Lloyd L. Handbook of Industrial Catalysts
Подождите немного. Документ загружается.

11
ENVIRONMENTAL CATALYSTS
The effects of atmospheric pollution from the combustion of fossil fuel have
been recognized for many years. Acid rain from industrial areas of Europe and
North America devastated forests. The pea-souper fogs in Donora, Pennsylva-
nia, London, UK and Belgium caused by emissions from power plants, steel-
works and metal smelters as well as domestic coal fires affected public health
and led to thousands of deaths. All this, together with the photochemical smogs
in Los Angeles, California, brought a growing demand for environmental protec-
tion.
1
Early measures were almost all restricted to imposing limits on particulate
emission and in the UK, the first Clean Air Act of 1956 resulted in the introduc-
tion of smokeless zones. Since then further legislation has led to the develop-
ment of catalytic processes that also reduce the concentration of nitrogen oxides,
hydrocarbons and carbon monoxide in the various exhaust emissions to speci-
fied levels.
Legislation was passed in the 1970s to limit the release of sulfur and nitro-
gen oxides from power plants in Japan and later in Germany. This led to the
investigation of new catalytic processes. Selective Catalytic Reduction (SCR)
was developed to enable power companies to reduce NOX emissions from coal
and oil based power plants. Since then another procedure has been developed to
remove NOX from the gaseous effluent produced by several other combustion
and chemical processes.
The Clean Air Act, introduced by the US Government during 1970, set spe-
cific targets for the control of emissions from automobiles to be achieved by
1975. Catalytic converters are now fitted to automobiles in many parts of the
world to remove nitrogen oxides, hydrocarbons and carbon monoxide in accord-
L. Lloyd, Handbook of Industrial Catalysts, Fundamental and Applied Catalysis,
DOI 10.1007/978-0-387-49962-8_11, © Springer Science+Business Media, LLC 2011
439
440 Chapter 11
ance with local regulations. An additional environmental benefit from the use of
catalytic converters has been the need to stop the addition of tetraethyl lead to
gasoline. Tetraethyl lead was used originally to increase the octane rating of the
gasoline, but lead is a powerful catalyst poison, and would cause a rapid deacti-
vation of the catalytic converter. This led to a need for major changes to be
made to the composition of the US gasoline pool and new Environmental Pro-
tection Agency (EPA) regulations covering the reformulated gasoline were de-
veloped. The use of low-boiling, high-octane hydrocarbons and toxic aromatic
components which may be released to the atmosphere is now restricted.
A third major group of emissions controlled by environmental legislation
comprises volatile organic compounds (VOCs) from all industrial and refinery
effluents. The US Clean Air Act amendments of 1990 called for the reduction in
the concentration of no fewer than 188 toxic chemical air pollutants before
2000. Many of these chemicals are VOCs and significant reductions have been
achieved by the introduction of a range of new catalytic oxidation processes.
The magnitude of the problems involved in environmental control and the po-
tential demand for catalysts is demonstrated by Table 11.1 that shows the esti-
mated volume of some effluents in the USA during 1995.
2
Reports show that about 1.27 million tons of major chemicals were released
in the US during 1993, some of which are listed in Table 11.2.
3
Sulfuric acid
made up about 73,000 tons of this total. During 1999 up to 22 million tons of
sulfur was recovered from crude oil fractions and hydrocarbon gases by the cata-
lytic Claus Process.
4
Although sulfur from power plant emissions has usually
been removed chemically as gypsum, it can be converted to useful sulfuric acid
by a modified form of the contact process.
5
TABLE 11.1. US Gaseous Emissions During 1995.
Emission Source %
NOX Transport 49
Combustion 46
Industrial Processes 3
Chemical Processes 1
Miscellaneous 1
Total 21.8 million tons
VOCs Non Chemical Processes 51
Transport 37
Chemical Processes 7
Fuel Combustion 3
Miscellaneous 2
Total 22.9 million tons
SOX (1986) Fuels 85
Transport 7
Industry 8
Total 18.5 million tons

Environmental Catalysts 441
TABLE 11.2. Major Chemicals Released to Atmosphere in 1993 in the United States.
Chemical Percentage of total
Ammonia 12.6
Hydrochloric Acid 8.0
Phosphoric Acid 7.6
Methanol 7.5
Toluene 6.4
Sulfuric Acid 5.7
Acetone 4.6
Xylenes 4.0
Carbon Disulfide 3.3
Methyl Ethyl Ketone 3.0
Chlorine 2.7
Zinc compounds 2.6
Dichloromethane 2.3
1,1,3 Trichloromethane 2.2
Total released 1.27 million tons
The catalytic processes used for environmental protection demand an enor-
mous capital investment but are relatively uncomplicated and more efficient
than physical procedures. Demands imposed by environmental legislation have
created a significant and rapidly expanding sector of the worldwide catalyst
business.
11.1 STATIONARY SOURCES
High temperature combustion of coal and oil to provide heat and generate elec-
tricity resulted in huge volumes of nitrogen oxides (NOX), sulfur dioxide and
carbon oxides being vented to the atmosphere. The most widely used fixed, or
stationary, sources which produce nitrogen oxides are steam generating boilers,
combustion turbines, process heaters, waste incinerators and reciprocating en-
gines. A number of chemical processes such as nitric acid, adipic acid, caprolac-
tam and acrylonitrile also produce effluents containing large volumes of nitro-
gen oxides.
It is now generally necessary to treat the flue or exhaust gases from new sta-
tionary sources to remove NOX to a statutory level by the most convenient and
economic procedure available (
Figure 11.1). This usually requires a reduction
process using added ammonia. Although some power plants have been retrofit-
ted with SCR equipment, many older installations have not been covered by the
legislation. This has been described as being ‘grandfathered’ but, as a typical
power plant has a life of at least 30-40 years, means that considerable NOX
would still release continue for several years.
442 Chapter 11
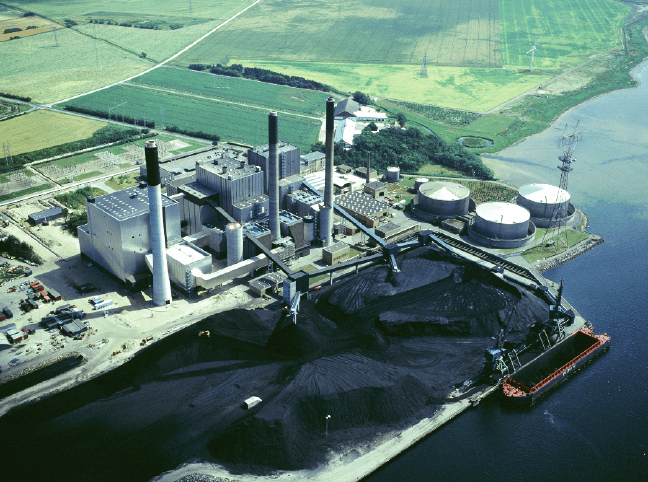
Figure 11.1. Coal-fired power plant fitted with SCR (Selective Catalytic Reduction) unit
to decrease NOX content of the flue gas. Reproduced with permission from Haldor
Topsøe A/S.
Coal and fuel oil can contain up to 5 wt% sulfur and during combustion
most is converted to gaseous sulfur oxides. This amounts to about 1800 volume
ppm sulfur dioxide in flue gas for 2.5 wt% sulfur in coal. Up to 95% of the sul-
fur in the flue gas can be removed by scrubbing with an aqueous slurry of lime-
stone to produce calcium sulphate, or gypsum. This procedure, however, re-
quired facilities for the disposal of the large amount of gypsum thus produced.
Lower sulfur dioxide emissions can be obtained where possible by using low
sulfur fuel. Alternatively, a mini sulfuric acid unit may now be installed to re-
cover several tonnes of acid per day from a single power plant unit.
5
Nitrogen oxides are formed at the high boiler temperatures, which can ex-
ceed 1500°C, from either the nitrogen compounds present in the fuel or by the
direct oxidation of elemental nitrogen in air as shown in Table 11.3.
6
The NOX
content of flue gas in modern power plants is usually in the range 800–1800
ppm as shown in Table 11.4.
7

Environmental Catalysts 443
TABLE 11.3. Formation of Nitrogen Monoxide.
Mechanism Reaction
Thermal (>1500°C) O + N
2
→ NO + N
Oxidation of molecular nitrogen
N + O
2
→ NO + O
N + OH → NO + H
Prompt (low temperature, fuel rich)
CH + N
2
→ HCN + N
Initiation by hydrocarbon radicals N + HCN converted to NO by reaction with
oxygen and hydrogen atoms in the flame.
This can be controlled to a certain extent by the use of low nitrogen fuels or
by modifying the combustion conditions. Low excess air addition, flue gas re-
circulation, low NOX burners or water/steam injection can provide 50-60% re-
ductions in NOX concentration. The scale of the operation is enormous and a
typical US power plant burning 9500 tons of selected coal per day will have to
remove about 135,000 tons of sulfur dioxide (95% conversion) and 45,000 tons
of NOX per year. During 2000 about 50% of US power was generated from coal
but by 2020 60% is expected to come from natural gas.
8
11.1.1 Selective Catalytic Reduction
Selective Catalytic Reduction (SCR) of nitrogen oxides with ammonia using
specially developed catalysts was investigated in Japan from 1974 and then in-
troduced commercially from about 1980.
9
It is now the most important process
for removing nitrogen oxides (NOX) from the effluent gas of power plants and
many other stationary sources. German power plants installed SCR units based
on Japanese licenses from about 1984 following the introduction of legislation
limiting the NOX content of effluent gases in 1983.
10
TABLE 11.4. NOX Concentration in Flue Gas.
A: Power Plant
Plant Type NOX Concentration (mg/m
3
)
Coal (Pre 1980) 1200–2800
Coal (Post 1984) 800–1800
Lignite 600– 800
Oil 500– 800
Gas 500–1200
B: Gas Turbines
Fuel
NOX Concentrations (mg/m
3
)
Normal Wet Injection Low NOX Burner SCR
Natural gas 100–400 25–40 15–25 5–10
Distillate 150–700 40–65 65 15–18

444 Chapter 11
Japanese power plants generally used low sulfur coal with dry fired boilers
and accepted relatively low NOX conversion with the SCR catalysts. This also
limited the catalytic oxidation of sulfur dioxide to sulfur trioxide and avoided
excessive deposition of sulfates in downstream equipment. German power
plants, with higher sulfur coals and slagging boilers, had difficulties resulting
from high sulfur dioxide conversion to sulfur trioxide as well as catalyst poison-
ing from volatile arsenic oxides. These problems were gradually overcome
through catalyst development and process modifications that included NOX
removal following sulfur oxide removal.
In the SCR process, NOX impurities are reduced with added ammonia in
the presence of some residual oxygen from the furnace. The main NOX reduc-
tion reactions are shown in Table 11.5 together with some of the undesirable
oxidation reactions, which can both produce sulfur trioxide and waste some of
the added ammonia. Between 0.6–0.9 moles of ammonia per mole of NOX are
added to limit the ammonia slip to downstream equipment where it would de-
posit as sulfates. NOX conversion is therefore limited to between 60-90%. At
low NOX levels, there is little conversion to nitrous oxide. Nitrous oxide
formation is also inhibited by water. Gas leaving the boiler is usually at a tem-
perature in the range 300–430°C and contains dust. Dust is removed in an elec-
trostatic precipitator with little heat loss before sulfur dioxide is removed as
gypsum by reaction with lime. Alternatively, sulfur dioxide can also be convert-
ed to sulfuric acid.
5
The effluent gas is then vented to atmosphere. In the first
power plants to be retrofitted with SCR units there were three possible locations
for the catalyst bed:
• At the boiler exit. In this position the temperature was suitable for the
NOX reaction and allowed some conversion of sulfur dioxide to trioxide.
Dust in the gas stream led to catalyst problems.
• Following the electrostatic precipitator. The gas temperature was still
high enough for both NOX removal and some sulfur dioxide oxidation.
Dust problems were, however, avoided.
• At the tail end following flue gas desulfurization. Dust problems and sul-
fur dioxide oxidation were avoided but the gas had to be reheated for the
catalytic NOX reduction to be effective.
TABLE 11.5. Reduction/Oxidation Reactions with SCR Catalysts.
Process Reaction
DENOX
4 NO + 4 NH
3
+ O
2
→ 4 N
2
+ 6 H
2
O
6 NO
2
+ 8 NH
3
→ 7 N
2
+ 12 H
2
O
OXIDATION
2 SO
2
+ O
2
→ 2 SO
3
4 NH
3
+ 5 O
2
→ 4 NO + 6 H
2
O
4 NH
3
+ 3 O
2
→ 2 N
2
+ 6 H
2
O

Environmental Catalysts 445
Figure 11.2. DeNOX catalyst for use in SCR (Selective Catalyt-
ic Reduction) plants. Reproduced with permission from Haldor
Topsøe A/S.
In the first processes, the choice was to use the catalyst in the most conven-
ient high dust location at the boiler exit where dust blocked the catalyst bed and
eroded the catalyst surface in a relatively short period of time. This led Japanese
operators to prefer the low dust location after the electrostatic precipitator. Ger-
man operators, however, decided to remove NOX after the sulfur dioxide re-
moval stage despite the need for heat exchange to reheat the gas when using
typical catalysts.
From 1980, a variety of catalyst shapes and compositions was developed to
overcome operating problems and provide better catalyst selectivity. Following
the US Clean Air Act Amendments in 1990, it became necessary to consider
SCR flue gas treatment from some coal based plants and to install suitable NOX
removal procedures with new boilers, turbines and furnaces.
11.1.2 Selective Catalytic Reduction Catalysts
The most important reactions taking place over SCR catalysts are shown in Ta-
ble 11.5. In power plants using coal or hydrocarbon fuels there is typically a
larger excess of nitric monoxide compared with nitrogen dioxide (~19:1) than
in, say, nitric acid plant tail gas (~1:1). A number of catalysts have been intro-
duced (Figure
11.2) that are able to operate in the available temperature range
from about 200°–430°C. The development, preparation and structure of the cata-

446 Chapter 11
lysts, the supports used and operating performance are described in several re-
views.
11
11.1.2.1 Catalyst Composition
SCR catalysts are prepared from vanadium pentoxide promoted with either mo-
lybdenum trioxide or tungsten trioxide and supported on the anatase form of
titanium dioxide. The kinetics of the reaction are diffusion limited, and conse-
quently, a thin layer of catalyst is usually deposited on a suitable high geometric
area framework or honeycomb. When the flue gas has high dust content, at the
boiler outlet, the honeycomb is formed from thin, notched, metal plates. These
can be stacked as layers in modules that provide appropriate channel openings in
the reactor. Alternatively, an extruded ceramic honeycomb with smaller chan-
nels and a higher surface area has been used when the gas has low dust content,
after having passed through the electrostatic precipitator or desulfurization unit.
Small honeycomb blocks are stacked together and loaded as beds in the reactor.
Details of catalyst composition and dimensions, together with operating condi-
tions, are summarized in Table 11.6.
10
Catalysts based on zeolites have also been used successfully to remove NOX
from the emissions of large and small-scale stationary sources. The zeolite can
be extruded directly as a monolith or applied as a washcoat on preformed cordi-
erite supports. The use of several zeolites—including wide-pore mor-
denite—for this reaction has been patented.
12
Up to 95% NOX conversion can
be achieved with mordenite, with no promoters, and some zeolites are stable at
temperatures up to 600°C. Long catalyst lives have been achieved in retrofitted
coal based SCR units with low sulfur trioxide formation and good poisons re-
sistance. Spent zeolite catalysts can be disposed of in approved landfill sites
because they have negligible heavy-metal content.
12
Promoted vanadium pentoxide/titania and zeolite based catalysts have been
used to reduce NOX emissions at low temperature from nitric acid plants, gas
turbines, industrial heaters, incinerators and boilers.
13
TABLE 11.6. SCR Catalyst and Operating Conditions.
Exit Boiler Exit deduster or low dust fuels
a
Gas Temperature (°C)
320–430 280–430
Dust content (g/SCF) > 6.5 0.02–6.5
Honeycomb Metal Titania/ceramic
Channel opening (mm) 4–6 3–4
Surface area (m
2
/g) 280–350 400–800
Honeycomb block size (cm) 65 x 46 x 46 10 x 15 x 15
Catalyst volume (m
3
per megawatt) ~0.6–1.2
Reactor volume (m
3
per megawatt) ~1.7–3.4
Catalyst composition V
2
O
5
/MoO
3
or WO
3
/TiO
2
a
Low dust fuels are hard coal, oil or natural gas.
Environmental Catalysts 447
11.1.2.2 Catalyst Operation
Most catalysts supported on titanium dioxide reach an optimum NOX reduction
temperature that depends on the catalyst composition and the treated gas. Activi-
ty then declines as the secondary reactions compete for the ammonia reductant
and sulfur dioxide oxidation becomes excessive. Typical operation is in the
range 300°–425°C although zeolite catalysts operate from 300°–600°C.
12,13
Catalysts may therefore be designed for use in specific duties. For power
plant, the design must balance the reaction rates of NOX reduction and sulfur
dioxide oxidation in the restricted range of temperature of flue gas leaving the
boiler, or at the dust and sulfur dioxide removal stages. A low activity catalyst
that reaches maximum NOX reduction between, say 380°–400°C, can be more
efficient than a catalyst that is more active between 300°–350°C because, over-
all, it produces less sulfur trioxide at the fixed operating temperature.
14
Vanadium pentoxide/titania catalysts, promoted with molybdenum trioxide
or tungsten trioxide, have lower sulfur dioxide oxidation activity under power
plant conditions than unpromoted catalysts. However, while catalysts containing
tungsten trioxide are initially more active, any arsenic oxides in flue gas more
easily poison them than similar catalysts containing molybdenum trioxide. Ar-
senic is a typical impurity in coal. Flue gas from slagging boilers contains up to
twenty times more than flue gas from dry ash boilers. Catalysts promoted with
molybdenum trioxide have been more widely used in Europe for this reason.
The vanadium pentoxide content of NOX reduction catalysts is probably less
than 1 wt% which maximizes the rate of nitrogen oxide reduction while limiting
the rate of sulfur dioxide oxidation. The total oxide content including promoter
would be less than 10 wt%.
15
11.1.2.3 Reaction Mechanism
Selective Catalytic Reduction catalysts are similar to the vanadium pentoxide-
anatase catalysts introduced by BASF and von Hayden in the 1960s for the oxi-
dation of methyl groups in ortho-xylene. They were also coated onto cordierite
supports.
15
Vanadium pentoxide reacts with surface hydroxyl groups on the tita-
nia to form active surface sites. In the case of oxidation catalysts, the mono-
vanadyl species are active. However, for NOX reduction, at least two vanadyl
groups, linked by an oxygen atom, form the selective site. These sites must be
maximized.
15
When the vanadium content of the catalyst is low, the monovanadyl surface
species predominates. At a concentration around 5%, the monovanadyl species
begin to polymerize, forming V-O-V bridges by dehydration mechanism, and at
concentrations above about 10%, crystalline vanadium pentoxide is deposited.
Preparation of the necessary active sites for maximum activity and selectivity
has been achieved by promoting the catalyst with an excess of molybdenum
