Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

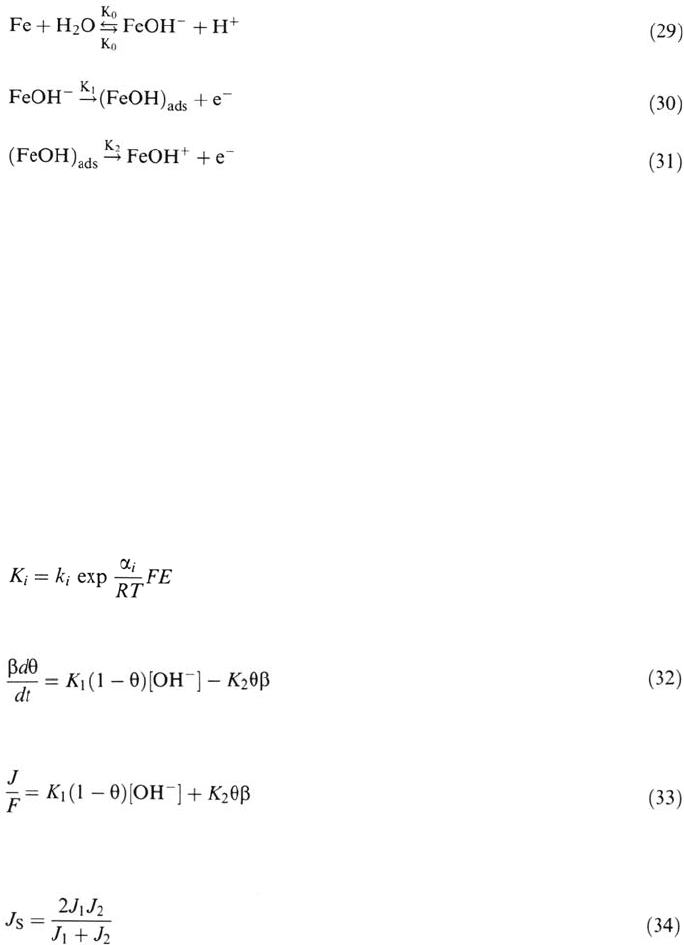
A rather convincing justification of the catalytic mechanism is reached in
terms of atomistic events, at least on conveniently oriented crystal faces. Now, in
turn, the problem remains of understanding the pH dependence of the step nucleation
probability [31]. Further investigation of iron dissolution by impedance analysis
has clearly proved that both catalytic and consecutive mechanisms are operative
with a relative contribution depending upon electrode potential and solution pH.
Advanced Study of Iron Dissolution by Impedance Techniques Early
application of impedance measurement to the anodic dissolution of iron in acidic
media demonstrated the inductive character of the faradic impedances. The reaction
scheme initially proposed [13,60] is modified according to:
116 Keddam
The initial fast chemical hydrolysis step is introduced to account for the
order of the first reaction with respect to OH
–
confirming the results of previous
authors [13]. This modification, introduced later [71,72], implies clearly that the
origin of chemisorbed hydroxyls is the dissociation of water.
Steady-state current-voltage characteristics and faradic impedance associated
with the mechanism (24) to (31) were derived and numerically simulated with the
following assumptions:
Step (29) in fast equilibrium, i.e., surface concentration of FeOH
–
proportional to
[OH
–
] and time independent
Two-dimensional (2D) surface coverage θ by (FeOH)
ads
governed by a Langmuir
adsorption isotherm
Rate constants K
1
and K
2
obeying Tafel kinetics with symmetry factors (0 < α
i
< 1)
but not constrained to 0.5:
With this particular mechanism, Eqs. (2) and (3) are written as
where β is the surface concentration of (FeOH)
ads
at full coverage (θ = 1).
The steady-state coverage θ
S
obtained by solving (32) for dθ/dt =0,
introduced in (33), gives a current-voltage relationship:
Copyright © 2002 Marcel Dekker, Inc.
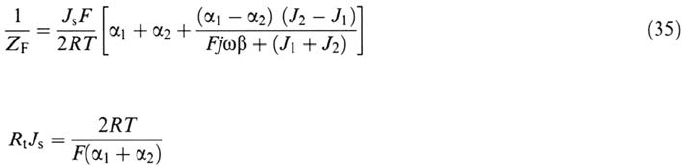
where J
1
and J
2
are the partial current densities flowing through steps (1) and (2):
J
1
= FK
1
[OH
–
] and J
2
= FK
2
β
Similarly to Eq. (6), the solution of the linearized form of (32) and (33)
yields the impedance expression
Anodic Dissolution 117
and an associated
A fair model-experiment fit is obtained for both DC and AC responses with
the same set of kinetic parameters and a value of β, 3 × 10
–9
mol cm
–2
, in good
agreement with one monolayer of adsorbed OH
–
ions on a low-index plane of the
iron lattice (2 × 10
–9
mol cm
–2
on {100}).
The next advance was achieved owing to the extension of the frequency
domain into the millihertz range by digital TFA [47]. The existence of a second
inductive loop was then established at lower frequencies. A branching reaction
mechanism was proposed by introducing in parallel with the consecutive mechanism
a catalytic dissolution path [75]. This branching reaction path was already
prefiguring the main features of the more recent models covering broader ranges
of pH and current density.
Later on, more accurate impedance measurements were reported by Lorenz
et al. [76] and Keddam et al. [61,62] in 1 M sulfate solutions at 0 ≤ pH ≤ 5 and
0 ≤ j ≤ 150 mA cm
–2
. Both groups agreed reasonably about the main impedance
shapes and particularly the presence of three relaxation processes showing up at
intermediate pH values (around 2). The models elaborated by these groups have
in common the interpretation of the frequency dependence in terms of degrees of
coverage by adsorbed species. However, very large differences lie in the basic
assumptions regarding
The actual nature of the surface species participating in the impedance response
and the use of an adhoc “surface relaxation time constant” [68]
The existence or not of a consecutive mechanism in one of the reaction routes
The ability to interpret DC and AC data by a unique model in the active, transition,
and prepassive range
A detailed discussion of the relative performances and flaws of these
approaches can be found in Ref. 62.
A systematic analytic screening of all the possible 40 reaction schemes in
which three Fe-containing surface species are involved completed by a numerical
simulation finally led to selecting the reaction pattern [20] shown below. The three
coverages determining the impedance properties in the active and transition
domains are related to Fe(I)
ads
, Fe*(I)
ads
, and Fe*(II)
ads
. The superscript (*) indicates
species involved in catalytic dissolution paths. Fe(II)
ads
is a precursor of the passive
film whose contribution is significant only near the second maxima and beyond.
Copyright © 2002 Marcel Dekker, Inc.
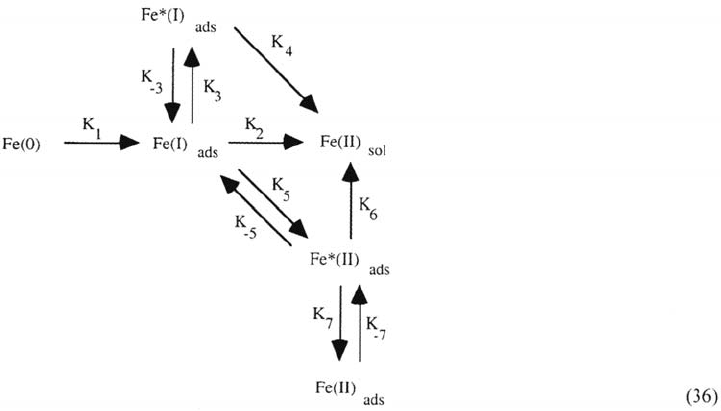
Numerical simulations showed that
The K
1
, K
2
path is the consecutive mechanism early established by impedance
measurements bounded to the hertz range and covering low pH and low
current density [60].
The K
1
, K
3
, K
4
path is a catalytic route mainly operative in the transition range, the
Fe*(I)
ads
coverage being responsible for the decrease of dissolution rate past
the first peak shown in Figure 5A in this range at higher pH.
The K
1
, K
5
, K
6
path is the main dissolution route at higher pH and current density
(j > 50 mA cm
–2
at pH 2 and j > 10 mA cm
–2
at pH 5) controlling the second
peak in Figure 5A.
Both catalytic paths introduced in this model were previously proposed by
Lorenz et al. [78–81] for interpreting the prepassive dissolution range.
Comparison of Figure 5A and B at pH 5 illustrates the outstanding power of
impedance measurements associated with modeling for mechanistic analysis.
The pH dependence of the rate constants proves that chemical bonds of Fe
atoms with solution species contribute to a determining extent to the generation of
active sites of dissolution, even in atomistic models where surface lattice features
(kinks, steps, terraces, etc.) are generally put forward. Large similarities of glassy
metals (amorphous) with crystalline ones [83,61] must also be regarded as arguing in
favor of chemical bonds as predominant entities with respect to lattice related sites.
Further support for the existence of a branching reaction pattern was obtained
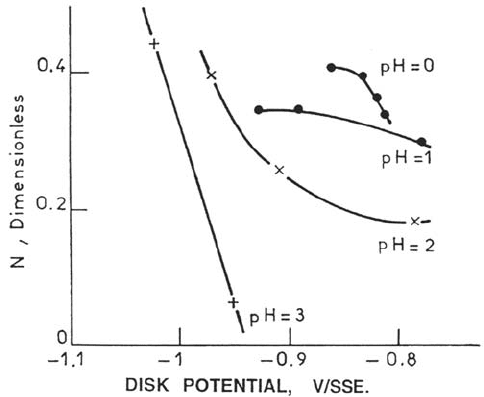
from RRDE investigations [84]. It is found, as shown in Figure 6, that the collection
efficiency N for the oxidation to the trivalent state of the divalent Fe species
released by the dissolution of the iron disk decreases with pH and current density.
This is interpreted by paths K
4
and K
6
producing less reactive Fe(II) species
than K
2
does. In agreement with this view, the lower the rotation speed, the larger
the collection efficiency [84] because a homogeneous chemical reorganization of the
118 Keddam
Copyright © 2002 Marcel Dekker, Inc.
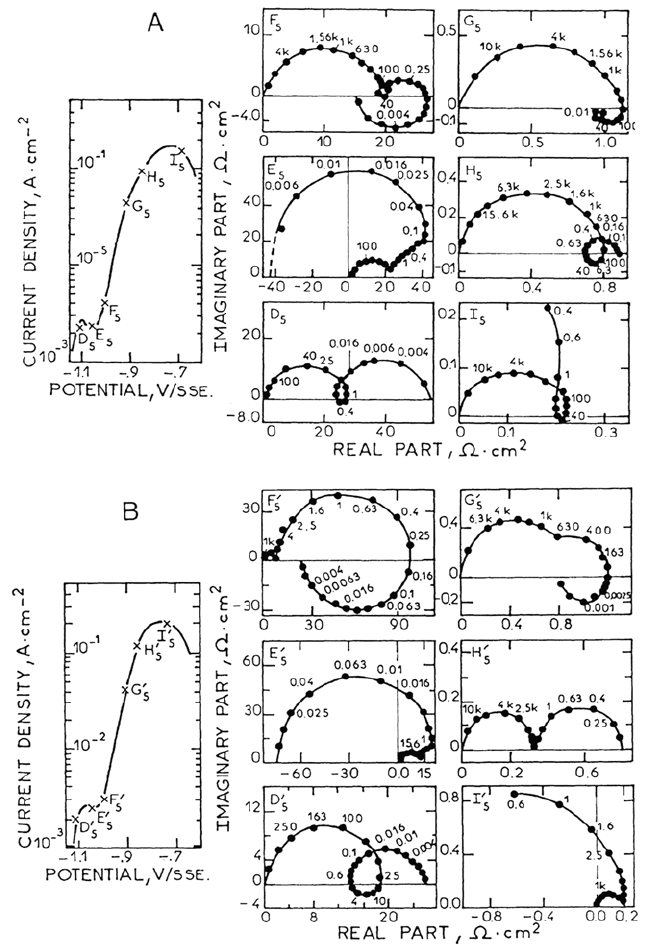
Anodic Dissolution 119
Figure 5. Experimental (A) and calculated (B) current-potential curves and complex
impedance diagrams at the corresponding points labelled on I(E) curves (Frequency in Hz)
(Johnson-Matthey iron, l M sulfate, pH = 5, 25°C). Numerical simulation (B) according to
the reaction mechanism (36). From ref [61].
Copyright © 2002 Marcel Dekker, Inc.
cation into a more reactive one is taking place between disk and ring. Such a
chemical step is formally accounted for in most reaction mechanisms by the
following step:
FeOH
+
+ H
+
→ Fe
2+
+ H
2
O
Similar results are reported in Ref. 85 from channel flow double electrode (CFDE).
Role of Anions in the Anodic Dissolution of Iron
The specific role of the electrolyte composition at a given pH is an extremely
common experience. The contribution of anions other than hydroxyl is expected
through the acid-catalyzed or anion-ligand mechanism [24,25]. It must be emphasized
that the role of pH in polyacids, such as H
2
SO
4
, may be obscured by the correlated
changes of electrolyte composition depending on acidity constants [86].
Specific anion dependence is expected to occur in the passive and transpassive
domains, and dissolution in the active range can be made to deviate from the
hydroxo-ligand mechanism [87] only by anions able to replace OH
–
, essentially
SH
–
[88] and the halide ions. In the case of iron, due to the well-known passivity
breakdown and subsequent localized corrosion by halide ions and particularly Cl
–
,
chloride effects have been investigated extensively. Complexing anions such as
acetate have also been considered to a lesser extent.
In the case of acetate, a series of papers [71,86,89] considered the participation
of the anion from the initial reaction steps of dissolution and it was also claimed that
this anion contributes only to the dissolution of aged solid phases [90]. Later studies
[86,89] in acetate solutions at neutral and slightly acid pH reported a clear negative
order with respect to A
–
or HA. A model is derived on the basis of the participation
of HA and OH
–
, which are assumed to play a symmetric role with the formation of
120 Keddam
Figure 6. Potential dependence of the collection efficiency: influence of solution pH
(ring reaction Fe
2+
→Fe
3+
) for iron dissolution (Johnson-Matthey iron 1M sulfate).
Theoretical collection efficiency [37]: 0.48. Shift to cathodic potentials at increasing pH is
consistent with Figure 5. From ref [84].
Copyright © 2002 Marcel Dekker, Inc.
(Fe(OH)
2
)
ads
, (Fe(OHA))
ads
, and (FeA
2
)
ads
. The kinetics of iron dissolution in the
active range in the presence of halide ions X
–
is largely dominated by the
competitive adsorption of X
–
[88] with the dissolution activating OH
–
. A critical
survey of the possible reaction paths in which Cl
–
competes with other anion
adsorption is given in Ref. 73. The mechanism is claimed to depend on the pH
range. The catalytic step of dissolution in Cl
–
-free media [63] is considered to
become at medium acidities (pH > 0.6):
Anodic Dissolution 121
In strongly acidic solutions the formation of the surface complex FeCl
–
H
+
and dissolution as FeCl
–
are proposed to interpret the accelerating effect of H
+
on
the dissolution rate [73].
According to other authors [91,92], Cl
–
and OH
–
would play a symmetric
role by forming (FeClOH)
–
ads
as an intermediate in a consecutive mechanism
followed by the rate-determining step
and in highly acidic solutions [92] the contribution of Cl
–
in the dissolution path
is depicted by the formation of (FeCl)
ads
and FeClH
+
as intermediate species.
Impedance measurements have been applied to iron dissolution in Cl
–
-con-
taining media [93–95]. Based on the mechanism for sulfate media and similarly to
earlier work [61,62], a branching pattern was proposed in (HCl/NaCl) involving
a consecutive and a catalytic dissolution path having (FeOH)
ads
as a common
bifurcation species. A third reaction path of the consecutive type with (FeClOH)
–
as an intermediate is in agreement with Ref. 91. An interpretation of the role of Cl
–
was advanced [94,95] in the framework of a progressive modification of the sulfate
mechanism [61,62] as a function of the chloride concentration, up to the sulfate-free
medium.
Mechanistic changes with pH are interpreted in a semiquantitative way as
follows: a gradual increase of Cl
–
content initially reduces the contribution of the
(K
1
, K
5
, K
6
) dissolution path in favor of a catalytic step similar to (K
4
), the Fe*(I)
ads
catalyst now being a Cl
–
-containing species.
Active Dissolution of Other Metals
The metals belonging to the so-called iron group (Co, Ni), which exhibit an
active-passive transition, have been far less extensively investigated than iron
itself. The state of the art can be found in Ref. 2. Little contribution is due to
impedance measurements and the mechanisms must be regarded as much less
solidly established than for iron.
Cobalt Cobalt seems to behave much similarly to iron and its dissolution is
assumed to take place through the formation of (CoOH)
ads
, a catalyst surface
species produced in an initial step of dissociative adsorption of water analogous to
Eq. (23). Regarding the structural description of the dissolution mechanism in
term of kinks at the lattice surface, cobalt and iron apparently follow the same
process in which atomic kinks are assimilated to catalytic sites (CoOH)
ads
.
Unfortunately, in the case of Co, microscopic evidence of the changes of surface
morphology and consequently estimation of the kink-kink distance are lacking.
Copyright © 2002 Marcel Dekker, Inc.
Nickel The mechanism of active dissolution of nickel remains highly
controversial. It seems that there is no agreement so far even on the actual shape
of the current-voltage profiles. Two-peak or single-peak curves are reported,
depending on the potential sweeping rate, the prepolarization conditions, the
structural properties of the metal, etc. This erratic behavior is tentatively ascribed
to either the remanence of stable natural oxides on the metal surface or strong
interaction with hydrogen. It must be pointed out that experiments performed in
situ on a surface continuously refreshed by mechanical abrasion never yielded
more reproducible results.
Chromium Due essentially to its role in the corrosion resistance of stainless
steels, the anodic behavior of Cr has been far more extensively considered. An
overview can be found in Ref. 96. Early studies were based on polarization curves
[97] and potential transient experiments [98].
Most of the significant data are established by impedance [43,99,100] and
RRDE [101,102] and reveal rather poor reproducibility, probably because of
interference with hydrogen overvoltage and its metallurgical dependence.
Simultaneous dissolution as Cr
2+
and Cr
3+
in the active and active-passive transition
ranges [103] and no pH dependence of the dissolution rate led, in contrast to Fe group
metals, to more direct dissolution mechanisms being proposed [104]. The
following oversimplified model [105,106] was considered with the purpose of
accounting for the Cr dissolution in the description of Fe-Cr alloys [107].
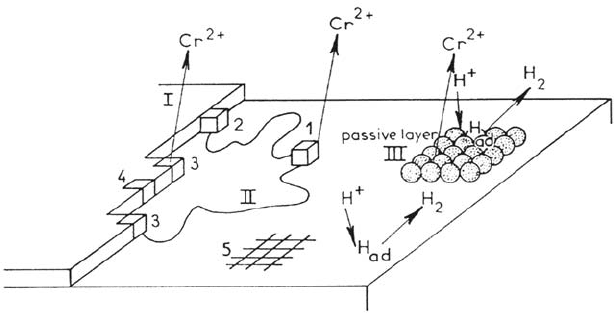
By means of CFDE the existence of adsorbed hydroxylated intermediates
CrOH and CrOH
+
was proposed [101] in the active dissolution range. From
impedance data on polycrystalline and (100) single crystal plane [96,99],
simultaneous hydrogen evolution was introduced in the anodic behavior of Cr by
assuming a strong dependence of the anodic reaction on the H–Cr bonding.
Evidence of diffusion-controlled kinetics in the impedance was tentatively
interpreted by surface diffusion of Cr(I)
ads
, and Cr dissolution and H evolution
were assumed to be competing on terraces. Figure 7 shows the main aspects of this
approach, which attempted to elaborate a kinetic description on the basis of an
atomistic picture of the metal surface.
One of the most innovative ingredients in this model is the coexistence of H
evolution and Cr dissolution on the passive areas, but divalent state of Cr in the
passive range appears quite contradictory to available data from X-ray photoelectron
spectroscopy (XPS).
Other Metals Titanium [108–110] and copper [111] are among the more
extensively investigated metals not pertaining to the iron group or being major
constituents of stainless steels.
122 Keddam
Copyright © 2002 Marcel Dekker, Inc.
Recent Advances in the Mechanism of Passivation by Downstream
Collector Electrode Techniques
In spite of the very elaborate approaches based on current-voltage techniques,
credible steady-state and transient mechanisms have hardly been derived for the
transition from the active to the passive range. An accurate description of the
whole set of elementary steps requires additional data on the relative contribution
of the oxidation current to the formation and growth of 2D and 3D layers and on
the release of cations in solution. Passivation mechanisms can be elaborated on the
basis of the active-passive transition in two complementary ways:
Analysis of passivation transients on an initially active surface either by applying
a steep potential jump into the passive range or by creating fresh surfaces at
constant applied potential by nonelectrochemical depassivation (chemical:
passivity breakdown; mechanical: scratching, ultrasonic waves, etc.; radiative:
laser beam impact [112,113]). These techniques have proved to be of
outstanding importance for the investigation of the mechanism of localized
corrosion associated with passivity breakdown [114,115].
Analysis of tiny changes of the anode maintained potentiostatically between
active and passive states in the negative slope of the dissolution peak.
Electrochemical impedance and frequency-resolved RRDE data are used
together.
Dissolution during Transient Passivation of Freshly Generated Iron
Surfaces Pulse depassivation of the upstream iron anode and collection of the
Fe(II) released into the solution on a downstream glassy carbon were performed
in a CFDE [116]. Transient currents I
D
on the Fe anode and collected currents I
R
for Fe(II) oxidation are shown in Figure 8a and b. After processing for double-
layer charging and collection efficiency, the charges involved in dissolution Q
d
and
Q
s
were discriminated from the overall charge Q
D
supplied to the disk. The results
as a function of pH and disk potential E
D
are shown in the Figure 8c and 8d. The
Anodic Dissolution 123
Figure 7. The corrosion processes occurring at a chromium-sulfuric acid solution
interface. From ref [96].
Copyright © 2002 Marcel Dekker, Inc.
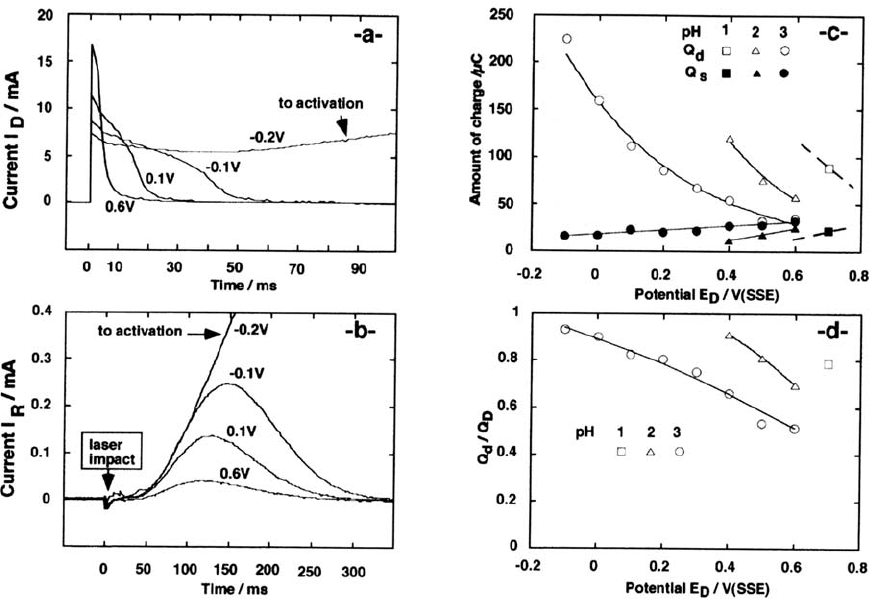
124 Keddam
Figure 8. “a”: transient currents on the Fe working (upstream) electrode. “b”: detection currents on the glassy carbon collector (downstream)
electrode 1M Na
2
SO
4
acidified to pH 3. “c”: potential dependence of the charge Q
d
for dissolution of ferrous cations and Q
s
for passive film
formation at various pH. Depassivated surface area 0.08 cm
2
. “d”: potential dependence of the charge ratio Q
d
/Q
D
(integral emission efficiency)
at various PH.
Copyright © 2002 Marcel Dekker, Inc.

whole set of data was interpreted by the reaction model (40), a simplified form of
the one derived for the faradic impedance of active iron (36). The kinetic equations
for the time dependence of the two coverages by Fe(II) and Fe(III) were integrated
numerically. Considering the wide range of current densities covered by the transient,
both noncatalytic and catalytic reactions paths were considered. Only Fe(II)
species participate in the dissolution mechanism and passivation is ascribed to
Fe(III). These assumptions are justified by the large degree of (super) saturation
in ferrous species during short transients.
That is supported by the results of simulations indicating in the first milliseconds
of the transient a fast buildup of the Fe(II) coverage to a maximum of about 0.5,
which then falls off during the growth of the passive film. The higher the pH and the
applied potential, the sooner and the steeper the transition from Fe(II) to Fe(III).
Impedance and AC RRDE Study of the Active-Passive Range of Iron
Impedance and the AC component of the ring current were simultaneously measured
at a series of polarization points in the negative slope region of Fe in 1 M sulfuric
acid [117]. This region immediately prior to the Flade potential is very meaningful
for understanding the imbrication of dissolution and passivation steps. The ring
potential E
R
was settled at two different potentials for collecting Fe(II) by oxidation
(0.80 V/SSE) and Fe(III) by reduction (–0.40 V/SSE).
Typical data obtained for one point of the polarization curve are shown in
Figure 9. Impedance shows the well-known behavior of a passivation range
characterized by the negative zero-frequency limit of the real part consistent with
the slope of the steady-state current-voltage curve. The low-frequency capacitive
arc displays the frequency response of the film growth. Emission efficiencies N
d
relative to Fe(II) and Fe(III) going to the solution contain two frequency domains,
i.e., a surface charge stored in two different species. For Fe(II) the largest changes
take place in the same frequency range as for impedance. Its positive imaginary
part establishes the passivating role of the faradic surface charge with respect to the
active path of iron dissolution (see earlier). A small loop at higher frequencies is
poorly visible. It reveals a feature not detected on the impedance spectrum and lies
in the half-plane of negative imaginary parts reflecting a surface charge stored in a
Anodic Dissolution 125
Copyright © 2002 Marcel Dekker, Inc.
