Lloyd L. Handbook of Industrial Catalysts
Подождите немного. Документ загружается.


408
Figure
the fusi
o
(1989)
b
10.1.2.
Cataly
s
flexibl
e
regula
r
b
y po
u
first b
e
loadin
g
all par
t
diamet
p
ossib
l
to achi
e
Pr
e
p
roper
l
while
m
catalys
to que
n
Chapter 10
10.3. Flow she
e
o
n route. Repri
n
b
y kind permiss
i
3 Loading C
a
s
t can be load
e
e
chute. The c
h
r
level in the
c
u
ring catalyst i
n
e
blocked to pr
g
a quench co
n
t
s of the bed.
T
e
r and a pred
e
l
e. Vibrators a
r
e
ve maximum
e
-reduced cat
a
l
y in hot clim
a
m
aking sure t
h
t bed should b
n
ch any hot sp
o
e
t for a plant to
m
n
ted from Catal
y
i
on of M. Twig
g
a
talyst to Con
v
e
d from a sma
l
h
ute should be
c
onverter. Tub
e
n
to the top of
t
event catalyst
n
verter it is im
p
T
his requires fi
e
termined wei
g
r
e often used t
activity.
a
lyst can be
r
a
tes. Loading
h
at friction or
e checked and
o
ts.
m
ake a pre-redu
c
y
st Handboo
k
, 2
n
g
.
v
erte
r
l
l hopper or a
moved aroun
d
e
-cooled conv
t
he opening ar
o
entering durin
p
ortant to mai
n
xed levels to
b
g
ht of catalys
t
o settle the be
d
r
e-oxidized if
should be co
m
vibration is l
i
access of air
r
ced ammonia s
y
nd
ed., Ed. by M
.
large bag fitte
d
during charg
i
erters can be
f
o
und the tube
s
n
g the loading
p
n
tain the desig
b
e marked aro
u
t
to be loaded
d carefully, w
h
heated in ai
r
m
pleted as qu
i
i
mited. The te
m
r
estricted.
N
itr
o
y
nthesis catalyst
.
Twigg, Copyri
g
e
d with a suita
b
ing to maintai
n
f
illed complet
e
s
. The tubes m
u
p
rocedure. W
h
g
n bulk densit
y
u
nd the conve
r
d
as uniformly
h
ich is necess
a
r
or not hand
l
i
ckly as possi
b
m
perature of
t
o
gen can be u
s
by
g
ht
b
le
n
a
e
ly
u
st
h
en
y
in
r
ter
as
a
ry
l
ed
b
le
t
he
s
ed
Ammonia and Methanol Synthesis
409
10.1.2.4 Catalyst Discharge from the Converter
Spent ammonia synthesis catalyst contains very small crystallites of iron, which
are extremely reactive, much more so than bulk iron metal. These residues are
potentially pyrophoric in air and can react with water to produce hydrogen. Ex-
treme care is therefore required when they are discharged from a converter.
The catalysts are not usually oxidized before being discharged from a con-
verter but, after cooling to ambient temperature, it is important that the converter
should not be exposed to air. The converter is usually purged with nitrogen. Cat-
alyst suppliers and contractors provide special instructions for the procedure.
Water should be sprayed onto catalyst as it is discharged from the converter.
10.1.3 Catalyst Reduction
gen or, more usually, synthesis gas can be used, at pressures in the range 70–100
bar. Gas leaving the bed being reduced should be cooled until reduction is com-
within a given bed for up to four beds can take about four to seven days.
10.1.3.1 Reduction of Oxidized Catalyst
A typical converter, in a plant with a dosage capacity 1000 tonnes per day am-
monia, will hold 100–200 tonnes of catalyst, which generates as much as 35–70
tonnes of water during reduction. Ammonia synthesis begins before all of the
catalyst has been reduced, the aqueous ammonia produced must be used in the
most convenient way. The reduction procedure is deliberately carried out at a
very low rate, to ensure optimum activity of the final catalyst. The presence of
high partial pressures of water vapour in contact with the freshly reduced cata-
lyst results in the growth of the small crystallites within the catalyst, via a re-
oxidation mechanism. This growth in crystallite size is inevitably accompanied
by a reduction of the metal surface area, and hence an irreversible loss of activi-
ty. The concentration of water vapour in the process gas is therefore limited to a
maximum of about 3000 ppm. Concentrations higher than these have been al-
lowed but this requires good gas mixing in the wide diameter beds and rapid
disengagement of water from the reduced particles. Back mixing of reducing
gas, containing water, can lead to re-oxidation and loss of activity. Gas flow
should therefore be as high as possible, the temperature of the bed carefully con-
trolled and water content of reducing gas at the converter inlet should also be as
low as possible. The cumulative volume of water collected during reduction can
be used as a practical indication of how reduction is proceeding and should be
carefully measured.
plete. Providing that there are no unexpected problems, the complete procedure
Ammonia synthesis catalysts must be carefully reduced before use. Either hydro-
410 Chapter 10
During the early stages of catalyst reduction, the maximum rate of gas flow
is limited by the thermal capacity of the start-up heater to maintain the tempera-
ture of the bed. As more catalyst becomes reduced, the exothermic synthesis
reaction begins. The heat from this exotherm supplements the output from the
start-up heater, and the gas rate can be increased. The rate of increase of a bed
inlet temperature should be limited to about 5°C per hour until a maximum out-
let temperature of 500°C is reached. During this procedure, the temperature of
the remaining unreduced catalyst beds should be just below the reduction tem-
perature that is about 350°C for typical catalysts. As more beds are reduced, gas
rates can be increased and the reduction rate is faster despite the larger catalyst
volumes in the lower beds.
10.1.3.2 Reduction of Pre-reduced Catalyst
Pre-reduced catalysts can be reduced much more quickly than oxidized catalyst
because only about 10% of the catalyst is re-oxidized during stabilization. The
procedure, therefore, only takes about one day. Early difficulties when pre-
reduced catalysts were unstable and could overheat on exposure to air have been
overcome and the catalyst is now very stable. It is wise, however, to take sensi-
ble precautions while loading a converter because of the large quantities of cata-
lyst involved.
The proportion of pre-reduced catalyst used depends on the time allowed
for reduction and how easily the by-product aqueous ammonia can be used. Op-
erating costs are balanced with the higher price of the catalyst. Using pre-
reduced catalyst in only the top section of a reactor simplifies the early stages of
reduction and speeds up commissioning. A full charge of pre-reduced catalyst
allows much earlier production of ammonia but is more expensive. Although
water evolution from pre-reduced catalyst is normally low, it is still usual to
reduce only one bed at a time.
10.1.3.3 Mechanism of Catalyst Reduction
α-Iron is produced when ammonia synthesis catalyst is reduced.
22
The catalyst is
initially non-porous, with a surface area of only 1–2 m
2
g
-1
, but on reduction, the
catalyst becomes porous and develops a surface area around 20 m
2
g
-1
. The re-
duction mechanism is unusual and is controlled by the wustite in the catalyst.
reduced to form iron at a lower temperature than that normally required for bulk
catalyst reduction. This provides iron nuclei for further reduction. Ferrous ions
then diffuse through the magnetite structure to the reaction centers where they
combine with magnetite to form wustite, which is also reduced, and the cycle
continues, gradually forming the pore structure needed. There is an optimum
wustite concentration so that diffusion of ferrous ions is not restricted.
Large pores form at the surface as wustite surrounding the magnetite crystals is

Figure
unredu
c
catalyst
of 230
0
Copyri
g
A
f
which
separa
t
alumi
n
it prev
e
surfac
e
nitrog
e
10.4. Scanning
c
ed catalyst sh
o
at different ma
g
, 7700 and 23,0
0
g
ht (1989) by ki
n
f
ter reduction,
the original st
r
t
ed by the new
l
n
a tha
t
has co
m
e
nts crystal gr
o
e
is covered b
y
e
n and weaken
s
electron micro
g
o
wing well-for
m
g
nifications sho
w
0
0. Reprinted f
r
n
d permission o
f
the catalyst c
o
r
ucture has be
l
y created por
e
m
e out of solid
s
o
wth as more
y
a film of
p
ot
a
s
the nitrogen-
n
Ammon
i
g
raphs (SEM)
o
m
ed crystals of
w
ing porous iro
n
r
om Catalyst Ha
f
M. Twigg.
o
nsists of por
o
en replaced b
y
e
s (Figure 10.
4
s
olution to be
r
magnetite is r
e
a
sh tha
t
p
romo
t
n
itrogen bond
i
a and Methanol
o
f ammonia sy
n
magnetite 230
X
n pseudomorph
s
a
ndboo
k
, 2
nd
ed.
,
o
us magnetite
p
y
small, plate-
l
4
). The structu
r
r
e-deposited i
n
educed. Up to
tes the adsorp
t
prior to reacti
o
Synthesis
4
n
thesis catalyst:
X
; (b)-(d) redu
c
s
at magnificati
o
,
Ed. by M. Twi
g
p
seudomorphs
like iron cryst
r
e is stabilized
n
the pores wh
e
90% of the i
r
t
ion of molec
u
o
n with adsor
b
4
11
(a)
c
ed
o
ns
g
g,
in
als
by
e
re
r
on
u
lar
b
ed
(a)
(c)
(b
(
b)
d)
(
(
412 Chapter 10
hydrogen. Ammonia is less strongly bound to the catalyst surface and rapidly
The formation of ammonia on the reduced iron surface is extremely struc-
ture sensitive and the 111 and 211 planes are by far the most reactive of the five
possible crystal surfaces. The close-packed 111 plane, for example, at the base
of each plate-like crystal can expose three layers of iron atoms. Potential active
sites may, therefore, have seven near neighbour iron atoms that are more active
and less easily poisoned than sites with fewer near neighbour atoms. The most
10.1.4 The Ammonia Synthesis Process
Since the Haber process was first introduced, there has been a gradual evolution
of both the process and converter designs as the production capacity of the
plants has increased. Until the 1950s, there were very few changes to either the
converter, synthesis loop or the catalyst formulations used by individual compa-
nies shown earlier in the chapter, in Table 10.3. This was partly because of iner-
tia and because most plants operated with several ammonia converters each
holding a few tonnes of a relatively cheap catalyst that could be conveniently
changed during shutdown periods. This situation altered during the 1960s as
larger capacity, single stream plants, using steam reforming and with a single
ammonia converter, were built in all parts of the world.
10.1.4.1 The Ammonia Synthesis Loop
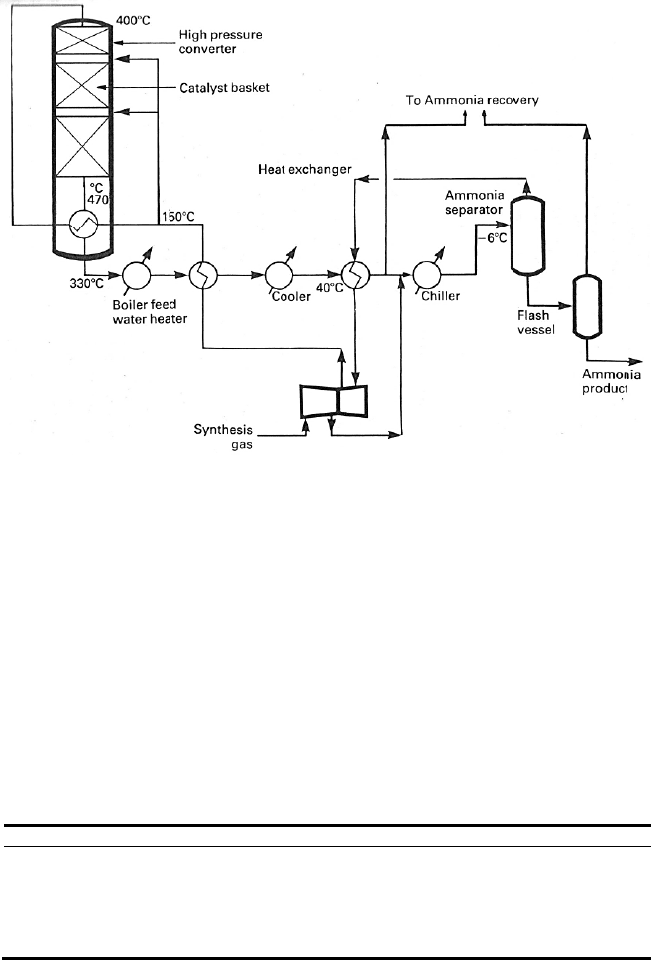
The ammonia synthesis reaction occurs in a loop where synthesis gas can be
circulated continuously through a converter containing the catalyst (Figure
10.5). Ammonia is condensed and removed from the loop while synthesis gas,
known as make-up gas, is added to maintain the design space velocity through
the catalyst. In most ammonia plants, the inert gases, such as methane and ar-
gon, accumulating in the loop are continuously removed in a purge gas stream,
so that they do not reach an unacceptable level. There is no longer any need in
modern plants to install a guard bed to protect the catalyst now that steam-
reformed synthesis gas is poison free. Fresh make-up gas is usually added before
ammonia is condensed. This means that poisons such as traces of compressor oil
or oxygen compounds are removed with the liquid ammonia. Typical composi-
tions of circulating gas, converter outlet gas and purge gas for a low-pressure
ammonia loop are show in Table 10.5.
A liquid nitrogen wash used to remove inerts from the make-up gas
which then contained less than 240 ppm methane was incorporated into some of
the early plants. However, it was found that the inerts were preferentially dis-
solved in the product ammonia and they did not accumulate in the recycle gas in
22, 23
24
25
desorbs.
active sites are known as C7 sites.
Figure
(1000 t
o
heat re
c
Ed. by
M
those
p
p
urge
w
A
s
sures,
i
then to
ated
w
Compos
i
Nitroge
n
Argon
Helium
Hydrog
e
Methan
e
Ammon
i
Notes:
2
10.5. Ammonia
o
nne day
-1
) pla
n
c
overy from th
e
M
. Twigg, Cop
y
p
lants which
w
w
as not requir
e
s
the synthesis
i
t became nec
e
incorporate a
w
ith two separ
a
TABLE 10.
5
i
tion vol%
n
e
n
e
i
a
1. Make up gas e
q
2
. Purge gas is us
e
synthesis loop.
n
t. Preheat of t
h
e
converter exit
y
right (1989) by
w
ere operated
e
d, and any ap
p
loops became
e
ssary to cool
refrigeration s
a
tors and mo
r
5
. Gas Composi
t
Circulating
G
21.0
3.1
0.4
63.4
10.0
2.0
q
uivalent to metha
n
e
d for hydrodesul
f
Ammon
i
This layout is t
y
h
e converter fee
gas. Reprinted
kind permissio
n
at high press
p
arent purge
w
simplified, a
n
the converter
tage using liq
u
r
e product am
m
t
ions in a Low P
r
G
as Con
v
n
ato
r
outlet gas c
o
f
urization and pri
m
i
a and Methanol
y
pical of a loop
f
d gas to 150
0
C
from Catalyst
H
n
of M. Twigg.
s
ure. In both
o
w
as entirely in
v
n
d were operat
e
exit gas with
u
id ammonia.
T
monia was re
r
essure Ammon
v
erter Outlet
18.2
3.4
0.5
54.9
11.0
12.0
o
ntaining <5 ppm
c
m
ary reformer fuel
Synthesis
4
f
or a large capa
c
allows high-gr
a
H
andboo
k
, 2
nd
e
o
f these cases
v
oluntary.
ed at lower pr
e
air or water,
a
T
he system op
e
moved from
t
n
ia Loop.
Purge
G
2
6
1
c
arbon oxides.
.
4
13
c
ity
a
de
e
d.,
, a
e
s-
a
nd
er-
t
he
G
as
2
1.0
3.6
0.5
6
3.0
1
2.0
–
414 Chapter 10
loop, resulting in a lower concentration of ammonia in the recycle gas. Since the
maximum conversion of the synthesis gas to ammonia is limited by the thermo-
dynamics of the reaction, the lower concentration of ammonia in the recycle gas
led to an overall increase in the conversion of synthesis gas to ammonia. It also
became possible to operate with a smaller converter, and still maintain the same
level of production. By the 1960s, refrigeration had become essential when op-
erating the low-pressure ammonia converters, as the equilibrium concentration
of ammonia at 50-bar pressure is only about 12%, and the concentration of am-
monia in the recycle gas was reduced to about 2%.
10.1.4.2 Converter Design
The majority of early ammonia plants used adiabatic beds of catalyst or tube-
cooled converters that acted like a heat exchanger with the cold synthesis gas
passing through the tubes to cool the catalyst. Tube-cooled reactors, such as
those introduced by the Tennessee Valley Authority (TVA), did not operate iso-
thermally and the exothermic reaction led to both axial and radial temperature
profiles developing. The temperature difference depended on the number of
tubes passing through the catalyst bed.
The converter design took this into ac-
count by maximizing the number of tubes although the temperature profile could
only be controlled by changing the inlet gas temperature. There were problems
in loading catalyst into the space between tubes to achieve the right packing
density as well as in discharging catalyst when it was deactivated. Large tube-
cooled converters were also expensive.
Modern converters are designed with several catalyst beds in which the hot
Quench cooling has usually been preferred in plants using multi-bed converters
some of the catalyst with a significant volume of the synthesis gas. The same
Problems were experienced in wide, multi-bed converters, with gas flowing
axially through the beds, because big catalyst particles were required to limit the
pressure drop through the catalyst. Since activity is inversely proportional to
increased the capital cost of a plant. By designing converters in such a way that
gas flowed radially through the catalyst bed, it was possible to decrease the
overall pressure drop and to use smaller catalyst particles that did not suffer
from the limitations of pore diffusion to the same extent, and thus showed great-
er activity per unit volume.
Gas distribution problems in the top bed—
resulting from a low-pressure drop—were overcome by the use of an improved
converter design. In one case, an axial-radial flow system was used. In another,
26
27
gas can be cooled at each bed exit either by heat exchange or by the addition of
particle size, increased volumes of catalyst were needed and the large reactors
cold synthesis gas, often referred to as quench cooling (Figures 10.6 and 10.7).
process is detailed for a three or four bed operation is shown in Table 10.6.
despite the disadvantage of using a larger catalyst volume and having to by-pass

Figure 10.6.
gas is inject
e
gravity disch
a
book, 2
nd
ed.,
of M. Twigg.
ICI quench co
n
e
d and mixed b
y
a
rge of the cata
l
Ed. by M. Twi
g
Ammon
i
n
verter for am
m
y
means of loz
l
yst bed. Reprin
t
g
g, Copyright (1
i
a and Methanol
m
onia synthesis.
z
enges which al
t
ed from Catal
y
989) by kind p
e
Synthesis
4
Quench
l
so allow
y
st Hand-
e
rmission
4
15

416
which
flow t
h
ammo
n
Chapter 10
Figure 10.7.
with permissi
was particula
r
h
rough the cat
a
n
ia converter
d
Topsøe conver
t
on from Haldor
r
ly useful in l
a
a
lyst beds, in a
d
esigns are out
l
t
ers for ammoni
a
Topsøe A/S.
a
rge capacity
a
horizontal co
n
l
ined in Table
1
a synthesis. Re
p
a
mmonia plan
t
n
verte
r
was us
e
10.7.
p
roduced
t
s, transverse
g
e
d. Some rec
e
g
as
e
nt
28
Ammonia and Methanol Synthesis
417
TABLE 10.6. Operation of Low-Pressure Ammonia Converters at 140–150 Bar.
Operation Bed 1 Bed 2 Bed 3 Bed 4
A:
Catalyst volume (m
3
) 12 22 32 —
Temperature inlet (°C)
410 405 400 —
Temperature outlet (°C))
500 470 450 —
Ammonia concentration (vol %) 12
Cooling Quench Quench
B:
Catalyst volume (m
3
) 10 14 18 22
Temperature inlet (°C)
410 400 400 400
Temperature outlet (°C)
480 480 480 450
Ammonia concentration (vol %) 12
Cooling Quench Quench Quench
Notes: 1. Total synthesis gas volume for 1000 tes/day production is >600,00 m
3
/h with 33—42%
being used as quench at 140°C.
2. At 220 atm pressure, 3 beds containing 7 m
3
, 12 m
3
, 22 m
3
(ΔT 95°C, 45°C, 40°C) gave a
conversion from 3% → 15% ammonia.
10.1.5 New Catalyst Developments
At the stage of development of the ammonia synthesis reaction as described in
the previous sections, the main problem was the low level of conversion of syn-
thesis gas to ammonia. This could be increased by operation at yet higher pres-
sure, but this would not be cost effective. The other option would be to operate
at lower temperature, under which conditions, the equilibrium concentration of
ammonia would be appreciably higher. Unfortunately, the catalysts available at
the time were not sufficiently active to operate at the lower temperature required
to make a meaningful difference to the concentration of ammonia in the product
TABLE 10.7. Ammonia Converter Design.
Contractor Converter Design
Kellogg a. Quench cooled, axial flow reactor with 3–4 beds using 6–10 mm catalyst.
b. Horizontal reactor, with down flow through three shallow beds and
quench cooling. Small 1.5–3.0 mm catalyst.
Topsoe Two radial flow beds with intermediate heat exchange and possibly quench
cooling in a single vessel. Small 1.5–3.0 mm catalyst.
Ammonia Casale Axial-radial flow through three catalyst beds with inter-bed heat exchange or
quench cooling. A second converter holding the third bed has been used.
Uhde Three catalyst beds; two in first converter with inlet/outlet heat exchange
and waste heat boiler and a third in a second converter with a waste heat
boiler.
C F Braun Three beds in separate converters with an inlet/outlet heat exchanger after
the first, and waste heat boilers after the second and third.
