Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

126
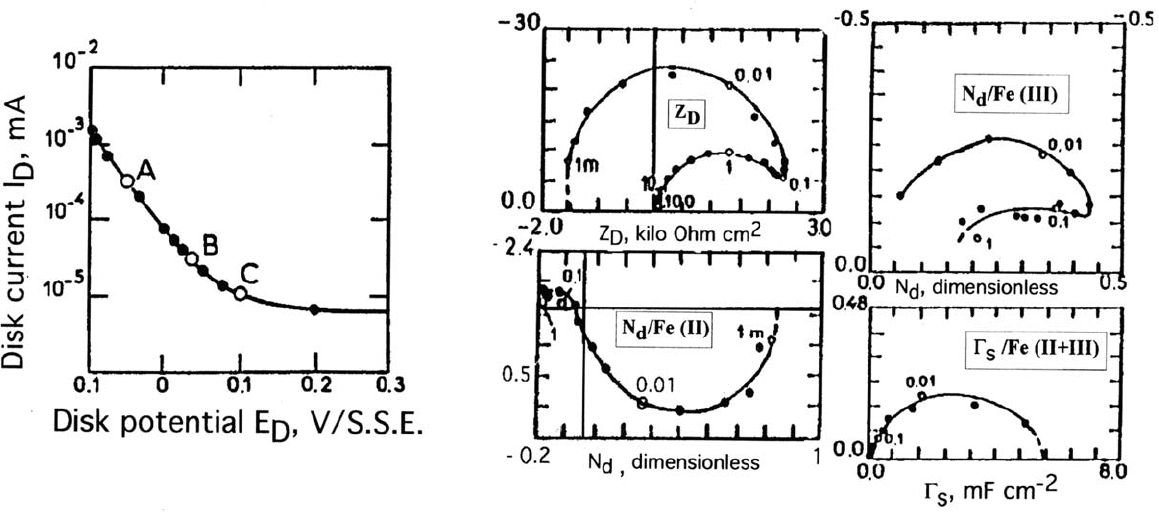
Figure 9. Impedance and AC-resolved RRDE of the active-passive transition of iron in 1 M H
2
So
4
. Rotation speed 900 rpm. Current-voltage
curve of the iron disk. Data at polarization “B”: Z
D
: disk impedance, N
d
/Fe(II) and N
d
/Fe(III): emission efficiencies respectively for Fe(II) and
Fe(III) species, ring potential respectively: 0.8 and –0.4 V/SSE, Γ
s
/Fe(II+III) : faradaic capacitance of the passivating layer.
Copyright © 2002 Marcel Dekker, Inc.

dissolution intermediate. The emission efficiency for Fe(III) contains the same
two domains of frequency but with quite different properties. Both are located in
the negative real part region with modulus maximum around the transition
frequency (0.1 Hz). Therefore both surface species participate as sources of
dissolution of Fe(III). This is consistent with the generally accepted concept of a
passivity current carried by the Fe(III) released by the film lattice. Finally, the
capacitance Γ
s
associated with the total charge as Fe(II) plus Fe(III) indicates that
around 5.5 mC V
1
cm
–2
are needed for passivation. Most of the current decay
takes place within 0.2 V; therefore the charge content of the passive film at the
Flade potential can be estimated as 1 mC cm
–2
. This value is practically equal to
the charge equivalent to one monolayer (one cell unit of a ferric oxide layer).
A kinetic model very close to that elaborated more recently for interpreting
the transient dissolution of laser beam–depassivated iron was proposed. It made it
possible to reproduce the main features of N
d
and Γ
s
shown in Figure 9.
Dissolution in the Passive State
Anodic dissolution in the active state generally results in equivalent corrosion rates
unacceptable in practical applications (order of mA cm
2
or more). Self-limitation of
the dissolution rate by the buildup of a thin surface layer under sufficiently oxidizing
conditions, known as passivation, is the only relevant phenomenon in terms of
corrosion control. The mechanism of dissolution in the passive state is dealt with in
the following. A thorough survey of the field can be found in the proceedings volume
of the last passivity symposia [118,122]
Film Relaxation and Dissolution in the Passive State
After completion of a full coverage through the initial stages of passivation, the
subsequent behavior of a passivated metal is entirely determined by the bulk
properties of the passive film and the reactions at its boundary interfaces with the
metal and the electrolyte. In spite of the enormous amount of research carried out
since Faraday’s time, relations between the solid-state chemistry of the barrier
layer and the electrochemistry of the passive metal remain largely unclear.
Concepts, Models, and Experimental Approaches Separation between film
relaxation (growth or dissolution) and metal corrosion in the passive state is
meaningless except at perfect steady state because both aspects of metal oxidation
are intimately interrelated by the charge and mass balances at the metal-film-
electrolyte interface.
Anodic Dissolution 127
The overall charge balance is expressed as
where I is the external current flowing to the electrode, I
G
the component of I
involve in the film growth, and I
C
the corrosion current measuring the flux of
metal cations released in the solution. Equation (41) must be considered algebraically;
i.e., a negative (cathodic) value of I will produce film destruction by cathodic
reduction and possibly a decrease of the corrosion current. Steady state is naturally
defined for a constant film thickness, I
G
= 0, and therefore an external current
equal to the corrosion current.
Most of the concepts introduced for modeling film growth and dissolution have
their origin in the theory of thermal (dry) oxidation initially adapted to thick (wet)
Copyright © 2002 Marcel Dekker, Inc.
oxides grown chemically or electrochemically on valve metals [123–125]. Much
more intricate problems arise from electrochemical conditions. Dissolution
processes in the passive state involve
Metal oxidation (ionization) at the metal-film interface
Transport of metal cation (and/or cation vacancies) across the film
Metal cation transfer into solution species (solvated, complexed) at the film-
electrolyte interface.
Film growth can proceed at interfaces:
Metal-film by transport of oxygen anions to the metal-film interface or of oxygen
vacancies toward the solutions
Film-electrolyte by transport of metal cations to the film-electrolyte interface or of
cation vacancies toward the metal
Models differ essentially by the active participation of the lattice, via defects,
to the transport properties and consequently to the growth and dissolution reactions
at interfaces.
For simplicity, the dissolution behaviour of passive metals can be split into two
groups:
Passive electrodes with a nearly constant corrosion current over an appreciable
potential range. They are generally dealt with in terms of high field migration.
Passive electrodes with a potential-dependent current, most commonly exhibiting
a minimum. Less advanced models are available; they involve the contribution
of defects (e.g., point defect model).
Non-steady-state techniques played an outstanding role in reference papers
in the field [4]. Galvanostatic and potentiostatic transients [126–129] are
exploited either at short times, for plotting current-voltage characteristics at
constant (frozen) film thickness, or at longer times as a function of increasing
(or decreasing) film thickness estimated from the total charge ∫ I dt. Transient
techniques were also extended to rapid changes of solution composition [130].
AC impedance in the very low frequency range (millihertz and submillihertz)
proved quite successful for achieving finely resolved information on corrosion
and film growth [4].
Passive Iron and the High-Field Migration The high-field migration model
(also known as hopping mechanism) was applied to interpret the steady-state and
non-steady-state behaviors of iron investigated by many different techniques during
the last 40 years. Pioneering works by the German school [131,132] have been
revisited in the past decade by more advanced experiments [133] and improved
modeling [134].
Salient features accounted for by this model are
A steady-state corrosion current independent of the electrode potential over an
appreciable range.
The existence of a specific potential, known as the Flade potential E
F
, at the
cathodic bound of passivity, to which all the film properties are referred in a
simple form. It can be regarded as the zero thickness origin.
128 Keddam
Copyright © 2002 Marcel Dekker, Inc.
A linear dependence of the film thickness (estimated coulometrically but also
supported by surface-sensitive techniques such as ellipsometry) on the anodic
overvoltage (E – E
F
).
In its original form [125] the model was based on a multibarrier thermally
activated cation transport giving rise to a current
Anodic Dissolution 129
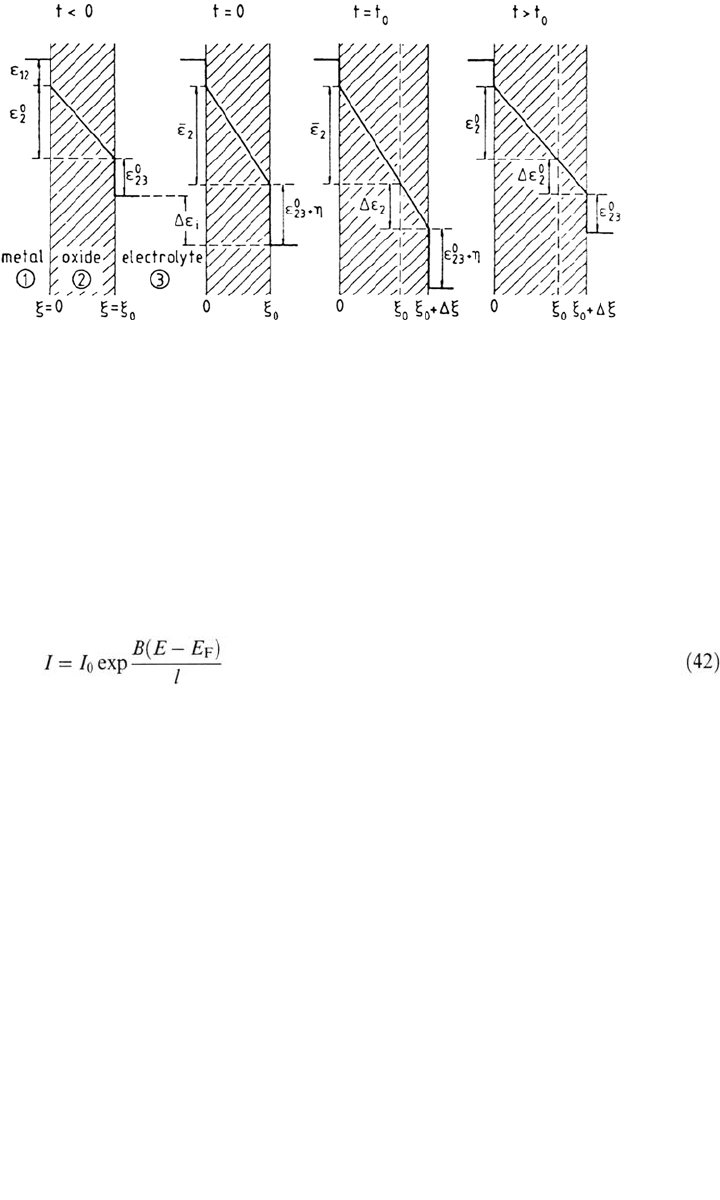
Figure 10. Positional changes of the potential from the metal (1) through the passive
film (2) to the electrolyte (3) for different times of a galvanostatic square pulse. According
to Vetter [131] and Wagner [123] at steady-state the film solution potential drop ε
2.3
is
potential independent (η = 0).
where B = zaF/RT with a the half-jump distance, z the number of elementary
charges on the mobiles species, l the film thickness, and F, R, and T the usual
physical constants. I
0
is a standard current assumed to depend on the density of
charge carriers in the film.
At steady state, the potential-independent corrosion current is totally supported
by a constant field strength in the film, i.e., a film thickness l proportional to
(E – E
F
). The conservative flux of cations Fe(III) across the interface requires a
potential-independent voltage drop at the film-electrolyte junction.
Most of the non-steady-state results were obtained by RRDE [135,136] and
monitoring of Fe(III) in solution by analytical chemistry [131,132] or radiotracer
techniques. Transfer of cations into the solution at the film-electrolyte interface
was found to depend on the overvoltage according to redox kinetics and to be
roughly proportional to the current forced in the electrode in excess of the steady-
state value. Accordingly, as sketched in Figure 10, any change of the steady current,
or potential, triggers a modification (positive or negative) of the film thickness
that finally restitutes the initial electrical field strength and potential drop at the
outer film surface, i.e., potential-independent DC current.
The overall problem of the non-steady-state passive iron has been reexamined
with the purpose of reaching a consistent interpretation of the empirical equation
Copyright © 2002 Marcel Dekker, Inc.
I = I
0
exp(βE – Q/B)
firmly established in acidic [128] and neutral [126,127] media and also for nickel
in acidic solutions [128] in classical papers. As stated earlier [134], this equation
contradicts the high-field mechanism because it predicts a direct logarithmic Q(t)
relationship consistent with a place exchange mechanism, whereas the high field
should generate an inverse logarithmic dependence [4].
It can be concluded that the key point lies in the potential distribution between
the film bulk and the film-electrolyte interface in the transient regime. Subsequently,
an attempt was made to incorporate the role of defects (cation vacancies) in the
high-field mechanism [137].
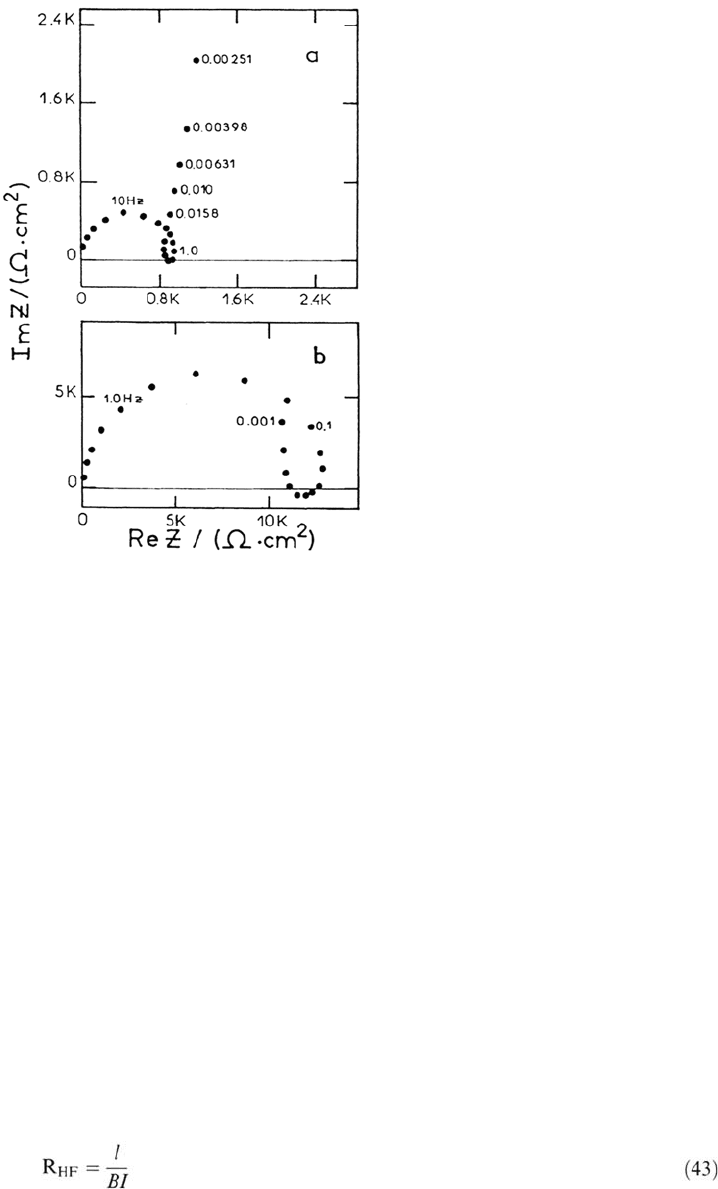
AC Impedance of Passive Iron in Acidic Media Film resistance to cation
migration and capacitance displaying the potential dependence of the charge
stored by the film growth are expected. Figure 11 shows two complex impedance
diagrams in the passive range in H
3
PO
4
and H
2
SO
4
solutions [133].
According to Eq. (42), the size of the HF loop is attributed to the derivative
of the flux due to high-field migration, with respect to E at constant film thickness:
130 Keddam
Figure 11. Impedance diagram of iron in the passive state. Johnson-Matthey iron. a. 1 M
H
3
PO
4
, 37°C, E = –0.65 V/SSE b. 1M H
2
SO
4
, 25°C, E = –0.35 V/SSE Frequency in Hertz.
From ref [133].
Copyright © 2002 Marcel Dekker, Inc.
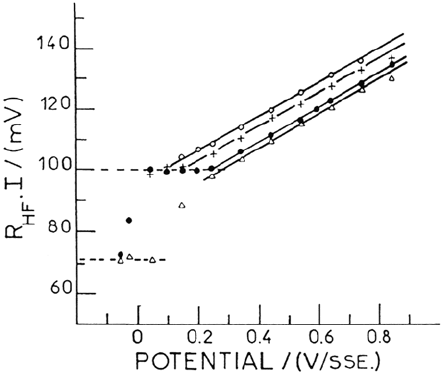
The DC current I being potential independent, a linear dependence of R
HF
I
on E is expected and fairly verified as shown in Figure 12 for various solution
compositions and pH. The straight lines begin at the Flade potential E
F
, where the
film is restricted to its inner and outer boundaries and above which the 3D film
starts growing.
From the slope, 0.06, of the straight line in Figure 12 and assuming a = 0.5 nm,
a calculated field strength of 3 × 10
8
Vm
–1
and a potential dependence of the film
thickness of 3.5 nm V
–1
are calculated, in agreement with literature data. The
inductive behavior at intermediate frequencies is physically equivalent to the over-
shooting transient considered in Ref. 137 and may be related to a relaxation of the
carrier density. The low-frequency capacitive branch is perfectly consistent with
the constant DC current. Its value is about one order of magnitude larger than esti-
mated by Faraday’s law applied to the thickness-potential dependence of the film.
Little dependence is found with respect to the solution composition, supporting the
interpretation of anion effect through the change of I
0
with the density of charge
carriers.
According to the high-field migration model, iron dissolution across the film and
film growth are only weakly coupled by the transient overvoltage η (see Fig. 10) at
the film-electrolyte interface. In contrast, any model based on defect mobility
necessarily implies a dynamic situation with two nonconservative reactions [138]
(film growth at the inner interface and film dissolution at the outer one). The whole
film is therefore translating outward with a velocity depending on the corrosion
current.
Investigation of the Transient Dissolution of Passive Iron by Impedance and
Electrogravimetric Transmittance One of the recurrent topics in passivity studies
is the transient behavior of the passive state. That is, how is the current shared
Anodic Dissolution 131
Figure 12. Potential dependence of the R
HF
.I product for passive iron in various solutions
(Johnson-Matthey iron. 25°C). Δ: 1M H
2
SO
4
, pH = 0.03. •: 1M H
3
PO
4
, pH = 1 +: 1M
(H
3
PO
4
+ KH
2
PO
4
), pH = 2.2. o: 1M (H
3
PO
4
+ KH
2
PO
4
), pH = 2.8. From ref [133].
Copyright © 2002 Marcel Dekker, Inc.
between film growth and metal dissolution during the restoration of a new steady
state after a change in the applied potential? As stated before, the predictions of
the high-field model in the transient regime are extremely sensitive to the distribution
of a potential increment between film bulk and interfaces. No ab initio answer is
available and the efforts have been concentrated on the analysis of time or frequency
domain experiments. Emphasis was put on the presence of an overshoot or inductive
response, but the interpretation of the inductance remained quite ambiguous without
no additional data. Quartz crystal microbalances are very well adapted to this
problem because the low corrosion rate provokes no drift of the mean mass of the
electrode while for the same reason the emitted flux of cation is below the detection
limit of collector electrodes. Impedance when associated with frequency-resolved
electrogravimetry is able to discriminate between the fraction of the overall charge
Q taken up in film growth (mass gain) and consumed for dissolution (mass loss).
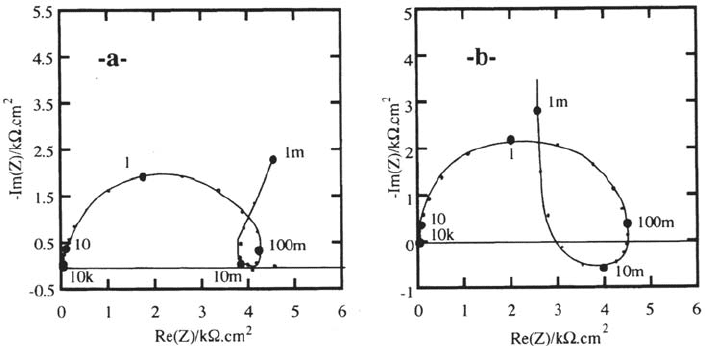
It was shown analytically that the high-field migration model is not able on
its own to explain the inductive response shown in Figure 13a. Correlatively, the
calculated mass transmittances Δm/ΔE and Δm/ΔQ simply reflect Faraday’s law
[55]. In particular, Δm/ΔQ is a real quantity determined by the antagonistic
contributions of transitory film growth and metal dissolution. A new time constant
besides that related to the relaxation of the film thickness was introduced [139,140]
by deriving the impedance and AC mass response for the model proposed by
Kirchheim [137]. It assumed that the density of cationic vacancies [or the density of
mobile Fe(III) cations] in the film depends on the excess of current with respect
to the corrosion current I
c
. Consequently, the film resistance R
HF
is lowered,
producing an inductive-like transient of the migration current. Impedance is
foreseen to contain, in addition to the series capacitance at low frequencies
standing for the film growth, an inductance generated by the relaxation of the film
conductance.
132 Keddam
Figure 13. Impedance of iron in 1M H
2
SO
4
, potential 0.5 V/SSE. Experimental: “a” and
numerically simulated : “b”, with a model based on a relaxation of the charge carriers
density in the passive film. From ref [139,140].
Copyright © 2002 Marcel Dekker, Inc.
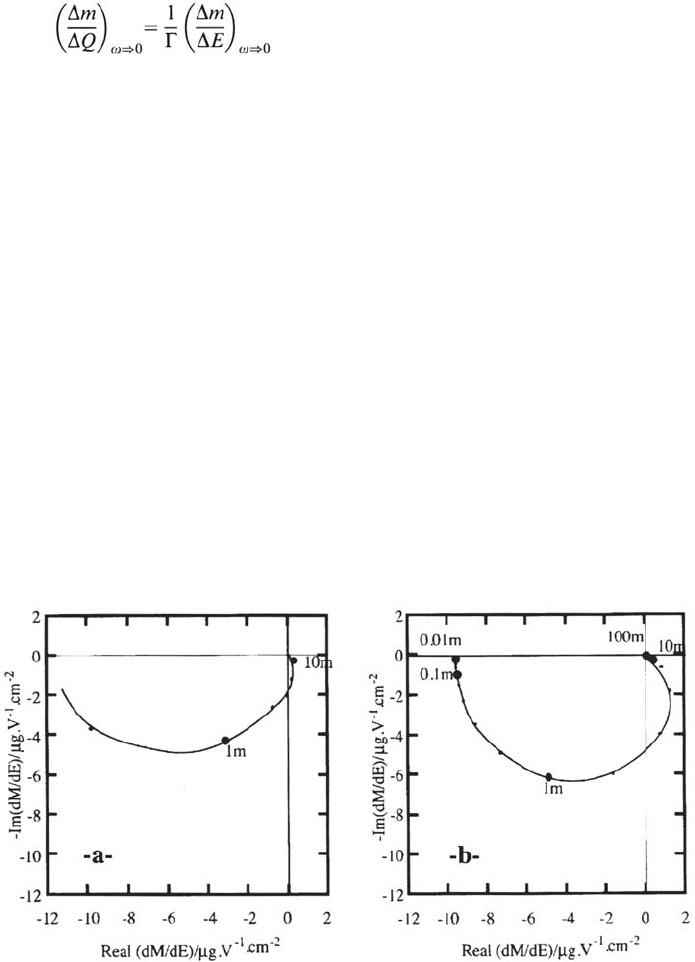
Figure 13 displays experimental and computed Nyquist plots of the impedance.
Similarly, the electrogravimetric transmittance was derived. It exhibits the mass
counterpart of the relaxation of the film conductance. Again, consistent with
Faraday’s law, the low-frequency limit
Anodic Dissolution 133
is fixed by the mass equivalent to the film growth. Experimental and simulated
complex electrogravimetric transmittances are shown in Figure 14.
The negative real limit at low frequencies reveals that the mass loss by the
corrosion current I
c
is much larger than the mass gain by the film growth. In
agreement with values of the capacitance Γ, this limit, –12mg V
–1
cm
–2
is about
10-fold that of a 2.5 nm V
–1
thick layer of Fe
2
O
3
. In contrast, the humped shape
in the positive real part region at high frequencies visualizes the mass gain due to
film growth. It is noteworthy that this takes place in the same frequency range as
that covered by the inductive loop. With this set of parameters, which may be not
optimal, both features are larger on simulated than on experimental curves. Paired
contribution of these transmittances confirmed that
Transient is controlled by film interface with respect to bulk.
One intensive property of the film probably its ionic conductance, is at the origin
of the inductive behavior.
The major part of the charge supplied to the film produces corrosion rather than
film growth.
Passive Nickel and the Point Defect Model The passive state of nickel shows
much more complicated behavior than for iron in the same solutions. A minimum of
Figure 14. Electrogravimetric transfer function of iron in 1M H
2
SO
4
, potential 0.5 V/SSE.
Experimental :“a” and numerically simulated : “b”, with a model based on a relaxation of
the charge carriers density in the passive film. Same set of parameters as for Figure 13a.
From ref [139,140].
Copyright © 2002 Marcel Dekker, Inc.
dissolution current is most often observed in the middle of the passive range, and
increasing dissolution takes place at higher potentials (transpassive range). It is
now well established that passive and transpassive dissolutions are so tightly related
that they cannot be dealt with separately. According to this behavior, the film is
expected to undergo important modifications in its structure and thickness as a
function of potential and solution composition.
An advanced form of the point defect model [123,124] has been applied to the
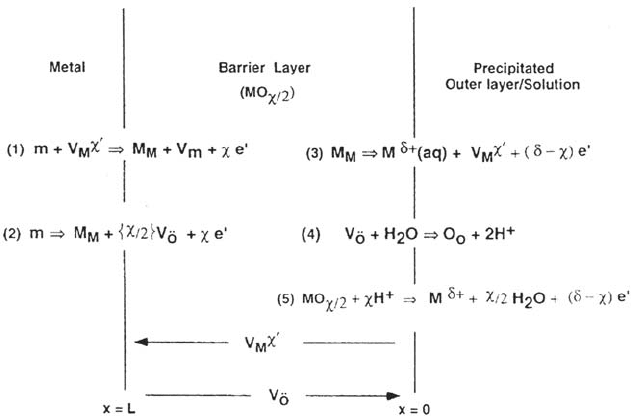
passivation of Ni in neutral solutions in a series of papers [138,141,142]. Figure 15
shows the main features of the model and the five reactions considered; two of
them, (2) and (5), are nonconservative and contribute directly to metal dissolution
through translation of the film growing at the inner interface (reaction 2) and
dissolving at the outer interface (reaction 5). The model involves many more
assumptions and parameters than the high-field migration and is able to account
for a broad spectrum of electrochemical responses to potential, pH, and cation
concentration. Some aspects of the model were investigated by impedance in spite
of the extremely low frequencies involved in the processes and control by cation
vacancies was concluded [141,142].
Passive and Transpassive Dissolution of Nickel in Acidic Solutions The
kinetics of nickel dissolution in the passive and transpassive ranges M remained
totally unclear until the application of a very low frequency impedance technique.
A general model was proposed on the basis of an extensive study of anion effects
[143]. In the passive state, the frequency domain had to be extended far below 1 mHz
and long-term stability was obtained only by using single-crystal electrodes [144].
134 Keddam
Figure 15. Schematic of the physicochemical processes that occur within a passive film
according to the point defect model. From ref [138].
Copyright © 2002 Marcel Dekker, Inc.
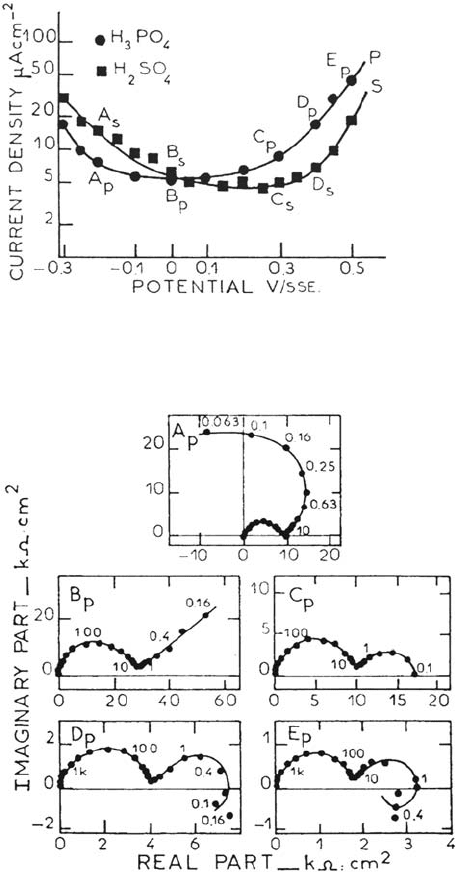
Figure 16 shows two current-voltage profiles in molar sulfuric and phosphoric
solutions. The behavior contrasts drastically with that of iron; a minimum is reached
close to 5 μAcm
–2
, thus suggesting the contribution of two antagonistic processes.
This is substantiated by the impedance data of Figure 17 for the phosphoric
medium (the lower frequency limit is less than 100 μHz). Diagram Ap has the
characteristic shape associated with passivation kinetics (see earlier)
Anodic Dissolution 135
Figure 16. Steady-state polarization curves for nickel, (111) single crystal, in 1 M H
2
SO
4
and 1 M H
3
PO
4
solution. 25°C. From ref [144].
Figure 17. Impedance diagrams of passive Ni, (111) single crystal, in 1M H
3
PO
4
solution. Potentials labelled in Figure 16. (Frequency in millihertz). From ref [144].
Copyright © 2002 Marcel Dekker, Inc.
