al. [151–153] using
impedance analysis and steady-state RRDE. Additional in situ information
concerning the conductance properties of the films is obtained by CER (contact
electric resistance). Many-parameter models were proposed combining reaction steps
138 Keddam
Figure 19 (Continued)
Copyright © 2002 Marcel Dekker, Inc.

coupled by adsorbed species and bulk conduction in 3D layers controlled by the
mass balance of vacancies.
Anodic Dissolution Under Mass Transport Control
In corrosion practice, the rate of the heterogeneous anodic processes dealt with in
the foregoing sections is ideally low enough that mass transfer is never a limiting
factor. However, fast dissolution kinetics may occur in the following circumstances
of obvious concern to corrosion:
Active dissolution as a transient regime in the initial stages of metal passivity
Dissolution of locally depassivated metals following passivity breakdown (e.g.,
pits, crevices, grain boundaries)
Because of the irreversibility of the dissolution reactions at high overvoltages,
the coupling to mass transfer cannot be simply understood in the framework of
classical transfer-diffusion theory:
A high concentration of dissolved cations in the Nernst boundary layer is
incompatible with the supporting electrolyte approximation.
Limitation of the reaction rate by mass transfer must imply either back diffusion
of an acceptor of metal cations (complexing or solvating species) toward the
electrode surface or growth of a new phase on the surface with limitation of
the reaction rate by ohmic drop or space charge overvoltage.
Little is understood at a mechanistic level because of an almost total lack of
knowledge of the local chemistry within the boundary diffusion layer or solid films.
The problem is essentially investigated in the framework of pitting, crevices, and
stress corrosion cracking [154] by modeling and experiments on occluded cells
(artificial pit designing).
Mass Transport Control and Corrosion
It is generally accepted that transport effects (diffusion and electromigration) are at
the origin of a buildup of metal cation concentration concomitant with a depletion of
H
+
and a overconcentration of anions (for electroneutrality reasons). Homogeneous
chemical reaction can add their own contribution, e.g., hydrolysis of Cr(III) resulting
in a local acidification of neutral media [155].
The most relevant consequence of the change of interfacial chemistry for
corrosion is undoubtly the modification of the active-passive transition and the
possible generation of self-sustained large-amplitude oscillations between active
and passive states [156,157]. These phenomena were tightly associated with the
manifestations of passivity from the earliest research, particularly in the case of
iron in acid solutions. Theoretical investigations imply difficult nonlinear
mathematics. A significant renewal of the field was observed in relation to the
increasing interest in nonlinear phenomena and such concepts as bifurcation theory
and chaos in chemistry [158–160].
In spite of this new sophistication, the basic model is still that due to Franck and
FitzHugh [156] in which the following reactions are supposed to participate in a
cyclic sequence.
An oversimplified form of dissolution and passivation reaction is considered:
Anodic Dissolution 139
Copyright © 2002 Marcel Dekker, Inc.
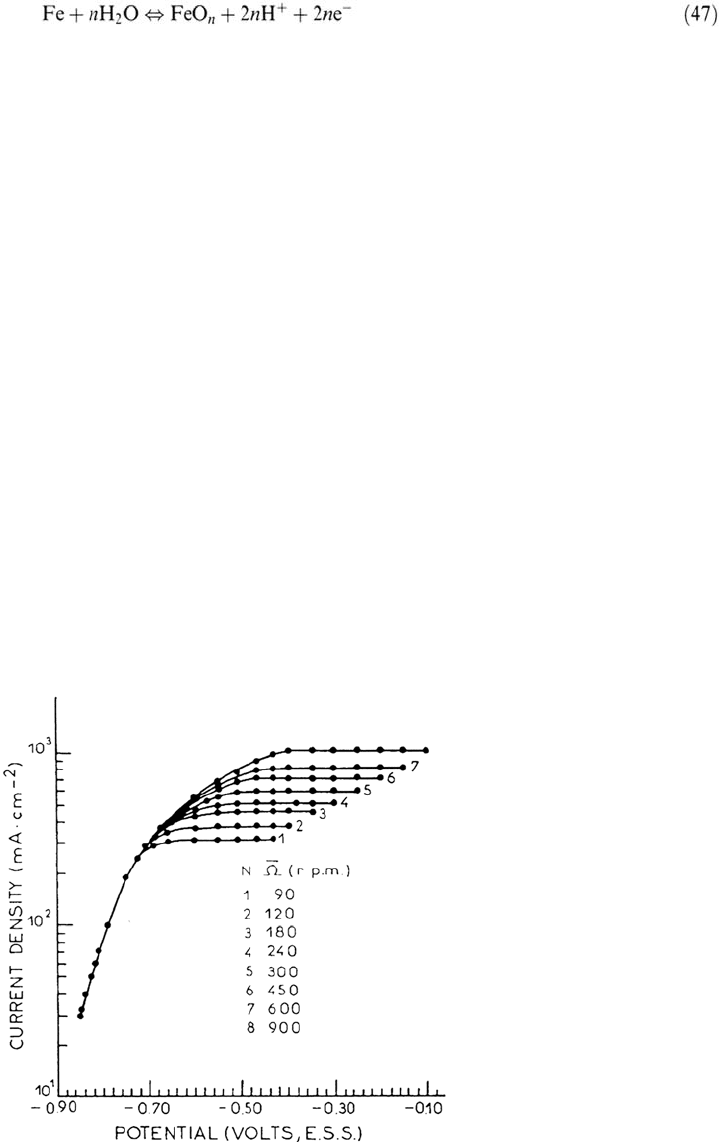
Reaction (46) at high dissolution rates tends to decrease the pH by H
+
electromigration and to shift the passivation equilibrium reaction (47) to the right.
At the same constant potential, the iron surface turns into the passive state, and
back diffusion of H
+
takes place and restores the active dissolution by displacing the
passivation equilibrium to the left. The same sequence restarts, generating a periodic
regime. It should be observed that this model ignores the likely participation of salt
layers (FeSO
4
,7H
2
O) and of ohmic drop and is unable to explain the coexistence
at a same electrode potential of side-by-side active and passive areas, i.e., of stable
localized corrosion coexisting with a passive sample. The latter self-stabilizing
process of dissolution is very generally ascribed to the depassivating role of Cl
–
,
enriched by electromigration toward the active areas. However, similar
behavior can be observed in chloride-free solutions [33,74].
Active Dissolution of Iron under Mass Tranport Control
The anodic dissolution of iron in sulfuric acid medium exhibits, with well-defined
convection on a rotating disk electrode, a current plateau as shown in Figure 20,
attributed to the limiting rate of transport between the dissolving surface and the
solution bulk [161]. The curves shown in Figure 20 suggest that some critical
phenomenon is associated with the common branching point from which all the
curves merge toward their plateau region, whereas they overlap perfectly below.
The nature of the process controlling the heterogeneous steps was investigated by
a detailed analysis of the EHD impedance.
140 Keddam
Figure 20. Current-potential curves for iron at different rotation speeds. Johnson-Matthey
iron. 1.8 M H
2
SO
4
, pH = 0, 25°C. From ref [161].
Copyright © 2002 Marcel Dekker, Inc.
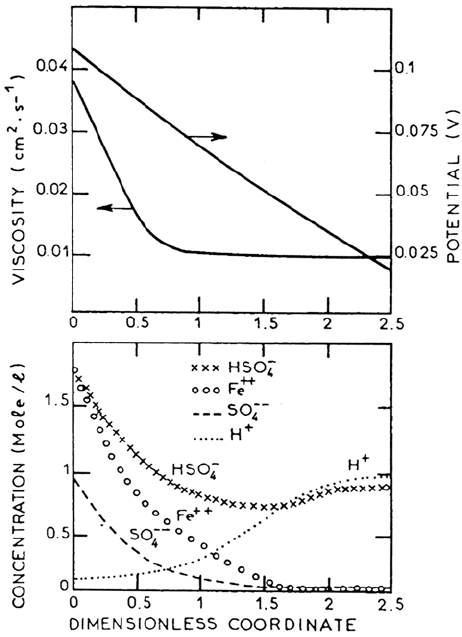
EHD impedances are interpreted in terms of the existence of a viscosity
gradient in the boundary layer estimated to be four- to fivefold between solution
bulk and electrode surface. On this basis, the profiles of species participating in
the mass and charge transport have been computed and are plotted in Figure 21.
The interfacial concentration of Fe
2+
is in good agreement with solubility
data. The increase of pH by H
+
migration is reflected in the profile of SO
2–
4
.
It is concluded that no barrier layer is involved in the mass transport control of
iron dissolution. The heterogeneous reaction rate is reported as not dependent
on the HSO
–
4
concentration; therefore, the only acceptor species likely to limit
the dissolution rate is water [74]. This is compatible with the modified form (29)
of the initial step of dissolution in which one water molecule is dissociated and
the depletion of free water at the electrode surface by Fe(II) hydration and
electromigration of hydrated Fe(II) away from the surface.
Anodic Dissolution Controlled by Transport in the Presence
of Solid Layers
In many practical cases of corrosion, the anodic dissolution takes place on a surface
partially or totally covered by thick (order of micrometers) solid layers grown by
Anodic Dissolution 141
Figure 21. Concentration field calculated under the conditions of Figure 20. Top :
viscosity and potential profiles. From ref [161].
Copyright © 2002 Marcel Dekker, Inc.
several possible mechanisms (heterogeneous nucleation and growth, dissolution-
reprecipitation) and usually regarded as porous. Until the past decade the mechanism
by which this layer interferes, with the anodic dissolution, and particularly the
transport phenomena, remained poorly understood. The last developments deal
with iron and copper in HCl solutions and take advantage of steady-state RDE and
RRDE associated with AC impedance (EIS and EHD) techniques. Mass transport
and solution chemistry [162–163] let to a dissolution model [164] in which the
electrochemical monoelectronic surface step:
142 Keddam
is followed by a series of complexation reactions, the more likely being
More advanced RDE studies were interpreted by the formation of a porous 3D
layer of CuCl
ads
[156] in a step such as
Because of the reversibility of reactions (48) and (49), both Cl
–
and CuCl
–
2
diffusing
in opposite directions may control the dissolution rate. In the plateau region it was
shown [164] by RRDE that compact coverage is attained. Therefore in the model
elaborated in Ref. 165 the contribution of the layer to the rate of dissolution is
twofold and constitutes the central ingredient of the model: the CuCl film is
considered at the same time to dissolve at its outer boundary with the electrolyte:
CuCl
film
+ Cl
–
→ CuCl
–
2
and to limit the rate of Cl
–
diffusion to the Cu surface to form the film by reaction
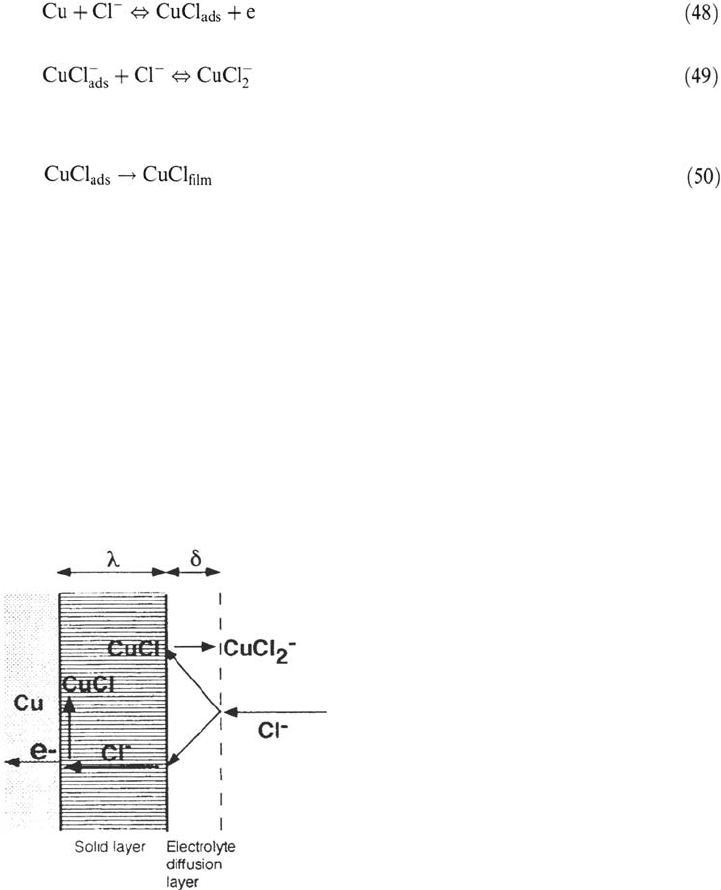
(48). This form of the model is sketched in Figure 22.
Figure 22. Model of dissolution of copper through a solid layer growing by a Tafel
kinetics at the metal interface and dissolving by a chemical kinetics at the solution
interface. Adapted from ref [165].
Copyright © 2002 Marcel Dekker, Inc.
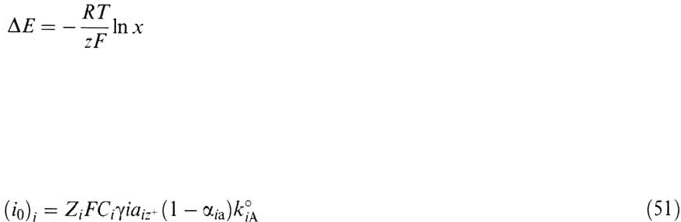
It is noteworthy that this model is formally similar to the point defect model
(transport of a metal acceptor toward the metal surface, film growth at its inner
interface, dissolution at its outer one) shown in Figure 15. EIS and EHD responses
associated with this model have been derived [165] taking into account the diffusion
of both Cu
–
and CuCl
–
2
in the solution (diffusion layer δ) and of Cl
–
through the
film (diffusion layer λ) and allowing for the modulation of the layer thickness by
the AC potential, an effect discarded in Ref. 164. Comparison with the experiments
substantiated the model. The mass transport by diffusion and migration and the
potential drop at soluble anodes covered by salt films have been dealt with [166,167]
by assuming compact (saturated) or porous (supersaturated) coverages (wet) on iron.
Even more intricate situations on nickel have been tentatively approached [168].
ANODIC BEHAVIOR OF ALLOYS
A detailed understanding of the electrochemistry of alloys is still far from being
achieved on the basis of the rather crude theory of pure metals. The complexity is
expected to increase more rapidly than the number of components (cross-
interactions) and it is doubful whether a unique mechanism can be at work in view
of the extreme variety of compositions. As far as mechanistic information is
concerned, only homogeneous (single phase) and binary alloys are considered in
this section.
The Basic Concepts: A Survey
Thermodynamics and Rate Constant Approaches
It was thought very early that the change of rest potential and of dissolution rate with
alloy composition cannot be understood without assuming surface modifications
induced by the anodic processes themselves [169]. The nature of these modifications
has been the aim of many electrochemical and surface analysis studies but is still
not fully elucidated.
In an ideal case, in the absence of any free energy of mixing, the free energy
of a metal in an alloy with respect to that of the pure metal is given by
ΔG = RTln x
where x is the molar fraction of the metal in the alloy, and the corresponding shift of
electrode potential is
Anodic Dissolution 143
This equation is obeyed only by a few alloys and dilute amalgams [10]. The
individual dissolution rates of Cu and Ni in Ni-Cu were investigated as a function
of alloy composition [169,170]. In the framework of the activated state theory, a
standard rate constant of the solution of the ith component of an alloy, k°ia, is
calculated from the exchange current density at the standard potential:
where γ
i
C
i
= activity of the element in the alloy
Copyright © 2002 Marcel Dekker, Inc.

a
iz
+
= activity of the element cation in the solution
α
ia
= transfer coefficient of the metal i–cation equilibrium
The k°ia is found independent of the alloy composition up to 60% Cu, above
which a new phase is invoked.
This purely formal kinetic treatment is of very little utility because it is quite
unable to dissociate the more elementary contributions to the alloy electrochemistry,
namely from metal solid-state physics to surface physical chemistry:
1. Change of elementary rates of charge transfer by electronic interaction in
alloy structure (alloying effect)
2. Change of the surface composition of alloy by selective dissolution of the less
noble element
3. Growth of 2D or 3D layers resulting from chemical or electrochemical
reactions of the alloy components with the solution
Following Rambert and Landolt [172], dissolution of single-phase alloys can be
classified in two categories:
Simultaneous dissolution, where at steady state the alloy elements go into the solution
at a rate proportional to their atomic concentration in the alloy. Well-known
examples pertain to the iron-base stainless steels and especially ferritic Fe-Cr,
on which this type of dissolution is repeatedly found [173,174].
Selective dissolution, where the less noble element dissolves selectively leaving
behind a “porous” metal phase enriched in the more noble constituent,
resulting in dealloying. The more common case is the selective dissolution
of Zn (dezincification) from α-brass (Cu-Zn). Selective dissolution is in
some instances associated with circumstances of stress corrosion cracking
(SCC) [175].
Simultaneous Dissolution and Associated Formalism
The basic formulation was elaborated sometime ago [175,176]. A linear relationship
of the form
144 Keddam
was suggested for representing the dissolution current of an alloy AB as a function
of the elementary current densities of A and B, γ
0
and (1 – γ
0
) being the atomic
proportions of A and B [commonly denoted A – (1 – γ
0
)B].
It was simultaneously assumed [178] that, as a result of the alloy dissolution,
its surface composition, (γ, 1 – γ), can deviate from the bulk one. Consequently,
the proportionality of the atomic fluxes of A and B going into the solution to their
alloy content is expressed by
where Z
A
and Z
B
are the dissolution electrovalences.
In principle, Eq. (53) yields a γ
0
value matching simultaneous dissolution at
any potential, I
A
and I
B
being known from the individual curves of A and B in the
same electrolyte. In general, there is no particular reason why this γ
0
would restitute
Copyright © 2002 Marcel Dekker, Inc.
the correct value of I
AB
. In the case of Fe-Cr alloys, the essential features of the
current-voltage characteristics in the transition range (7 to 12%) are not satisfactorily
reproduced [178] and nonlinear interactions must be introduced [105,106].
Application of EIS to this problem in the case of Fe-Cr alloys in the active and
passivation ranges is illustrated in the following. In the full passive domain,
compositional changes in the passive film, determined by XPS, play a role similar
to the adjustement of the surface composition of the alloy phase to achieve the
simultaneous dissolution [179]. According to Ref. 180, percolation phenomena,
apparently relevant to selective dissolution, could also explain some features of the
dissolution of Fe-Cr in the incompletely passivated state.
Selective Dissolution
Different mechanisms have been proposed to explain the selective dissolution of
alloys and the formation of a porous dealloyed layer. A dissolution-redeposition
mechanism [180] was proposed for α-brass dealloying. A larger group of models
requires the description of atomistic processes of restructuring of the more noble
atoms A in order to allow the dissolution of the more soluble element B to proceed
across an A-enriched porous layer. A critical review of the mechanisms likely to
participate in these surface phenomena can be found in Ref. 32. It was suggested
[182] that the rate-determining step is a solid-state diffusion of the less noble
atoms via divacancies. Surface diffusion can also be taken into account. Roughening
by a mechanism of “negative” aggregation known to generate fractal interfaces
[161] constitutes a fruitful approach. In the 1980s, a series of papers [175,180,184]
underlined the existence of critical compositions and of a threshold concentration
of the less noble constituent below which no dealloying appears. This sharp
dealloying threshold is not consistent with any of the diffusion-based models of
selective dissolution, which are supposed to produce essentially continuous
behaviors. A model based on percolation phenomena was proposed and extensively
worked out. Its main background and developments will be exposed.
Simultaneous Dissolution: Fe-Cr Alloys
In contrast to selective dissolution, evenly dissolving alloys can be dealt with, up
to a sophisticated level including non-steady-state responses, by macroscopic, i.e.,
kinetic descriptions. As shown in Figure 23, Olivier [1851] pointed out that the
steady polarization curves of Fe-Cr alloys in a 0.5 M sulfuric solution display the
decay of active and passive currents with increasing Cr content and the emergence
of the transpassive dissolution of Cr to the hexavalent state.
The studies have focused on two aspects of the behavior of Fe-Cr: the
modifications brought about by the addition of chromium to iron in the active
dissolution and prepassive ranges on the one hand and in the passive state on the other
hand. Electronic interaction by filling up of the d level of Fe [186] was put forward.
Most of the subsequent contributions concluded in some progressive change from
an iron-to a chromium-like behavior without a further detailed mechanism in the
absence of a satisfactory model for pure iron on its own. A particular ability of Cr to
enhance the passive state of iron, even with low surface coverage, was repeatedly
reported [187,188]. Regarding the passive state, advances of in situ surface analysis
[Auger electron spectroscopy (AES) and XPS] associated with electrochemical
Anodic Dissolution 145
Copyright © 2002 Marcel Dekker, Inc.
