Lloyd L. Handbook of Industrial Catalysts
Подождите немного. Документ загружается.

378 Chapter 9
and about twice the normal volume of catalyst had to be used to compensate for
the lower activity of iron sulfide.
The catalysts were originally produced from readily available materials
such as copperas, the ferrous sulfate waste product from steel works, and
chrome tan, an industrial form of chromic acid. The process involved precipita-
tion from ferrous sulfate solution with sodium carbonate. The precipitate was
carefully washed, to remove soluble impurities but early catalysts still contained
up to 1-2% insoluble ferric and chromic hydroxyl sulfates, which formed hydro-
gen sulfide during catalyst reduction. It was possible for the concentration of
hydrogen sulfide in the process gas to reach more than 250 ppm, gradually fall-
ing over a period of several days, before the concentration fell to a an equilibri-
um level. Of course, on most occasions hydrogen sulfide levels were lower than
this. When catalysts were used in naphtha based town gas plants during the
1960s and, later, in single stream ammonia plants with copper based LTS cata-
lyst, the HTS catalyst production method had to be modified. With low sulfur
catalysts it was possible to produce sulfur free gas following reduction in less
than twelve hours.
A further undesirable feature of catalysts containing chromium is that dur-
ing the calcination stage of preparation, a proportion of the chromium oxide is
oxidized to hexavalent chromium at temperatures in the range 250°–350°C. The
concentration of hexavalent chromium falls to an acceptable level of less than
1% when the calcination temperature is increased to about 430°C. High levels of
hexavalent chromium in the catalyst lead to a significant exotherm during reduc-
tion, which can lower the activity of the catalyst. Furthermore, handling of the
catalyst during manufacture can be a very dusty procedure, and contact with the
dust containing hexavalent chromium is hazardous to the workforce, since it
believed to have carcinogenic properties.
9.5.2.1 Operating Conditions
In modern, single stream ammonia plants there is little scope in the design to
make significant changes to the operating conditions in any of the individual
catalyst reactors. Operating conditions for the carbon monoxide conversion reac-
tion are shown in Table 9.14. The only practical variable is operating tempera-
ture which can be slowly increased as catalyst loses activity.
The composition of the catalyst can affect its performance in many ways.
For example, changes in the formulation to lower the amount of hydrogen sul-
fide evolved during reduction led to a significant physical weakening of the
catalyst, which became much more prone to damage caused by the condensation
of water or contamination by potash. Any water that condensed during plant
start-up could also wash out any soluble chromium and lead to loss of stability.
Most problems led to an increase in pressure drop or maldistribution of gas. Cat

Synthesis Gas
379
TABLE 9.14. Carbon Monoxide Conversion Catalysts Operation.
Inlet HTS Outlet HTS Inlet LTS Outlet LTS
Gas rate (m
3
/h) 133,000 146,000
Steam ratio 0.6 0.46
Pressure (atm) 29 28
Catalyst volume (m
3
) 60 64
Temperature (°C) 360
±
20 430
±
20 210
±
10 230±10
Gas composition (%):
CO 13 3.5 <0.5
CO
2
8 16 18
H
2
56 60 61
N
2
22 20 20
CH
4
/A ~0.3 0.5 0.5
alysts were usually, however, able to operate for a period ranging from two to
four years.
With the drive to more energy-efficient ammonia plants in the 1980s, the
steam ratio fed to the primary reformer was significantly decreased. Apart from
the effects in the reformer which have already been described, a number of
changes were detected in the operation of the HTS catalyst. The lower steam
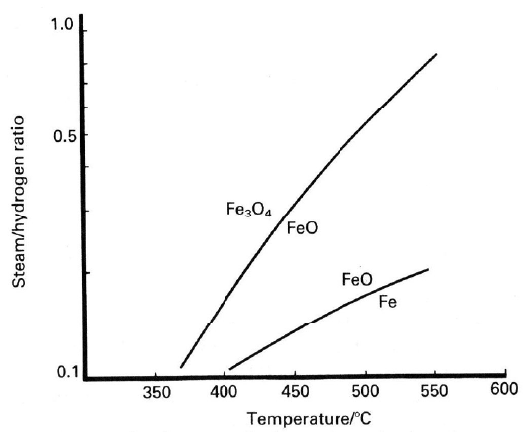
ratio was still greater than the level calculated from the thermodynamics at
which magnetite could be reduced to the metallic state (Figure 9.5). However,
the lower concentration of steam resulted in a higher level of carbon monoxide
in the process gas, as described earlier in this chapter, and this increase in the
carbon monoxide/carbon dioxide ratio gave rise to a more reducing atmosphere.
This, in turn, led to the formation of more carbon by the disproportionation of
carbon monoxide, also known as the Boudouard reaction. The end result was an
increase in pressure drop, and further disintegration of the catalyst.
Carbon monoxide also reacted with magnetite forming carbides which cata-
lyzed the production of hydrocarbons by Fischer-Tropsch reactions. It has since
been shown that both reactions can be suppressed by the addition of copper to
the existing iron-chromium catalyst. This has allowed operation of HTS cata-
lysts in existing plants down to a steam ratio as low as 0.4 compared with about
0.6 in the early single stream plants.
In more recent ammonia plant designs, in which the large steam reforming
furnace has been replaced with gas-heated reformers or combined autothermal
reformers, the HTS catalyst can be replaced by a temperature resistant copper
catalyst that can operate at temperature as low as 260°–270°C.
9.5.3. Low Temperature Carbon Monoxide Conversion
As the industry moved towards cleaner feedstocks, such as naphtha and naturals
gas, and the use of increasingly efficient gas purification systems, the synthesis
380
gas fr
o
made
i
conver
p
er ox
i
200°–
2
to 0.2
–
catalys
ation
o
cedure
chlori
n
import
a
It
i
lyst w
h
large a
m
obvio
u
obvio
u
Chapter 9
Figure 9.5.
M
conventional
Fe
3
O
4
at H
2
O
~400
0
C. Rep
r
sion of M. T
w
o
m the reform
e
i
t possible to
c
sion and to si
m
i
de-zinc oxid
e
2
50°C, lowere
d
–
0.3%. Conditi
o
t from the con
o
f the new cop
was improve
d
n
e, was under
s
a
nt part of the
i
s important t
o
h
ich can oper
a
m
monia plant
s
u
s penalty is t
h
u
s cost is the o
v
M
inimum ratio
HT shift cataly
s
O
/H
2
> 2x10
3
a
r
inted from Cat
a
w
igg.
e
rs contained
o
c
onsider the
u
m
p
lify plant d
e
e
catalyst in 1
d
the concentr
a
o
ns were only
d
ensation of
w
per catalyst w
d
however an
d
s
tood the low
ammonia synt
h
o
understand t
h
a
te at maximu
m
s
. In a typical
h
e daily cost
o
v
erall effect o
f
of steam to hy
s
ts. Fe
2
O
3
b
eco
m
a
t ~550
0
C and
a
a
lyst Handboo
k
,
o
nly trace am
o
u
se of copper
c
e
sign even furt
h
963, operatin
g
a
tion of carbo
n
limited
b
y th
e
w
ater at low te
m
as not reliabl
e
d
the effect o
f
temperature s
h
h
esis process.
h
e commercial
m
conversion
f
1000 tonnes p
e
o
f an unexpe
c
f
an increase i
n
y
drogen for red
u
m
es stable with r
a
bout half this
2
nd
ed., by kin
d
o
unts of catal
y
catalysts for
c
t
her. The intro
d
g
at temperat
u
n
monoxide in
e
need to prev
e
m
perature. Un
f
e
. Once the m
a
f
catalyst poi
s
h
ift (LTS) ca
t
significance
o
f
or long, predi
e
r day ammo
n
c
ted plant clo
s
n
the volume
o
u
ction of
r
espect to
value at
d
permis-
y
st poisons. T
h
c
arbon monox
i
d
uction of a c
o
u
res in the ra
n
the synthesis
g
e
nt damage to
t
f
ortunately, op
a
nufacturing p
r
s
ons,
p
articula
r
t
alyst became
o
f an active c
a
i
ctable
p
eriods
n
ia plant the m
o
s
ure. Anther l
e
o
f carbon mon
o
h
is
i
de
o
p-
n
ge
g
as
t
he
er-
r
o-
r
ly
an
at
a-
in
o
st
e
ss
o
x-
ide leaving the low temperature reactor. One volume of hydrogen is lost for every
Synthesis Gas
381
again an extra three volumes of hydrogen are lost as the carbon monoxide is
removed in the methanator.
CO + H
2
O Æ H
2
+ CO
2
1 vol. H
2
not made (9.13)
CO + 3 H
2
Æ CH
4
+ H
2
O 3 vol. H
2
consumed (9.14)
The additional methane formed must then be purged from the synthesis loop
which leads to even more hydrogen loss as significant hydrogen is also removed
during the purge. Overall, the equivalent of failing to convert just 0.1% of car-
bon monoxide is more than 4000 tons of ammonia every year, or about 1% of
the design production.
Every catalyst in an ammonia plant is important but, because low-
temperature carbon monoxide conversion catalyst is likely to be the most sensi-
tive to poisons, good operation of this catalyst is probably the most significant.
Apart from using the best available catalyst, it is usual to make an allowance for
catalyst poisoning by increasing the catalyst volume in the reactor.
9.5.3.1 Operation
An LTS catalyst should be sufficiently active to give a high conversion for a
given volume of catalyst at the minimum practicable temperature. It should also
be thermally stable and operate for the design period with maximum carbon
monoxide conversion. With proper design and good upstream poisons removal,
a typical catalyst lifetime is about three years.
The catalyst must be activated by careful reduction to convert the copper
oxide component to metallic copper before use (Figure 9.6). The reduction reac-
tion is exothermic and the reduction process must be carried out using an inert
carrier gas to which a low concentration of hydrogen has been added, so that the
temperature of the bed does not exceed 250ºC. After reduction and when a new
charge of catalyst is commissioned, the bed should be heated to a temperature
greater than the dew point of the feed gas so that liquid water does not condense
onto the catalyst when the feed is first admitted. The temperature is then in-
creased gradually until the reaction begins. At this stage, the temperature can be
adjusted until the required degree of conversion is achieved and a satisfactory
temperature profile is seen in the bed.
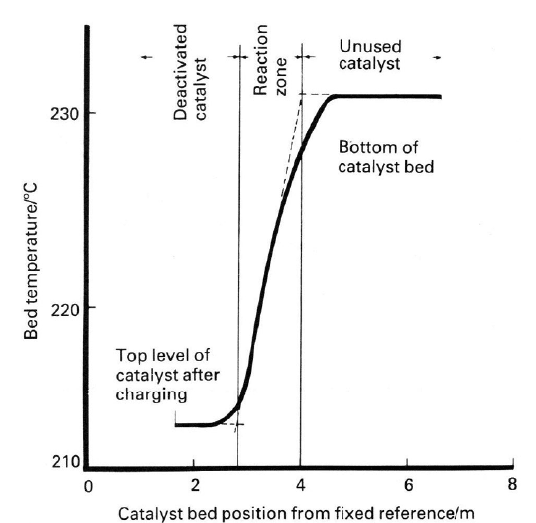
As the catalyst ages, the peak temperature moves slowly down the bed
(Figure 9.7). The rate of movement depends both on the thermal stability of the
catalyst and on the total amounts of different poisons that accumulate on the
bed. The temperature at the inlet to the bed can be increased gradually to com-
pensate for deactivation of the catalyst. Eventually the catalyst must be changed
because the concentration of carbon monoxide leaving the bed at equilibrium
volume of carbon monoxide which is not converted by the LTS catalyst and
Figure 9.6. Sch
e
Catalyst Handb
o
e
matic arrangement
o
o
k
, 2
nd
ed., by kind
p
for the
r
eduction of
p
ermission of M. Tw
i
LT shift catalyst us
i
i
gg.
i
ng a typical once-t
h
h
rough system. Repr
r
inted from
382 Chapter 9
increa
s
the de
g
p
eratu
r
life ca
n
any co
n
into th
e
It
i
no wa
y
guard
b
p
oison
s
oxide
w
seriou
s
The u
s
carbon
Figure 9.7. T
53-1 LT shift
kind permissi
s
es as the temp
g
ree of slippag
r
e profile in t
h
n
be estimate
d
n
densation of
w
e
active layer.
i
s possible to
m
y
of removin
g
b
ed can be ins
t
s
can be remo
v
w
as used as a
s
poison for L
T
s
e of catalyst
a
monoxide co
n
ypical temperat
u
catalyst. Repri
n
on of M. Twigg
erature is incr
e
e of carbon m
o
h
e bed should
b
d
or any malo
p
w
ater in the c
a
m
inimize the e
g
them and a
t
alled in a sep
a
v
ed. This bed
n
guard to rem
o
T
S catalyst is
c
a
s guard has
t
n
version.
u
re profile thro
u
n
ted from Catal
y
.
e
ased, and a p
o
o
noxide is not
b
e measured
r
p
eration of th
e
a
talyst bed mi
g
ffects of pois
o
short catalyst
a
rate reactor
b
n
eed to be cha
n
o
ve sulfur co
m
c
hlorine, ordi
n
t
he advantage
Synt
u
gh a bed of ICI
y
st Handboo
k
, 2
n
o
int is eventua
l
economically
r
egularly so t
h
e
plant detect
e
g
ht wash solu
b
o
ns in the
p
roc
e
life is unacc
e
b
efore the mai
n
n
ged
r
egularly
m
pounds but,
b
n
ary LTS catal
of always en
s
t
hesis Gas
3
Catalyst
nd
ed., by
l
ly reached w
h
viable. The te
m
h
at the remain
i
e
d. For exam
p
b
le poisons do
w
e
ss gas if ther
e
e
ptable. A s
m
n
bed so that
a
. Originally, z
i
b
ecause the m
o
l
yst is prefera
b
s
uring maxim
u
3
83
h
en
m
-
i
ng
p
le,
w
n
e
is
m
all
a
ny
i
nc
o
st
b
le.
u
m
384 Chapter 9
9.5.3.2 Catalyst
The first LTS catalysts were based on copper and zinc oxides, (CuO)/2ZnO),
and were prepared by calcination of the precipitated hydroxycarbonates of zinc
and copper. By 1965, it was found that incorporation of some alumina into the
formulation led to an increase in thermal stability and improved resistance to
poisons. Depending on the plant conditions, the operating life of the catalyst was
increased from about six months to more than two years. The most successful
catalysts were produced by simultaneous precipitation of the copper, zinc and
aluminum hydroxycarbonates, at about pH = 7, to optimize the particle size and
distribution of the oxides. They were similar in composition to the successful
low-pressure methanol catalysts produced in the same way although the copper
content was significantly lower. Other catalysts were introduced which had high
copper content in an effort to achieve a high activity. High copper catalysts were
more susceptible to poisoning in some plants. Another catalyst, based on copper
and zinc oxides but with high chromium oxide content, was expected to be re-
generable, to be able to compensate for the effects of poisons. However, it was
very susceptible to poisoning by sulfur, needed a long period off line for regen-
eration, and its use was not successful. This catalyst has now proved to be an
acceptable chlorine guard for a much more stable copper-zinc-aluminum cata-
lyst. Details of approximate catalyst compositions are given in Table 9.15.
Most spent catalysts contain varying amounts of sulfur, chlorine and silica
poisons depending on the age of the catalyst and the purity of synthesis gas. The
effects of each poison can therefore be considered separately in relation to the
differing operating conditions. In some cases, up to 3–4% sulfur has been meas-
TABLE 9.15. Carbon Monoxide Conversion Catalyst Compositions.
Catalyst Composition
Low Temperature Shift:
Composition (wt%) loss free Low Copper High Copper Chromium
Copper oxide 32 42 20
Zinc oxide 55 47 35
Alumina 13 10
Chromia – – 45
Ignition loss 900°C
<20 <10 <10
Bulk density (kg liter
-1
) 1 1.4 1.2
High Temperature Shift:
Composition (wt%) loss free High Steam Ratio Low Steam Ratio
Ferric oxide 89–91 85–89
Chromia 9–10 7–11
Water soluble CrO
3
<2
Copper oxide – 2
Ignition loss 900°C
<10 <10
Bulk density (kg liter
-1
) 1.3 1.3
Synthesis Gas
385
ured in samples from the top of catalyst beds when the life has been as long as
seven years. The level of chloride in the same samples has been less than 0.02-
0.04%, and it can be concluded that sulfur is held firmly by the catalyst and may
not be a serious problem. A possible explanation for this observation is that alt-
hough sulfur compounds are initially adsorbed by the copper crystallites, they
are rapidly transferred to the small zinc oxide crystals. Zinc sulfide, which is
thermodynamically more stable, is easily formed. This mechanism is dependent
upon the manufacturing process to provide small crystallites in the catalyst
structure. Similarly, silica deposits of up to 1.5wt% at the top of the LTS cata-
lyst bed have been detected after satisfactory lives of two to four years.
On the other hand, while chlorides accumulate near the top of the catalyst,
they are more mobile and can be detected in significant concentrations, up to
0.05%, at all levels in a deactivated bed. Although reasonable lives of at least
two years can often be achieved in the presence of chloride there is more rapid
movement of the peak in temperature profile, and the concentration of carbon
monoxide in the outlet gas increases more rapidly. Surface chlorides, which are
formed by reaction with zinc oxide, are mobile and sinter the catalyst surface.
Chlorides are also soluble in condensed steam and can be washed down onto
lower, more active catalyst layers.
Sulfur and chlorine are both present in the hydrocarbon feed, the process
steam and the lubricating oils used while sulfur may also come from the high
temperature shift catalyst. A major source of chlorine is from the air used in the
secondary reformer. Silica is present in process steam but also comes from the
refractory linings or support materials used in the reforming section.
9.6 METHANATION
Carbon monoxide and carbon dioxide cause the temporary deactivation of am-
monia catalysts. Carbon dioxide can also lead to further problems because it
forms ammonium carbonate in the make-up gas compressor and the synthesis
loop. The removal of these impurities is, therefore, a vital step in the purification
of synthesis gas. Removal of carbon dioxide has generally been via absorption
in some suitable solvent, whereas at the present time, the concentration of car-
bon monoxide is reduced to a low level by reaction with steam in the water gas
shift reaction, prior to almost complete removal by an additional procedure.
Methanation, the conversion of carbon monoxide and dioxide to methane,
was described by Sabatier in 1905, during his work on hydrogenation catalysts,
and since then it has been used in several industrial processes. Since about 1955,
as the use of large, single-stream\m ammonia plants became more widespread,
the methanation reaction has become the preferred way to remove the final trac-
es of carbon oxides from the process gas.
386
Fig
u
Thi
s
2
nd
e
9.6.1
When
0.7%
o
A sim
p
250°–
3
ides o
f
for ev
e
Chapter 9
u
re 9.8. Photo
g
s
vessel contain
e
e
d., by kind per
m
Operation
the plant is o
p
o
xides of carb
o
p
le, single-
b
e
d
3
20°C dependi
n
f
carbon (Figur
e
e
ry 0.1% carb
o
g
raph of a met
h
e
d about 25 ton
n
m
ission of M. T
w
p
erating norm
a
o
n, which need
d
reactor can
b
n
g on the cat
a
e
9.8). There i
s
o
n monoxide
a
h
anator in a typ
n
e catalyst. Repr
i
w
igg.
a
lly, the synt
h
to be remove
d
b
e used at an
a
lyst activity a
n
s
an adiabatic
t
a
nd 6°C for e
v
ical ammonia
s
i
nted from Cata
h
esis gas cont
a
d
during the
m
inlet tempera
t
n
d the concen
t
t
emperature ri
s
v
ery 0.1% car
b
s
ynthesis plant.
lyst Handboo
k
,
a
ins around 0
.
m
ethanation sta
g
t
ure in the ra
n
t
ration of the
o
s
e of about 7.4
b
on dioxide c
o
.
3–
g
e.
n
ge
o
x-
°C
o
n-
Synthesis Gas
387
verted. Although the catalyst should not normally be operated at temperatures
exceeding 450°C it is frequently allowed to overheat to temperatures as high as
600ºC during periods of maloperation, but seems to incur little deactivation..
There is probably more chance of damage to the reactor in these situations. At a
space velocity in the range 5000-7000 hours
-1
methanation is virtually complete
at a space velocity in the range 5000-7000h-1 and the outlet carbon oxides level
is usually less than 5ppm. Good methanation catalysts have a normal life of
about ten years.
The most common catalyst poisons are derived from traces of solvent car-
ried over from carbon dioxide removal process. These simply block the catalyst
pores and, providing no sulfur or arsenic compounds are used, can easily be re-
moved by washing the catalyst with water to restore activity. If sulfur enters the
catalyst bed from any other source it will rapidly poison the methanation cata-
lyst. The low temperature shift catalyst, up-stream of the methanator, usually
acts as a very efficient sulfur guard!
9.6.2. Catalyst
Different suppliers have produced methanation catalyst in a wide range of
shapes and sizes, ranging from spheres and pellets to extrusions and granules,
containing between 15–30% nickel oxide. Some typical support materials react
with nickel oxide to form relatively stable spinels, which cannot readily be re-
duced to give an active catalyst, and these materials are not used. It is better if
the nickel oxide can be precipitated in solid solution with a thermally stable ma-
trix similar to those supports used in reforming catalysts. Typical supports are
alumina, silica and magnesia, sometimes bound by alumina cement. The most
important catalyst property is that it can be reduced easily at a temperature at or
below 300°C to give an active and stable catalyst. The nickel can be combined
with the support by either impregnation or precipitation, provided that the final
catalyst is thermally stable.
The nickel oxide component of the catalyst is reduced to the metal with
process gas, by gradually increasing the temperature of the bed to about 300ºC.
If necessary, the reduction procedure can be assisted by allowing a small propor-
tion of the process gas to bypass the low temperature shift converter. This results
in a temporary increase in the concentration of the oxides of carbon in the reduc-
ing gas to around 1–2%, which are then hydrogenated into methane over the
active part of the catalyst. The resulting exotherm from the reaction increases
the temperature of the bed to about 400
o
C, so that any remaining nickel oxide
can be reduced fully. During the reduction period the carbon monoxide and car-
bon dioxide content of outlet gas is analyzed at regular intervals. Full operation
can start when the level falls to less than 20 ppm. This normally takes 10–15
hours. If the LTS reactor cannot be by-passed the catalyst reduction should be
