Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

In a similar way, for the reaction
4 Landolt
the Nernst equation reads
At 25°C this becomes
In this equation E
°
Zn (OH)
2
/Zn
= –0.439 is the standard potential for the formation of
a hydrated zinc oxide [2].
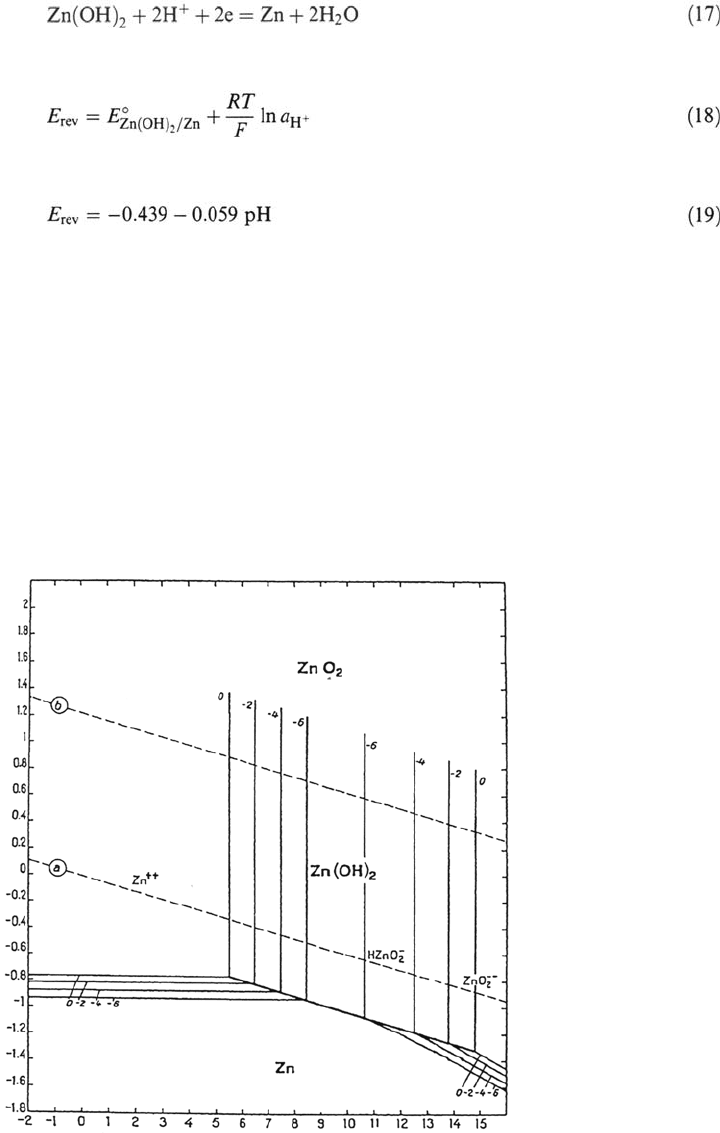
Pourbaix Diagrams
The graphical representation of the reversible potential as a function of pH is called
the potential-pH diagram or Pourbaix diagram. In order to trace such diagrams one
must fix the concentration of the dissolved species. Figure 1 shows a simplified
Pourbaix diagram for zinc [2]. The numbers indicate different concentrations of
Figure 1 Potential-pH diagram of Zu (From Ref. 2).
Copyright © 2002 Marcel Dekker, Inc.
dissolved species, for example, 10
–2
, 10
–4
mol/L. The diagram shown takes into
account the formation of zinc hydroxide, of Zn
2+
and of the zincate ions HZnO
2
–
and ZnO
2
2–
. At high potentials ZnO
2
may possibly be formed, but because the
corresponding thermodynamic data are uncertain they are not presented in the
diagram. The broken lines indicate the domain of thermodynamic stability of
water. Pourbaix diagrams are widely used in corrosion because they easily permit
one to identify the predominant species at equilibrium for a given potential
and pH. On the other hand, being based on thermodynamic data, they give no
information on the rate of possible corrosion reactions.
KINETICS OF CHARGE TRANSFER REACTIONS
At the electrode-electrolyte interface, a charge separation between the metal surface
and the electrolyte occurs. The spatial region corresponding to the charge separation
is called the electrical double layer. It is usually separated into two parts, the
Helmholtz layer or compact double layer and Gouy-Chapman layer or diffuse
double layer. The relative importance of the diffuse double layer increases with
decreasing concentration. In very dilute solutions it may extend over a distance of
several nanometers, whereas the compact layer never exceeds two to three tenths
of a nanometer. The charges at the interface establish an electric field. Within the
compact double layer the electric field reaches values of the order of 10
8
to 10
9
V/m. Charge transfer reactions occur across the compact double layer and the
influence of the diffuse double layer is usually neglected.
Let us consider the transfer of n electrons between two species, B
ox
and B
red
.
Electrochemical Basis of Corrosion 5
According to Faraday’s law, the current density across the interface corresponding
to this reaction is equal to the difference of the anodic rate v
a
and the cathodic rate
v
c
multiplied by nF.
Here k′
a(E)
and k′
c(E)
are potential-dependent rate constants and c
red,s
and c
ox,s
rep-
resent the surface concentrations of B
red
and B
ox,
respectively. The rate constants
k′
a
and k′
c
obey the Arrhenius equation.
Here ΔG
#
a
and ΔG
#
c
represent the activation energies for the anodic and cathodic
partial reactions, respectively, and k′
a,0
and k′
c,0
are preexponential factors. The
presence of an electric field at the electrode-electrolyte interface modifies the
activation energy of the partial oxidation and reduction reactions as shown
Copyright © 2002 Marcel Dekker, Inc.
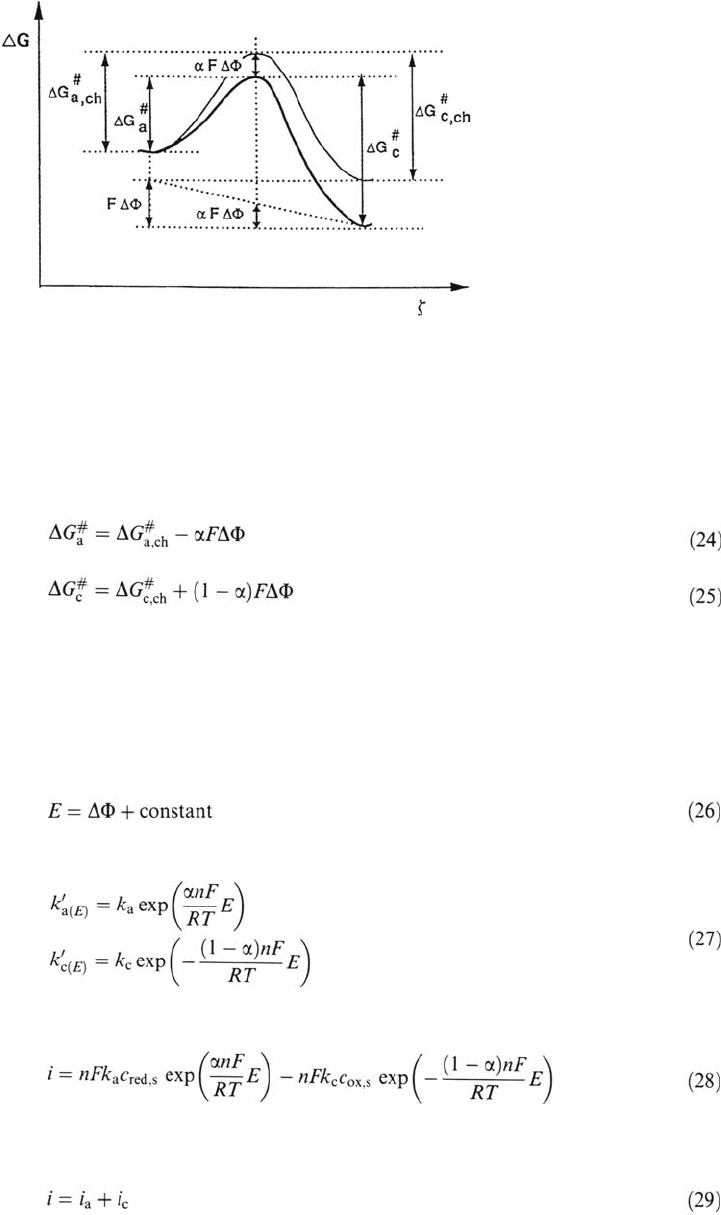
schematically in Figure 2. The electric field in this case diminishes the activation
energy of the anodic partial reaction and increases that of the cathodic partial
reaction.
6 Landolt
Figure 2 Free energy as a function of the reaction coordinate showing the influence of
the electrical potential on the activation energy of a charge transfer process.
Here ΔG
#
a,ch
and ΔG
#
c,ch
represent the potential-independent parts of the activation
energies and ΔΦ represents the potential difference across the interface. The pro-
portionality factor α is called the charge transfer coefficient. Its value is usually
close to 0.5 [3]. The absolute value of ΔΦ is not measurable. It differs from the
electrode potential E measured with respect to the standard hydrogen reference
electrode by a constant.
Combining all potential-independent terms in the rate constants k
a
and k
c,
one gets
With Eqs. (21) and (27) one obtains Eq. (28).
The current i corresponds to the sum of the partial anodic and cathodic current
densities:
Copyright © 2002 Marcel Dekker, Inc.
The anodic and cathodic partial current densities, i
a
and i
c
, respectively, are
given by
Electrochemical Basis of Corrosion 7
At the reversible potential, E = E
rev
, the current density i is zero and the surface
concentration of the reacting species is equal to the bulk concentration: c
red,s
= c
red,b
and c
ox,s
= c
ox,b
. This allows one to define the exchange current density of a reaction
by the relation:
With (32) and (28) one gets
This expression shows that the value of the exchange current density depends on
the concentration of the participating species. Defining the overvoltage η =E – E
rev
,
Eqs. (33) and (28) yield (34).
If the rate of an electrode reaction is entirely controlled by charge transfer, the
concentrations of reactants and products at the electrode surface are equal to the
bulk concentrations. Equation (34) then reads
This is the usual form of the Butler-Volmer equation, which describes the relationship
between current density and potential for a simple electrode reaction controlled by
charge transfer.
Normally, multielectron transfer reactions proceed in subsequent one-electron
transfer reaction steps. For n > 1, Eq. (35), therefore, does not describe the physical
mechanism. Indeed, depending on which reaction step is rate limiting, the value
of the exponential terms may change. For example, the cathodic reduction of protons
corresponding to the overall reaction
proceeds in two steps. The Volmer-Heyrovsky mechanism applies to most metal
substrates:
On certain noble metals of the platinum group, however, the so-called Volmer-Tafel
mechanism applies:
Copyright © 2002 Marcel Dekker, Inc.

In this case the second step is a chemical recombination reaction of adsorbed
hydrogen atoms produced in the first step, which according to the reaction
stoichiometry must proceed twice. The two mechanisms presented here and the
rate-determining steps can be distinguished by measuring Tafel slopes. For a more
detailed discussion of this point the reader may refer to the literature [3–6].
In corroding systems the detailed mechanisms of the partial electrode reactions
are frequently not known. Therefore, it has been found useful to introduce the
empirical Tafel coefficients β
a
and β
c
defined by
8 Landolt
Comparing these definitions with Eqs. (30) and (31), one finds β
a
= RT/αnF and
β
c
= RT/(1 – α)nF. Equation (35) thus becomes:
The Butler-Volmer equation written in the form (44) is frequently used in corrosion.
It describes the relationship between current density and potential of an electrode
reaction under charge transfer control in terms of three easily measurable
quantities: i
o
, β
a
, and β
c
. For large anodic overvoltages (η/β
a
>> 1) Eq. (44)
reduces to (45).
Taking the logarithm and defining a
a
= –2.303β
a
ln i
o
and b
a
= 2.303β
a
, this yields
the Tafel equation for an anodic reaction:
Analogously, for large cathodic overvoltages (η/β
c
<< –1):
Taking the logarithm and defining a
c
= –2.303β
c
ln i
o
and b
c
= 2.303β
c
yields the
Tafel equation for an cathodic reaction:
The Tafel equation applying to large overvoltages predicts a straight line for the
variation of the logarithm of current density with potential.
Copyright © 2002 Marcel Dekker, Inc.
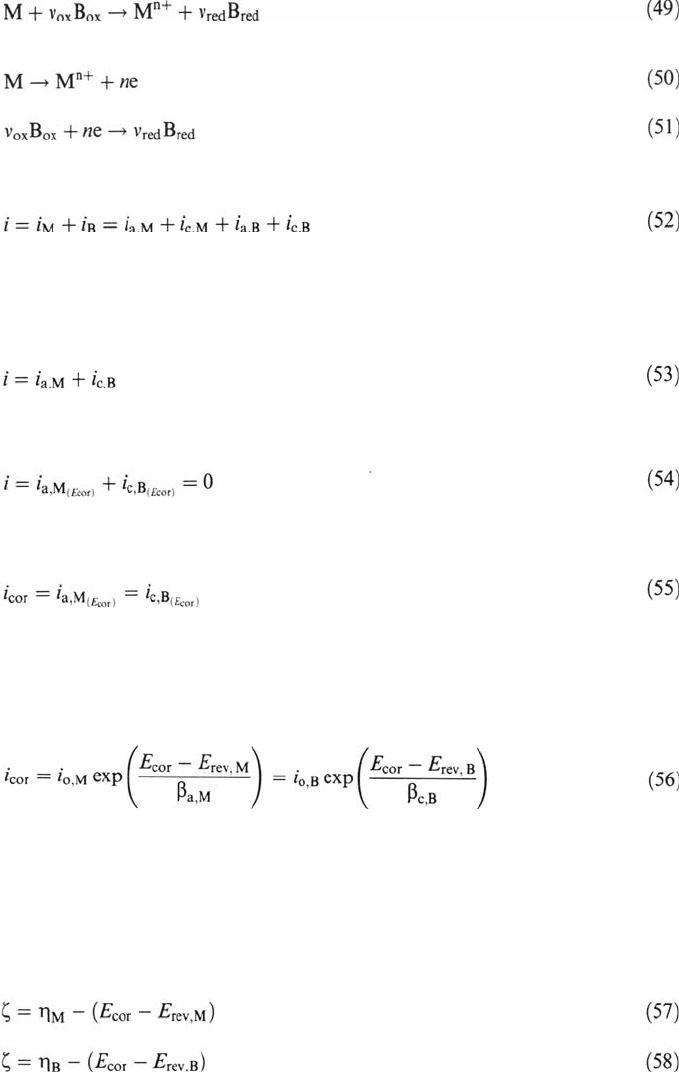
CHARGE TRANSFER REACTIONS AT MIXED ELECTRODES
If a metal is in contact with an electrolyte containing on oxidizing agent, two or more
electrode reactions can take place simultaneously. Such a system is called a mixed
electrode. Suppose a metal M dissolves while a species B
ox
is reduced to B
red
:
Electrochemical Basis of Corrosion 9
The two partial reactions are
The total current density at the metal-solution interface is equal to the sum of the
anodic and cathodic partial current densities of the two electrode reactions.
The subscripts M and B designate the partial reactions and the subscripts a and c
stand for anodic and cathodic, respectively. Usually in a corroding system one can
neglect the cathodic partial current of the metal and the anodic partial current of
the oxidizing agent: i
c,M
≈ 0, i
a,B
≈ 0. Therefore (52) can be simplified:
At open circuit the mixed electrode is at its corrosion potential, E = E
cor,
and there
is no net current. Hence:
This allows one to define the corrosion current density i
cor
, which according to Eq.
(4) is proportional to the corrosion rate.
If charge transfer is rate limiting, the Butler-Volmer equation (35) described the
current-potential relationship of each partial reaction. With the overvoltage of the
metal dissolution reaction, η
M
= E – E
rev,M
, and for the reduction reaction, η
B
= E – E
rev,B
, one gets the following expression for the corrosion current density:
Equation (56) shows that the corrosion current density for a charge transfer–
controlled reaction is entirely determined by the kinetic parameters of the partial
electrode reactions involved.
By definition, the polarization ζ =E –E
cor
indicates the deviation of the
electrode potential from the corrosion potential. The polarization ζ is related to the
overvoltage of the partial electrode reactions by (57) and (58).
Copyright © 2002 Marcel Dekker, Inc.
With these expressions one can easily derive the Butler-Volmer equation of a
mixed electrode corresponding to the corrosion reaction (49):
10 Landolt
At the corrosion potential the absolute values of the two partial current densities
are equal. The reciprocal of the slope of the curve i = f
(E)
is called polarization
resistance. A measurement of the polarization resistance at the corrosion potential,
r
cor
, allows one to determine the corrosion current density provided the anodic and
cathodic Tafel coefficients are known. By definition:
With Eq. (59) this yields
The value of the corrosion current density can also be obtained from a logarithmic
plot (Fig. 3) by extrapolating the anodic or the cathodic partial current density to
the corrosion potential. Indeed, for the anodic and cathodic Tafel regions,
respectively, Eq. (59) yields
Semilogarithmic plots of the partial anodic and cathodic current densities of mixed
electrodes as shown in Figure 3 are called Evans diagrams. The determination of the
Figure 3 Logarithmic presentation of current-potential curve of a mixed electrode
(Evans diagram) showing the corrosion potential E
cor
and the corrosion current density
i
cor
. The broken lines indicate the partial current densities.
Copyright © 2002 Marcel Dekker, Inc.
corrosion rate from electrochemical polarization experiments was introduced by
Stern and Geary [7], but the development of the theory of mixed electrodes, due
to Wagner and Traud [8], dates much earlier.
MASS TRANSPORT-CONTROLLED ELECTRODE REACTIONS
For a corrosion reaction to take place, oxidizing agents must reach the electrode
surface. Similarly, reaction products must be eliminated from the electrode surface
by transport toward the bulk solution. If mass transport of reactants and products
is slow compared to charge transfer, their concentration at the electrode surface
differs from that in the bulk solution: the concentration of reactants diminishes,
that of products increases. Figure 4 shows the concentration profile of a reactant.
Three mechanisms contribute to mass transport: diffusion, convection, and migration.
For neutral species such as dissolved oxygen and for ionic species present in small
amounts in a supporting electrolyte, the contribution of migration is negligible.
Transport then occurs by diffusion and convection only. To describe transport
by diffusion and convection, one frequently uses the Nernst diffusion layer model,
which postulates that the electrolyte volume can be divided into two regions.
Near the electrode surface convection is negligible and transport occurs by
diffusion only. In the bulk electrolyte, on the other hand, the concentration is
uniform and no diffusion takes place. The thickness δ of the so-called
Nernstdiffusion layer is obtained by extrapolating the gradient at the surface (
υ
= 0)
to the bulk concentrations (Fig. 4). Its value depends on convection conditions and
is typically of the order of a few micrometers [4].
Let us assume that the reaction rate for the reduction of an oxidant B
ox
is
partly mass transfer controlled. If one neglects migration, the flux N
B
at the
electrode surface is given by Eq. (64).
Electrochemical Basis of Corrosion 11
Figure 4 Concentration of a reacting species as a function of distance from the electrode.
Copyright © 2002 Marcel Dekker, Inc.
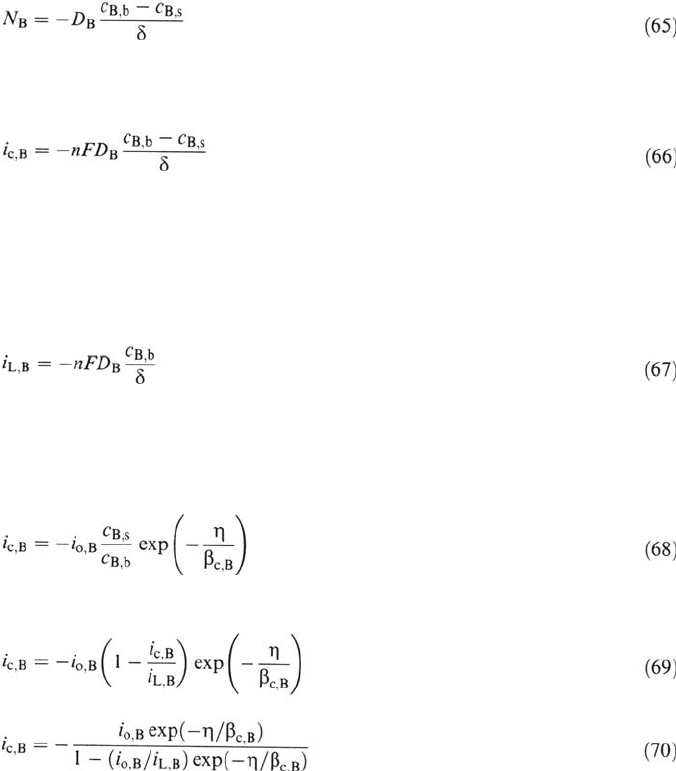
Integration over a constant diffusion layer thickness δ yields
12 Landolt
In these equations D
B
is the diffusion coefficient of B
ox
and c
B,b
and c
B,s
indicate
the bulk and surface concentrations of B
ox
, respectively. With Faraday’s law one
obtains for the cathodic partial current density:
This expression is valid at all potentials. If the potential becomes increasingly
cathodic the absolute value of the current density i
c,B
increases and, consequently,
c
b,s
decreases. Eventually, a condition is reached where c
B,s
becomes zero. This
situation corresponds to the maximum possible reaction rate, and the corresponding
current density is called the limiting current density i
L
.
Below the limiting current density, mass transport as well as charge transfer
determines the overall reaction rate. For η/β
c,B
<< – 1 the Butler-Volmer equation
(34) reduces to (68), where β
c,B
is the cathodic Tafel coefficient for the reaction of
species B.
Combining this expression with (66) and (67), one obtains (69) and (70).
If i
o,B
/i
L,B
exp(–η/β
c,B
) <
<
l, Eq. (70) reduces to Eq. (47) describing a charge
transport–controlled cathodic reaction obeying Tafel kinetics. On the other hand,
if the absolute value of i
o,B
/i
L,B
exp(–η/β
c,B
) is large compared with 1 Eq. (70)
becomes i
c,B
=i
L
,B
. At the limiting current density the reaction rate is entirely
controlled by the rate of mass transport and no longer depends on potential.
Figure 5 shows on a semilogarithmic plot a cathodic partial reaction
corresponding to Eq. (69). At low current densities the reaction follows Tafel
kinetics. As the current density increases, transport becomes more important and
eventually a limiting current plateau is reached. The figure also shows a charge
transfer–controlled anodic partial reaction. The corrosion potential is situated at
the intersection of the two curves, which is located on the limiting current
plateau of the cathodic partial reaction. The corrosion current density, therefore,
is equal to the limiting current density of the cathodic partial reaction. The rate of
corrosion in this case is mass transport controlled. Corrosion in neutral media in the
presence of dissolved oxygen frequently follows this mechanism.
Copyright © 2002 Marcel Dekker, Inc.
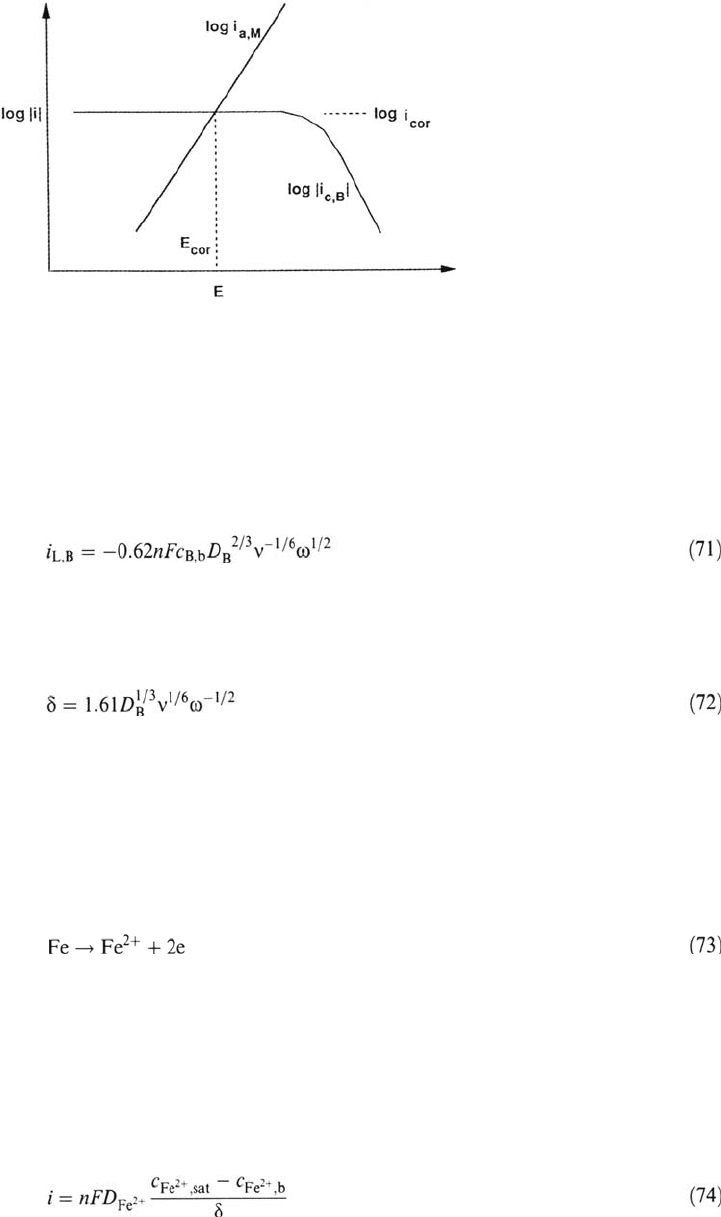
In the laboratory one frequently uses rotating-disk electrodes for the study of
mass transport effects. According to the Levich equation [9], the limiting current
density for a cathodic reaction at a rotating-disk electrode under laminar flow
conditions varies linearly with the square root of the rotation rate.
Electrochemical Basis of Corrosion 13
Figure 5 Evans diagram showing a cathodic partial reaction controlled by mass transport.
In this equation
ν
represents the kinematic viscosity and ω the rotation rate in
rad/s. The other symbols have their usual meaning. Combining (71) with (67)
yields for the Nernst diffusion layer thickness:
According to (72), the value of δ depends on the diffusion coefficient of the reacting
species. This clearly shows that the Nernst diffusion layer is not a physical entity
but a theoretical concept permitting us to treat separately the contributions of
convection and diffusion to mass transport.
Under certain conditions the rate of transport of anodically formed dissolution
products may become rate limiting. For example, the dissolution of iron in
concentrated chloride media according to the reaction
leads to accumulation of ferrous ions in the diffusion layer. At sufficiently high
dissolution rates the concentration at the electrode surface reaches the saturation
concentration of ferrous chloride, causing the precipitation of a salt film on the
surface. This condition manifests itself by the appearance of a transport-controlled
current plateau on the current-potential curve. Neglecting migration, the limiting
dissolution current density is given by Eq. (74) where c
Fe
2+
,sat
indicates the
saturation concentration of ferrous ions at the electrode surface.
Copyright © 2002 Marcel Dekker, Inc.
