Marcus P. Corrosion mechanisms in theory and practice
Подождите немного. Документ загружается.

support anion or mixed anion-cation movement. The network formers are
non-crystalline, while the intermediates tend to be microcrystalline at low
temperatures. The Cabrera-Mott inverse logarithmic expression [Eq. (2)] best fits
the oxides that are noncrystalline, although a fine-grained oxide may be uniform
enough to simulate a noncrystalline one. The metals that are in the modifier class
have been observed to grow crystalline oxides by cation transport. These oxides are
thicker and less protective. Their kinetics of growth fit direct logarithmic kinetics
rather than the inverse type.
Impurities
Water is a major impurity that affects the rate of oxidation at low temperatures. It
can, of course, be the sole source of oxygen at higher temperatures where
dissociation of water can ocuur [17], but at low temperatures it serves to modify
the structure of oxides. It has been found, for instance, that the rate of aluminum
oxidation is increased [18] and that of copper is decreased [19] by the presence of
water in oxygen. The details of the process as it affects low-temperature oxidation
have yet to be elucidated. However, it may be postulated that water can act as a
modifying oxide when added to network-forming oxides and thus weaken the
structure. This would allow oxidation to proceed at a faster rate. On the other
hand, water incorporation into modifiers may result in polymeric species [20] that
are more stable than the polycrystalline oxides. They would form a stable protective
gel layer.
The presence of other impurities such as sodium chloride, sulfur dioxide, or
nitrogen oxides can also change the rate of thin-film formation. Examples of this
are found in the literature of atmospheric corrosion, which is covered in Chapter
15 of this book.
TECHNIQUES FOR MEASURING THIN FILM GROWTH
A primary goal of the analytical work is to combine several methods so that a
comprehensive picture of the metal-oxide system results. The features of particular
interest include oxide structure and kinetics of the oxidation reaction.
Kinetics of Oxide Growth
Measurement of the rate of oxide growth on a metal surface is critical to sorting
out the effects of time, temperature, oxygen pressure, metal structure, and
impurities. The early use of manometric techniques has been supplemented by
recording microbalances. Either method can give a continuous record of the weight
gain or loss of a metal treated in a controlled-atmosphere furnace. Sensitivities in the
submonolayer range are possible.
Resistivity measurements can supplement the information obtained from
weight gain. Fehlner [5c] has pioneered a method that uses discontinuous metal
films. The gaps between islands of metal accentuate the effect of surface oxidation.
Oxide Structure—Chemical
The initial stages of three-dimensional oxide growth can be studied using X-ray
photoelectron spectroscopy (XPS) and Auger electron spectroscopy. The valence
Thin Oxide Film Formation on Metals 177
Copyright © 2002 Marcel Dekker, Inc.
state of the atoms can be determined from energy shifts of the characteristic peaks.
The escape depth of excited electrons from the oxide limits applications to the first
1–2 nm. This is, however, an important stage of growth where valence changes of
impurities can be critical.
Examination of the composition of thicker films relies on depth profiles using
XPS, Auger, or secondary ion mass spectrometry (SIMS), as well as Rutherford
backscattering (RBS) and other nuclear techniques. In SIMS, an ion beam is used
to bore a hole through the oxide. Simultaneously, a mass spectrometer records the
ions that are generated during the boring. Thus, a continuous record in depth is
obtained of the atomic composition. RBS also gives a depth profile of the oxide,
but in a nondestructive manner. The energy distribution of backscattered ions such
as helium is analyzed to reveal atomic mass as well as depth information. In both
techniques, some interferences can be encountered for certain atomic masses.
Oxide Structure—Physical
Again, the methods can be divided into those best used for the initial stages of three-
dimensional oxide growth and those useful in the latter stages. Low-energy electron
diffraction (LEED) and reflection high-energy electron diffraction (RHEED) fall
into the former class. They reveal the periodic structure and changes in that struc-
ture as an adsorbed oxygen layer evolves into the three-dimensional oxide. Thicker
oxide is best examined using transmission electron microscopy (TEM) to look at
both the plane of the film and the cross section. The physical features can be seen
directly. The crystallography is studied using transmission electron diffraction.
These techniques can be enhanced by applying selective etching to the samples.
Scanning tunneling microscopy (STM) and atomic force microscopy (AFM) are
new developments that allow the atomic arrangement on a surface to be monitored.
Changes in structure with oxidation time can be observed using these techniques.
EXAMPLES OF METAL OXIDATION AT LOW TEMPERATURES
The usefulness of a thin oxide film is often the reason for studying its growth rate
and properties. For this reason, each of the examples below will be introduced by
an application.
Silicon
Thin silica films of tunneling dimensions (≤ 5 nm thick) are useful to the electron-
ics industry. Formerly, such films interfered with good electrical contact at
metal-to-metal junctions. Now, however, lateral dimensions on integrated circuits
are reaching the submicrometer range and film thicknesses must scale according-
ly. Metal oxide semiconductor (MOS) transistors with tunneling oxides as gate
dielectrics are fabricated [21]. In the field of solar energy, the use of a thin oxide
film between the silicon solar cell and the positive electrode has led to a conver-
sion efficiency of 22% [22]. This equals the semi-empirical limit predicted by
Bolton [23] and may be compared with the thermodynamic limit of 30%. We see
then that thin silicon dioxide films are certainly useful.
178 Fehlner and Graham
Copyright © 2002 Marcel Dekker, Inc.
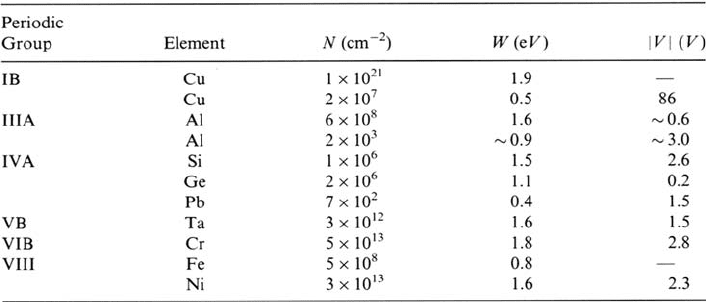
Logarithmic growth of oxide on silicon is found at temperatures up to
approximately 500°C for pressures of 1 atmosphere. Higher temperatures over
1000°C can be used in reduced oxygen environments provided the total pressure
is 1 atmosphere. Kamigaki and Itoh [24] worked with oxygen-nitrogen mixtures,
and Ahn et al. [25] used nitrous oxide. In the latter case, some nitrogen was
incorporated at the oxide-silicon interface. Other methods for forming thin oxides
on silicon include rapid thermal oxidation [26], plasma oxidation [27], and moist
oxygen in the presence of ultraviolet light [28]. The detailed mechanism of
oxidation involving oxygen transport is still under discussion [42–44].
A noncrystalline oxide is formed on silicon. This is because the high oxide
bond strength as given in Table 1 results in a very stable material. At the same
time, it makes self-diffusion of oxygen or silicon difficult. It has been found that
at high temperatures diffusion of oxygen molecules through the silica accounts for
continued oxide growth. Oxygen atoms may be involved in the interfacial reaction
with silicon. Under high electric fields, i.e., at low temperatures or during
anodization, oxygen anions are postulated to be the mobile species. Fehlner [5d]
discusses this point further.
The integrated form of the Cabrera-Mott expression [Eq. (2)] has been
applied to the dry oxidation of silicon [29–31]. Values of N, W, and V have been
calculated and are included in Table 2, which lists values for a number of
elements. The values of W and V are reasonable but that of N is surprisingly low
[9]. It may be related to the fact that anions are the mobile species in silica and the
number of entry sites is limited.
Iron
This metal is used in many facets of industry, commerce, and home life. As such,
the fact that it rusts away assumes vast economic importance. Scientists have
labored to explain the mechanism of corrosion so that the destruction can be
controlled. Both the nature of the corrosion film and the kinetics of the reaction
have proved useful in understanding and avoiding the losses.
Thin Oxide Film Formation on Metals 179
Table 2 Values of N, W, and |V| Obtained from Kinetic Data by Fitting the Cabrera-Mott
Expression [9]
a
a
Note: Repeat runs for the same metal indicate two sources of experimental data.
Copyright © 2002 Marcel Dekker, Inc.
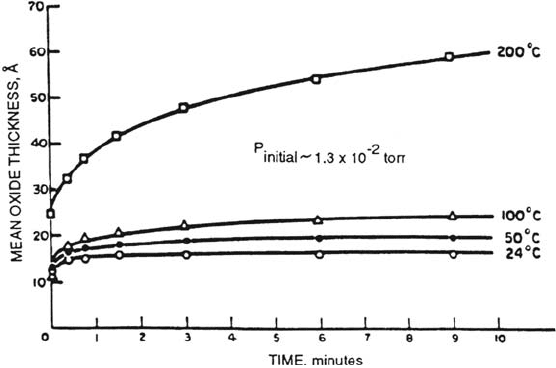
Iron can assume a valence of two or three in oxides. The former acts as a
modifier as listed in Table 1, and the latter can be a network former, at least in
mixed oxide glasses [32].
A logarithmic rate law is followed for the oxidation of polycrystalline iron in
the temperature range 24 to 200°C. Results of Graham et al. [33] are shown in
Figure 3. They were unable to distinguish between a direct and inverse logarithmic
expression. Nevertheless, Eq. (2) has been applied to the data and values of the
significant variables are included in Table 2. The number of active sites N on
the metal surface, assuming cation transport through the oxide, may be related to the
grain boundary population. The value of W is believable, but V was unreliable and
is not included in the table.
Single-crystal studies show that the rate of oxidation varies with crystal face.
The order was found to be polycrystalline > (110) > (112). Observations of the
low-temperature oxide by electron diffraction were interpreted as epitaxial Fe
3
O
4
.
Recently, XPS has been used to determine the composition of thin oxide films on
iron [45–47].
A parabolic oxidation rate was found for temperatures above 200°C. It was
controlled by oxide grain size as dictated by the grain size of the prior oxide. This
was concluded from a study of the effect of surface pretreatment on the rate of
oxidation [34]. Surface preparation is found to play an important role in the
oxidation of many metals and alloys.
Nickel
This metal is less reactive with oxygen than iron. As such, it is used as a
protective coating on base metals and as an additive in iron alloys to improve their
corrosion resistance.
180 Fehlner and Graham
Figure 3 Iron oxidation. Oxide growth on polycrystalline iron as a function of temperature
in an oxygen partial pressure of ~ 1.3 × 10
–2
torr. (From Ref. 33.)
Copyright © 2002 Marcel Dekker, Inc.
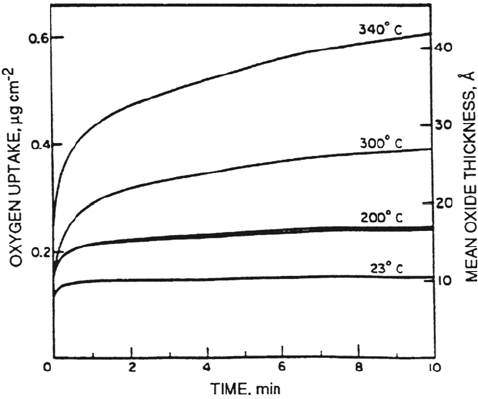
Graham and Cohen [35] reported on the low-temperature oxidation of
polycrystalline nickel. Their results for the temperature range 23 to 340°C are
shown in Figure 4. A direct logarithmic law was followed for oxide thicknesses up
to 3 nm. Parabolic kinetics were followed for thicker films. The crossover
temperature was approximately 300°C.
The valence of nickel in the oxide is two. As a result, the oxide is a modifi-
er (Table 1) and direct logarithmic kinetics are expected. The application of Eq.
(2) to NiO as shown in Table 2 gave reasonable values but the applicability of
the equation is in question. This may be a case where the factor ξx in Eq. (5)
is insufficient to dominate the electric field so that inverse logarithmic kinetics
still apply.
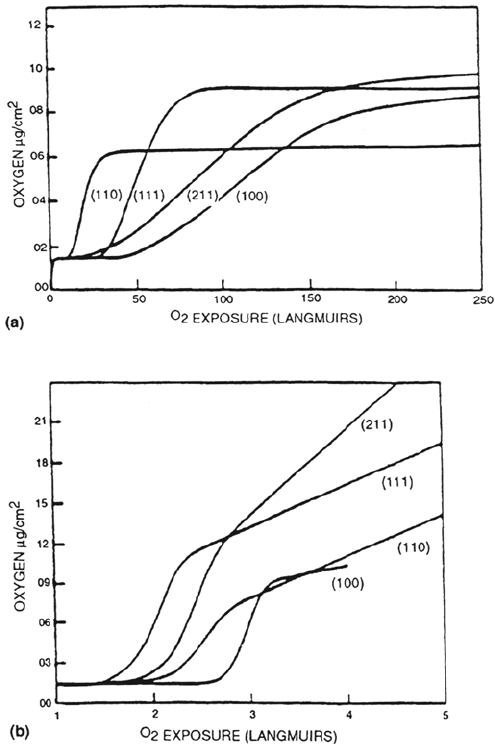
The nucleation and growth of oxide on nickel single crystals have been
extensively studied. The results of Mitchell and Graham [3] are shown in Figure 5,
which illustrates the transformation from oxygen chemisorption to three-dimen-
sional oxide formation. At 40°C (Fig. 5a) the uptake of oxygen at low pressures
shows two distinct plateaus, one corresponding to fractional monolayer adsorp-
tion and the other at longer exposures to the formation of an oxide film two or
three atomic layers thick. A dramatically higher rate of oxidation has been report-
ed for atomic oxygen-induced oxidation [48]. At 200°C (Fig. 5b) the second
plateau is not observed. Instead, continuous uptake of oxygen occurs, indicative
of continuing oxide growth. The kinetics of oxide formation can be explained in
terms of oxide nucleation and growth, the density of nuclei depending on the
particular crystallographic orientation, temperature, and oxygen pressure.
Continuing oxide thickening, as on polycrystaline nickel, follows a logarithmic
rate law.
Thin Oxide Film Formation on Metals 181
Figure 4 Nickel oxidation. Oxide growth on zone-refined, polycrystalline nickel sheet
at 5 × 10
–3
torr oxygen as a function of temperature. (From Ref. 35.)
Copyright © 2002 Marcel Dekker, Inc.
Chromium
This metal by itself is very corrosion resistant. It is therefore used as a coat-
ing for base metals to maintain their integrity. An example is decorative trim
forappliances. Of even greater importance is the use of chromium in alloys.
It is the key component of stainless steel. McBee and Kruger [36] have
shown that the addition of chromium to iron in an alloy causes the oxide film
to go frompolycrystalline to noncrystalline as the amount of chromium
increases. Ingeneral, oxidation of an alloy causes the more base component
to segregate to the surface while the more noble component concentrates in
the bulk of the alloy.
Low-temperature oxidation of polycrystalline chromium has been studied by
Young and Cohen [37]. They found that a transition from inverse logarithmic to
182 Fehlner and Graham
Figure 5 Nickel oxidation. Oxidation kinetics on various faces of nickel at (a) 40°C and
(b) 200°C. One Langmuir equals 10
–6
torr s. (From Ref. 3.)
Copyright © 2002 Marcel Dekker, Inc.
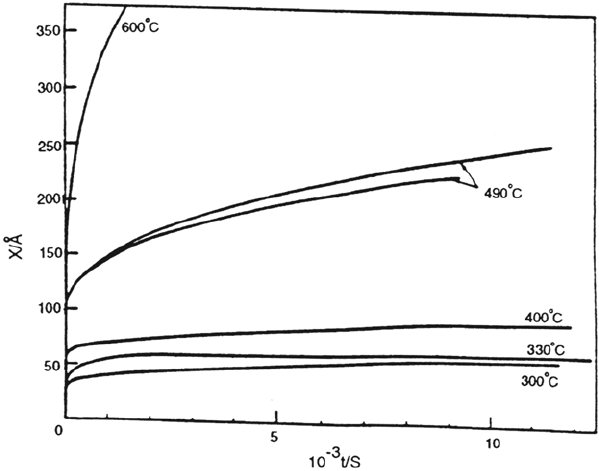
parabolic kinetics occurred at approximately 450°C for the data shown in Figure 6.
However, the transition was a function of both time and temperature. It also
correlated with a change in the oxide structure, from a fine-grained, almost vitreous
oxide to a strongly preferred fiber texture. Ionic transport by cations was assumed
and values for N,W, and V were calculated as shown in Table 2. However, Arlow
et al. [38] present evidence that oxygen diffussion is the main mechanism for
oxide film growth at temperatures below 300°C. Fortunately, Eq. (2) applies to
both cation and anion movement. Only the value of V would change if the charge
on the mobile ion was assumed to be different. At higher temperatures, mixed
anion-cation movement takes place. These findings concerning ion motion agree
with the fact that Cr
2
O
3
can be an intermediate oxide, i.e., have properties between
those of network-forming and modifying oxides, according to Table 1.
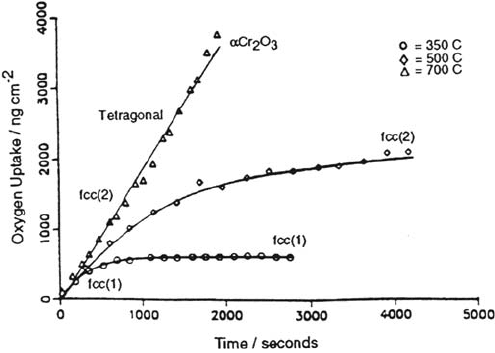
Arlow et al. [38] examined in detail the structure of thin oxides formed on a
Cr(100) single-crystal surface. RHEED patterns distinguished the evolving atomic
arrangements as shown in Figure 7. Two face-centered cubic (fcc) phases and a
tetragonal phase were observed before the precipitation and growth of α-Cr
2
O
3
.
There is also a transition from initial linear kinetics to a logarithmic reaction rate
that approaches zero at some limiting thickness.
Recent work on Cr(110), under conditions where the kinetics of oxide growth
are governed by chromium ion diffusion, shows that below 450°C Cr
2
O
3
grows on
a layer-by-layer basis whereas at higher temperatures the oxide surface roughens
and cavities are formed at the metal-oxide interface [49–51]. In situ STM and Auger
studies reveal that the oxidation of Cr(110) proceeds in three stages [52]. After an
initially formed superlattice is saturated, it rearranges and characteristic oxide stripes
Thin Oxide Film Formation on Metals 183
Figure 6 Chromium oxidation. Oxide growth on polycrystalline chromium at 4 × 10
–3
torr oxygen as a function of temperature. (From Ref. 37.)
Copyright © 2002 Marcel Dekker, Inc.
form at terraces or above 700°C at steps on the surface. Subsequently, the stripes
coalesce and bulk Cr
2
O
3
growth continues.
Copper
Usefulness of the red metal is in the same class as that of iron. It is a mainstay of
the modern industrial world. Electrical applications dominate, but it is also an
important ingredient of alloys used for structural and decorative applications. The
formation of a thin oxide on copper does not pose the electrical problem encountered
with aluminum or silicon. Current passes quite easily through the oxide. Extensive
corrosion of alloys, however, is a continuing problem.
Copper is not expected to follow Cabrera-Mott inverse logarithmic kinetics
since its oxide is a modifier according to Table 1. In fact, copper follows direct
logarithmic kinetics. This was emphasized by results (Table 2) from the analysis
of experimental data [5e,9] including the results shown in Figure 2. No attempt is
made here to apply the Fehlner-Mott direct logarithmic expression, Eq. (5) above.
This is because the evidence for oxide recrystallization with time is very complex.
Onay [39] reported on the formation of multiphase, multilayer scales on copper at
300°C. He found that they result from the dissociation of compact cuprous oxide
scale that has lost contact with the copper substrate.
Recent in-situ transmission electron microscopy (TEM) studies by Yang et al.
[53–56] present evidence that the “passive” oxide film on copper nucleates and grows
as oxide islands, not as a uniform layer. Figure 8 from Ref. 53 shows the oxidation
data of Young et al. [13] for (100) Cu (Fig. 2) together with a dark-field image from
the Cu
2
O reflection, where the bright specks are Cu
2
O islands. These islands form at
both atmospheric [53] and very low oxygen pressures [54,55] and in the latter case
184 Fehlner and Graham
Figure 7 Chromium oxidation. Oxygen uptake by Cr(100) as a function of time at a
pressure of 4.7 × 10
–8
torr oxygen at 350, 500, and 700°C. Indicated on the kinetic curves
are the temperature/mass regions over which the various oxide phases are observed. The
two face-centered cubic phases are denoted as fcc(1) and fcc(2). At 700°C, the sticking
factor is unity over the range shown. (From Ref. 38.)
Copyright © 2002 Marcel Dekker, Inc.
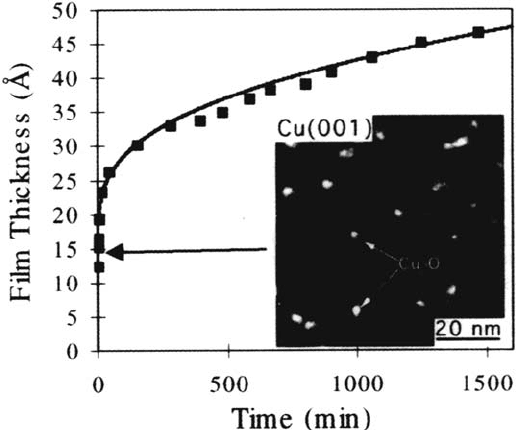
can be observed to grow and coalesce with time. Because the oxide island coverage
is approximately 30%, the local oxide island thickness in Figure 8 is estimated to
be ~ 400 Å, outside the range where an electron tunneling model would apply, and
Yang et al. [53–55] consider that oxygen surface diffusion is the dominant
mechanism for the transport, nucleation, and initial oxide growth on copper. In
contrast to previous speculations, these authors [56] did not observe clear evidence
that surface steps are preferential oxide nucleation sites.
Tantalum
This example illustrates the similarities between anodization and low-temperature
oxidation. Tantalum has formed the basis for a solid-state capacitor that is exten-
sively used in high-performance electronics. The dielectric is formed by
anodization, a process studied by Vermilyea [40], The kinetics are expected to
follow inverse logarithmic kinetics because the oxide is a network former, as
seen in Table 1.
Ghez [8] used Eq. (2) to fit Vermilyea’s results for the temperature range 150
to 300°C, and the resulting values of N, W, and given V are given in Table 2. All
three are reasonable. The results of thermal oxidation of tantalum from 25 to 275°C
[41] were also interpreted in terms of Cabrera-Mott theory.
CONCLUSION
The growth of thin oxide films on metals and semiconductors can be a useful as well
as a destructive phenomenon. The study of the reactions involved leads to a measure
of control. Specific oxide thickness and properties can be achieved for electronic
Thin Oxide Film Formation on Metals 185
Figure 8 Copper oxidation. Oxidation of Cu (100) (Fig. 2 from Ref. 13) and dark-field TEM
image from the Cu
2
O reflection, where the bright specks are Cu
2
O islands. (From Ref. 53.)
Copyright © 2002 Marcel Dekker, Inc.
applications. Materials that grow network-forming oxides are better for these
purposes.
Metals that grow network-modifying oxides more easily undergo degradation
by corrosion. The existence of grain boundaries or other paths of easy ion move-
ment in the oxide allows continued film growth beyond the electron tunneling
limit. A partial solution to this problem is to alloy the metal with one that forms a
network oxide. The alloying metal tends to oxidize preferentially. It can segregate
to the surface as a vitreous oxide film that protects the alloy from further attack.
The need for thin oxides on metals is increasing. Our ability to control the
properties of the oxides will depend on our understanding at the atomic level of
the processes involved.
REFERENCES
1. N. Cabrera and N. F. Mott, Rep. Prog. Phys. 12:163 (1948–49).
2. F. P. Fehlner and N. F. Mott, Oxid. Metals 2:59 (1970).
3. D. F. Mitchell and M. J. Graham, Intern. Corrosion Conf. Ser. NACE-6, p. 18 (1981).
4. D. F. Mitchell and M. J. Graham, Surf. Sci. 114:546 (1982).
5. F. P. Fehlner, Low-Temperature Oxidation: the Role of Vitreous Oxides, Wiley, New
York, 1986, (a) p. 110, (b) pp. 148ff, (c) pp. 94ff, (d) pp. 228ff, (e) p. 199.
6. U. R. Evans, An Introduction to Metallic Corrosion, 2nd ed., Edward Arnold,
London, 1963.
7. H. H. Uhlig, Corrosion and Corrosion Control, 2nd ed., Wiley, New York, 1971.
8. R. Ghez, J. Chem. Phys. 58:1838 (1973).
9. F. P. Fehlner, J. Electrochem. Soc. 131:1645 (1984).
10. W. D. Kingery, H. K. Bowen, and D. R. Uhlmann, Introduction to Ceramics, 2nd ed.,
Wiley, New York, 1976, (a) p. 240, (b) pp. 91ff.
11. J. A. Davies, B. Domeij, J. P. S. Pringle, and F. Brown, J. Electrochem. Soc.
112:675(1965).
12. T. N. Rhodin, Jr., J. Am. Chem. Soc. 72:5102 (1950); 73:3143 (1951).
13. F. W. Young, Jr., J. V. Cathcart, and A. T. Gwathmey, Acta Metall. 4:145 (1956).
14. R. Ramesham, S. DiStefano, D. Fitzgerald, A. P. Thakoor, and S. K. Khanna, J.
Electrochem. Soc. 134:2133 (1987).
15. D. J. Siconolfi and R. P. Frankenthal, J. Electrochem. Soc. 136:2475 (1989).
16. K.-H. Sun, J. Am. Ceram. Soc. 30:277 (1947).
17. O. Kubaschewski and B. E. Hopkins, Oxidation of Metals and Alloys, 2nd ed.,
Butter-worths, London, 1962, pp. 266ff.
18. J. Grimblot and J. M. Eldridge, J. Electrochem. Soc. 128:729 (1981).
19. W. E. Campbell and U. B. Thomas, Trans. Electrochem. Soc. 91:263 (1947).
20. C. S. G. Phillips and R. J. P. Williams, Inorganic Chemistry, Vol. I, Oxford University
Press, New York, 1965, p. 533.
21. W. E. Dahlke and S. M. Sze, Solid-State Electron. 10:865 (1967).
22. Solid State Tech., August 1992, p. 12.
23. J. R. Bolton, Solar Energy 31:483 (1983).
24. Y. Kamigaki and Y. Itoh, J. Appl. Phys. 48:2891 (1977).
25. J. Ahn, W. Ting, T. Chu, S. N. Lin, and D. L. Kwong, J. Electrochem. Soc. 138:L39
(1991).
26. C. A. Paz de Araujo, R. W. Gallegos, and Y. P. Huang, J. Electrochem. Soc. 136:2673
(1989).
27. T. Sugano, Thin Solid Films 92:19 (1982).
186 Fehlner and Graham
Copyright © 2002 Marcel Dekker, Inc.
