Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

As illustrated in this chapter, Eq. (6), in parallel with the double-layer
capacitance C
dl
, generates identifiable shapes on the impedance curves in the Bode
or Nyquist plane making possible to determine the number of chemical entities C
s
j
and C
j
participating in the reaction mechanism and thus providing information on the
reaction pattern. In terms of dissolution-passivation processes, capacitive responses
and negative resistances are related to inhibition or passivation whereas inductive
behaviors arise from catalytic effects or activating intermediates [4–8]. Acquisition
and processing of the transient response of electrochemical systems are easily
performed by modern laboratory equipment [5,6,49] and do not deserve special
attention in this chapter.
Local Electrochemical Measurements Practically any real-life solid electrode
exhibits, for structural and/or geometric reasons, heterogeneities in surface properties
and therefore in reactivity. The characteristic dimension may range between the
nanometric scale and the macroscopic size of the electrode. The traditional
electrochemical measurements provide surface average quantities, in both current
and potential. The information can be extremely biased, in somes cases totally
obscured, when sharp differences in reactivity are present. This is often the case for
anodic dissolution under the influence of metallurgy and/or composition. In order to
overcome the problem, techniques have been introduced for collecting local values
of the potential and current densities at short distances above the electrode surface.
Potential probes have long been known for attenuating the ohmic drop (Haber-
Luggin capillaries). Current probes have been developped intensively in the last 15
years. They are based on the measurements of the ohmic drop along a short current
path in the solution. Most of the studies used the so-called SVET technology
(scanning vibrating electrode technique) or to a lesser extent twin electrodes.
Current mapping thus obtained, with a spatial resolution at best about 15 μm, is
essentially applied for imaging galvanic currents associated with local cells in
corrosion. The techniques were extended to transient regimes, and spatially resolved
impedance measurements (LEIS, local electrochemical impedance spectroscopy)
[50–54] are now available with valuable performance. Undoubtedly, serious
advances in the interpretation of the kinetics of anodic dissolution can be expected
in the near future from the growing application of these techniques.
Complex transmittances relevant to corrosion phenomena have been introduced
[36,38,39]. Examples are given in several parts of the chapter. Two of them deserve
a particular interest. The case of techniques pertaining to the rotating ring disk
electrode (RRDE) is dealt with here. Electrogravimetric transmittance, a frequency-
resolved technique based on the electrochemical quartz crystal microbalance
(EQCM), was presented in a paper in 1996 [55].
Background of Time/Frequency-Resolved Measurements with an
Upstream (Emitter)–Downstream (Collector) Electrode Setup
These techniques are based on the discrimination between the dissolution and the
surface film growth component of the working electrode current [56]. The following
derivation makes used of the RRDE parameters indexed D (disk) and R (ring). The
transposition to an upstream-downstream pair of electrodes in any kind of flow
cell (e.g., channel flow double electrode, CFDE) is straightforward.
106 Keddam
Copyright © 2002 Marcel Dekker, Inc.
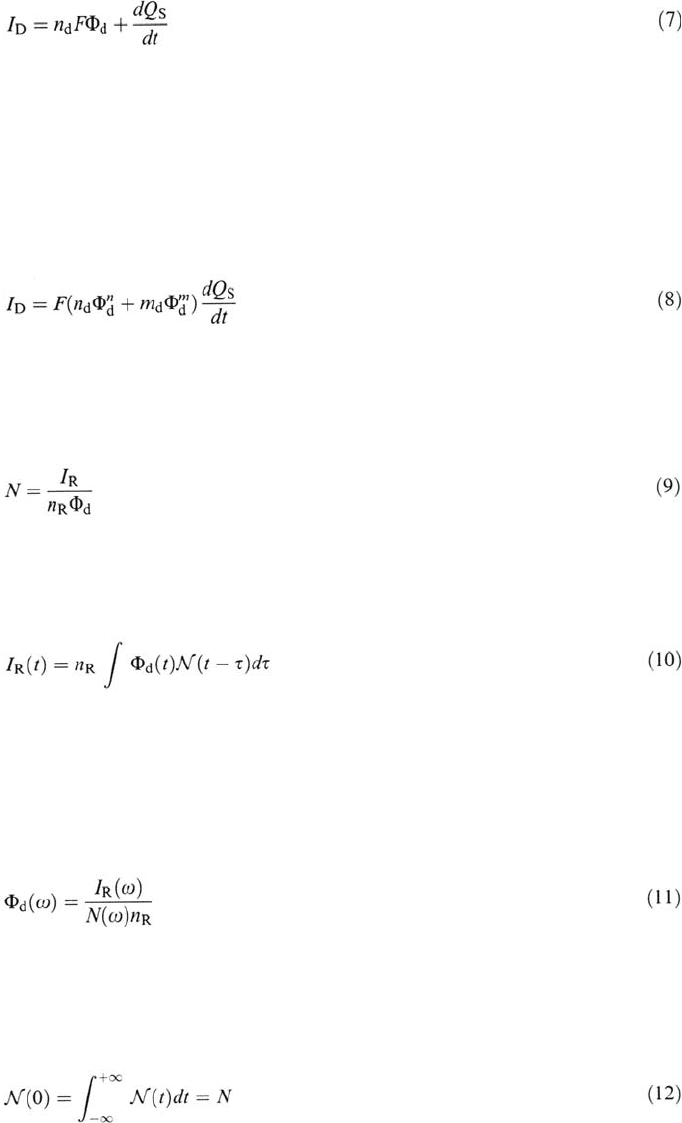
Electrical Charge Balance In the non-steady-state regime the electrical charge
balance equation at the disk surface is
Anodic Dissolution 107
with I
D
= the electrical current supplied to the disk (at the exclusion of the
double-layer charging)
n
d
= the dissolution valence of the disk metal
Φ
d
= the flux of n
d
-valent cations released in the solution
Q
S
= the faradic charge stored in surface layers
Equation (7) can be extended to the emission of more than one species with
different oxidation states: n
d
, m
d
,…
The Collection Efficiencies A fraction of the flux Φ
d
is collected and converted
into an electrochemical current I
R
on a downstream electrode owing to the transfer
of nR electrons in a convenient redox reaction. At steady state, the dimensionless
ratio
called the collection efficiency is determined entirely by the electrode layout. At the
non–steady state, Φ
d
is obtained:
In the time domain, through a deconvolution because one has
where N(t) is the characteristic transient collection efficiency of the device
experimentally defined for Φ
d
(t) = Dirac function δ(t). Unlike N, it depends
on both the electrode layout and the hydrodynamic parameters (fluid velocity,
viscosity, diffusivity).
In the frequency domain, through a simple division by the complex collection
efficiency N(
ω
), which yields
The collection efficiencies are most easily determined by calibration experiments
on redox systems. In practice, the deconvolution of (10) is carried out by FFT
(fast Fourier transform) using the classical equation N(
ω
) = Fourier transform
of N(t) and
Copyright © 2002 Marcel Dekker, Inc.
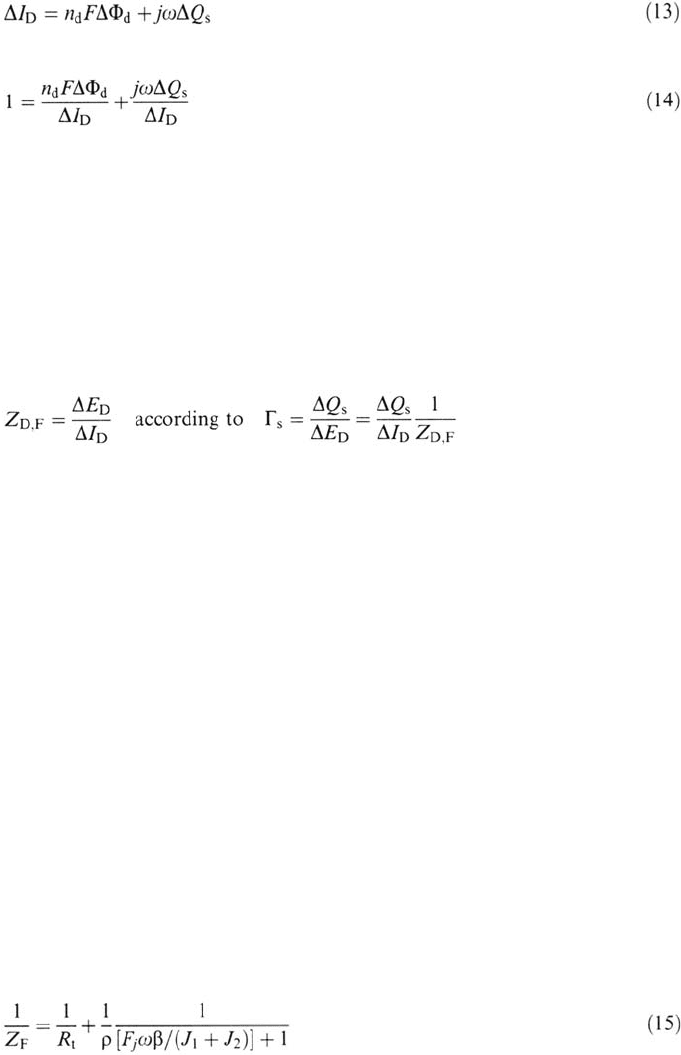
Emission Efficiency and True Capacitance Exploitation in the time domain
of the differential (7) or of the integrated form Q
d
= n
d
FP
d
+ Q
s
, where P
d
is the
amount of species generated by the disk dissolution, yields as the main information
the charge Q
s
involved in the surface layer. In the frequency domain, if Δ stands
for small-amplitude variables, (7) becomes
108 Keddam
or
where the ratio N
D
= (n
d
FΔΦ
d
) / (ΔI
D
) = 1 – (j
ω
ΔQ
s
)/(
Δ
I
D
) is the emission efficien-
cy of the disk, a complex dimensionless quantity representing the fraction of the
disk current actually consumed in dissolution. Graphs of N
d
in the complex plane
provide a direct criterion about the kinetic role of the surface charge Q
s
. For instance
(ΔQ
s
) / (ΔI
D
) < 0; i.e., charge buildup producing a decrease of the dissolution cur-
rent (e.g., an electrochemically grown passivating layer) is displayed as a positive
imaginary part.
In order to get the true electrode capacitance Γ
s
, related exclusively to the film
growth, Eq. (14) can be further coprocessed with the faradic impedance of the disk:
The application of the technique in the time and frequency domains is illustrated
hereafter for the passivation of iron in acidic solutions.
Anodic Dissolution of Metals and Inductive Behaviors
It has long been recognized, originally in the time domain (transient measurements)
and later in the frequency domain (impedance measurements), that the non-
steady-state response of anodically dissolving metals displays in most cases an
inductive-like behavior. With the galvanostatic step used in the early work, this
behavior showed up as a voltage peak (overshoot), its counterpart in potentiostatic
mode being a minimum of current density. In the frequency domain an inductive
loop of the Nyquist plot was first identified by Keddam et al. [43–46] in the case
of iron in acidic media and interpreted by the so-called consecutive dissolution
mechanism (see later).
Classical Interpretation It was known from the previous work by Gerischer
and Mehl [42] on hydrogen evolution that a reaction path involving two consecutive
steps coupled by an adsorbed intermediate species can generate either an inductive
or a capacitive reaction impedance depending on the kinetic parameters. The
detailed derivation is given in the next section devoted to iron dissolution. It can
be shown according to (6) that, for a Langmuir type of adsorption and single-electron
transfers, Z
F
is given by the equation
Copyright © 2002 Marcel Dekker, Inc.
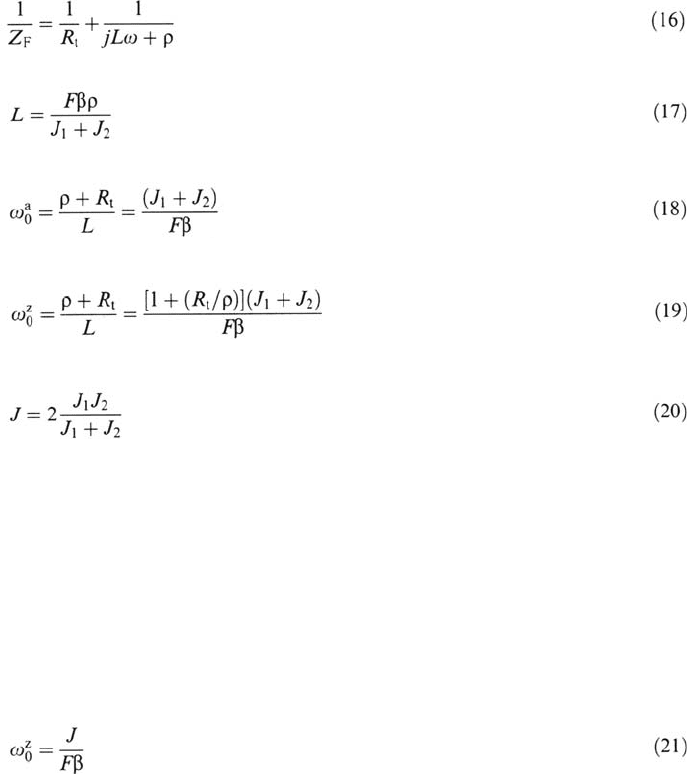
where R
t
and ρ are resistances depending on the kinetic parameters of the reaction
steps, β is the maximum surface concentration of the adsorbed intermediate, J
1
and
J
2
are the current densities of the first and second steps, and F is the Faraday con-
stant. Z
F
is inductive for parameter values such that ρ is positive; this is shown to
occur for J
1
< J
2
. In this case Z
F
can be split immediately into two parallel
branches by identification of (15) to (16):
Anodic Dissolution 109
the self-inductance L being given by
The characteristic frequencies associated are in the admittance plane
and in the impedance plane
Both quantities are increasing functions of the DC current density:
The ratio Fβ is about one monolayer of elementary electrical charges per area unit
of the interface. Many experiments were found to agree with this model even
though several authors mentioned values definitely exceeding one monolayer and
serious deviations from Eqs. (18)–(20). Inductive loops at lower frequencies with
little or no current dependence are ignored in this discussion.
Weak Points and New Model: This problem was revisited in two papers
[57,58]. The first one established from a thorough survey of the literature of metal
and semiconductor dissolution that the frequency of the inductive impedance, in
contradiction of Eq. (19), is remarkably proportional to the DC current over several
orders of magnitude:
The meaning of the deviation between (19) and (21) can be perceived by noting
that according to (18) the frequency is determined by the faster of the two reaction
steps wherever (21) relates it to the slower one. In the second paper, an original
interpretation is proposed. It is based as well on a two-step process between the
metal atom at the lattice surface and the cation in solution on the outer side of the
double layer. A detailed presentation of this approach is far beyond the scope of
this chapter. It will be only outlined briefly. Basically, the relaxation is ascribed to
the time dependence of the surface concentration of an intermediate state of
dissolution. In that sense, the model ingredients are quite similar to those of the
consecutive mechanism, namely:
Copyright © 2002 Marcel Dekker, Inc.
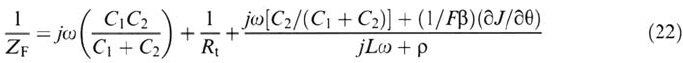
The ionization proceeds through a two-step path involving an intermediate state
(partially solvated).
The interface is (statistically) shared between the intermediate state (fractional
coverage θ) and the ground metallic state (1 – θ).
The model is still compatible with monovalent dissolution (Ag ⇒ Ag
+
, for
instance).
The time dependence is due to the difference between the fluxes of metal species
crossing the interface on the inner and outer sides of the intermediate state.
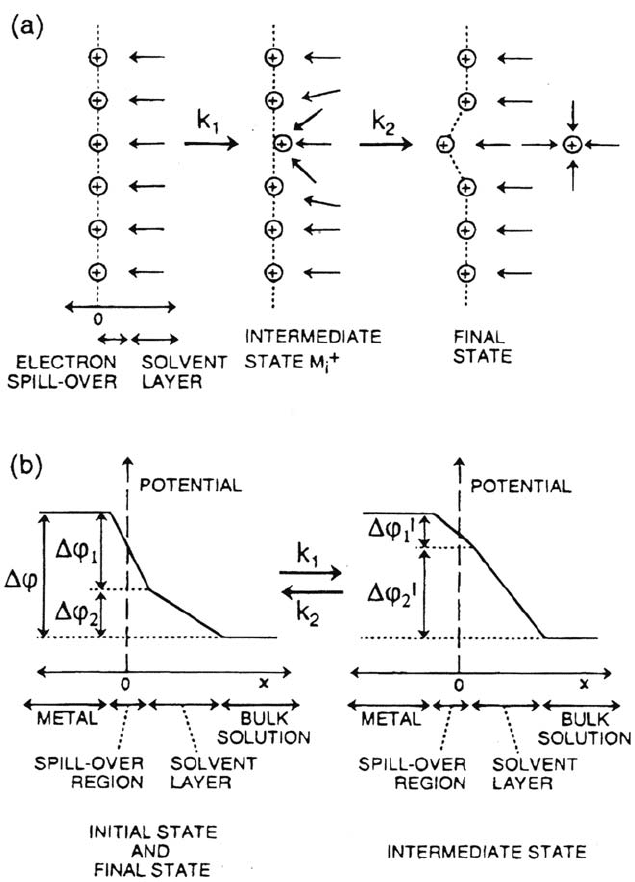
The new ideas lie essentially in the adjustement of the potential profiles in
the metal side (jellium) and the electrolyte side of the double layer in order to comply
with charge balance in conduction and electrostatic properties at the interface. This
is sketched in Figure 2. According to Ref. [58], the impedance has a structure similar
to (15). It can be cast in the form
110 Keddam
where C
1
and C
2
are the capacitances of the inner and outer parts of the double
layer, respectively. With this model, the linear dependence of frequency on DC
current can be simulated for acceptable sets of parameters including values of C
1
and
C
2
consistent with modern theories of the double layer. However, the ratio of one
monolayer is hardly obtained.
Actually, the model introduces a strong coupling between charge transfer and
double-layer structure, a feature generally discarded in spite of the high interfacial
concentration of cations released by the dissolution. In some way it may be regarded
as a ζ-potential approach to the problem. The topic of the kinetics content of the
inductive impedance of anodically dissolving metals is still an open question.
Obviously, it deserves further theoretical and experimental efforts.
ANODIC DISSOLUTION OF PURE METALS
Even though of little interest for practical corrosion problems, pure metals are
extremely basic to any approach to the mechanism of anodic dissolution, which is
believed to be much simpler than for alloys. However, the electrochemical behaviors
of high-purity metals can be deeply influenced by vanishingly amounts of impurities
able to segregate at the interface during anodic dissolution and also by metallurgical
factors. This may explain misleading interpretations and serious discrepancies
between materials from different sources with the same purity grade.
Mechanism of Heterogeneous Reaction:
Dissolution in the Active State
The case of iron will be taken as an example of heterogeneous reaction in the
active range of dissolution. Some other metals are also dealt with briefly.
Copyright © 2002 Marcel Dekker, Inc.
Active Dissolution of Iron in Acidic Solutions
The active dissolution of iron in acidic media has been the subject of a very large
number of papers for the last 40 years. All the reaction mechanisms are based on the
generally agreed upon experimental evidence that the dissolution rate increases with
the solution pH at potentials well below the onset of passivity processes. The
apparently unacceptable participation of hydroxyl ions in the reaction at these low pH
Anodic Dissolution 111
Figure 2. (a) Sketch of the two consecutive ion-tranfer steps in anodic dissolution (b)
Sketch of the distribution of the interfacial potential drop Δ
ϕ
across the electrochemical
double layer. From ref [58].
Copyright © 2002 Marcel Dekker, Inc.

values can be related to the strong dissociative power of transition metals with
respect to water, an assumption supported for Fe by experimental evidence in
ultra-high vacuum [59]. Both groups of mechanisms stem from a common initial
hydrolysis step assumed to be at equilibrium:
112 Keddam
thus accounting for the pH dependence of the subsequent dissolution step. They
diverge from each other by the nature of the dissolution steps leading to the soluble
divalent species.
According to the catalytic mechanism [22], (FeOH)
ads
enters in a catalytic
sequence of dissolution at the end of which (FeOH)
ads
is regenerated and Fe
dissolved as (FeOH
+
):
A reluctant criticism of the catalytic mechanism is directed against the quantum
mechanically unacceptable transfer of two electrons in a single step. It must be
pointed out that on considering even a very crude atomistic content of this two-
electron step it appears less definitely excluded. Interpretation in terms of kink Fe
k
shifted to the next neighboring atom, denoted Fe′, in the dissolving edge:
clearly shows that the two electrons are not transferred from the same Fe species.
According to the consecutive mechanism [23], (FeOH)
ads
enters in a
noncatalytic one-electron transfer step by which it is oxidized to (FeOH
+
):
Finally, FeOH
+
is assumed to react with solution protons:
Mechanistic Criteria Based on Steady-State and Transient Polarization Data
For a long time, with the exception of the pioneering impedance approach of
Epelboin and Keddam [60], the controversy about the validity of these mechanisms
remained based on kinetic criteria drawn from true steady-state and fast polarization
techniques. Tafel slopes and orders of reaction with respect to OH
–
are the two
main parameters taken into consideration.
In theory, the transient state is assumed to take place at times short enough
to keep the concentration of (FeOH)
ads
“frozen” at its initial value. Therefore the
initial transition is due only to the change of the reaction rate of step (26) or (27)
under the effect of potential at constant (FeOH)
ads
concentration. The same
discrimination between instantaneous and delayed contributions is at the origin of
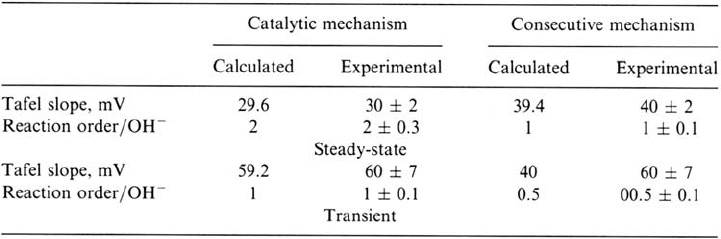
the frequency dependence of the faradic impedance. Table 1 shows the theoretical
and experimental values of the steady-state and transient kinetic parameters for
both mechanisms, according to Ref. 12.
According to Table 1, the delimitation between the two mechanisms relies on
a quite clear-cut difference in the set of kinetics criteria and would not involve
Copyright © 2002 Marcel Dekker, Inc.
controversy at all. In fact, a careful analysis of literature data putting the emphasis
on experimental conditions leads to the following remarks [61,62]:
Tafel slope values are spread over a wide range from less than 30mV to more than
100 mV depending on ill-identified parameters. The role of metallurgical
factors such as dislocations and subgrain boundaries was interpreted as
favoring the catalytic mechanism (30 mV), whereas annealed iron with a low
density of structural defects would obey the consecutive mechanism (40 mV)
[61,62].
In many early studies iron dissolution was investigated as part of corrosion and
inhibition at open-circuit potential under an H
2
-saturated atmosphere, no
account being taken of the interference of the H
2
/H
+
reaction with the Tafel
slopes [63,64]. Even under an inert atmosphere, the anodic parameters may be
affected by slow hysteresis phenomena resulting in steeper Tafel lines [65–69].
Reaction order measurements are not always very reliable and are sometimes
reported with fractional values [12].
Current transients are much too long to be consistent with the minimal exchange
current density still compatible with reaction (23) being at pseudoequilibrium
[70]. Alternative explanations based on local increase of pH have been
proposed [65] and further elaborated [70].
From the impedance approach it was then established that the iron mechanism
is far more complex than expected from early experiments and can hardly be
investigated correctly by current-voltage plots in spite of continued efforts
[71,72]. Thorough analysis over extended ranges of electrode potential, pH, and
frequency is presented in the following.
Atomistic Interpretation of the Catalytic Mechanism Anodic dissolution of
iron is probably the sole case for which an atomistic description was elaborated
with a somehow successful issue. An early attempt to explain the transient regime
of iron dissolution by relaxation of etched pits by a nucleation and growth model
can be found in Ref. 65 and is analyzed in Ref. 60. Starting in the middle of the
1970s, Heusler et al. have developed in a series of papers [29–31] an experimental
and modeling approach aimed at giving a crystallographic basis to the catalytic
mechanism. Correlation of kinetic and morphological data was performed on a
Anodic Dissolution 113
Table 1 Kinetic Data for Iron Dissolution at T = 298 K
Source: Ref. 12.
Copyright © 2002 Marcel Dekker, Inc.
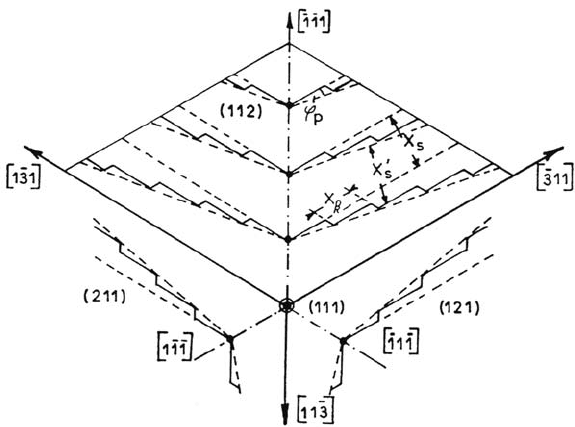
surface vicinal to {211} (misorientation ≅ 1°) of iron single crystals in acidic
solutions. These surfaces are found to develop a relatively simple steady-state
morphology made of triangular pyramids limited by nearly perfect {211} planes
and 〈311〉 edges. A schematic model of steps and kinks projected on a {111} plane
is given in Figure 3.
Steps are generated at the pyramid apex and move away on the three {211}
planes. The structure of the dissolving surface is determined by x
S
, the mean distance
of steps perpendicular to the 〈311〉 direction:
x
s
= bN
p
/N
S
with b = interatomic distance
N
P
= probability of generation of six steps at the apex
N
S
= probability of kink generation at the intersection of two monatomic steps
and x
k
, the mean distance of kinks:
x
k
= bN
k
/ N
P
where N
k
is the probability of removing an atom from a kink.
The current density j is the product of the current on one kink ze
0
N
k
by the
density of kinks: j = ze
0
N
k
/ x
s
x
k
.
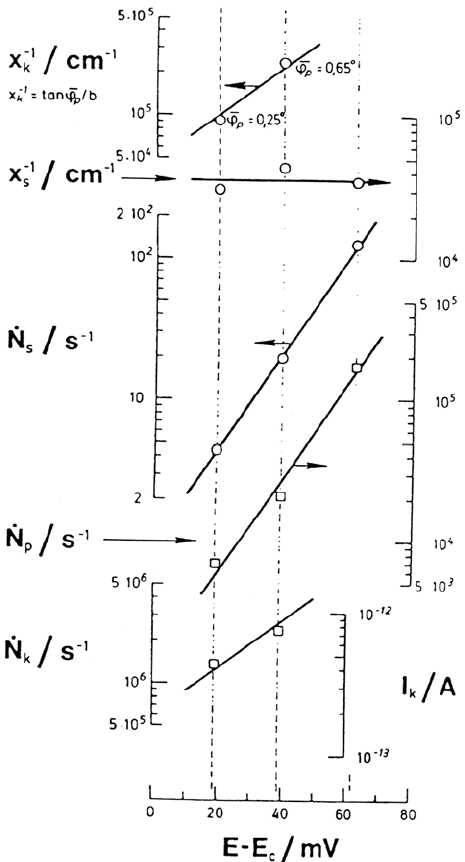
Scanning electron microscopy (SEM) examinations, [29] of the macroscopic
morphology of dissolution were then substantiated by transmission electron
microscopy (TEM) pictures of gold-decorated surfaces [30]. The better resolution,
even though not truly atomic, allowed an estimate of x
s
and x
k
and of their
dependence on the dissolution rate as shown in Figure 4. The surface concentration
of kinks (x
s
x
k
)
–1
is an exponential function of the potential with an exp(FE/RT)
114 Keddam
Figure 3. Schematic model of steps and kinks on crystallographic (211), (112) and (121)
planes projected into a (111) plane. From ref [30].
Copyright © 2002 Marcel Dekker, Inc.
dependence. The potential-dependent parameter is x
k
≅ exp – (FE/RT) while the
mean distance of steps, x
s
, remains constant. Consistent with the catalytic
mechanism, at steady state the dissolution rate of atoms at kinks obeys an
exp(l + 2α)FE/RT Tafel law and, at constant kink density, the elementary rate of
atom dissolution is proportional to exp(RE/RT), i.e., α = 0.5 and transfer of n = 2
electrons in a single step.
Anodic Dissolution 115
Figure 4. Potential dependence of structural parameters and mean rates of elementary
processes for three different polarization conditions. TEM measurements on replica of
gold-decorated surfaces after anodic dissolution. Johnson-Matthey iron. From ref [30].
Copyright © 2002 Marcel Dekker, Inc.
