Lloyd L. Handbook of Industrial Catalysts
Подождите немного. Документ загружается.


368 Chapter 9
The presence of excess steam in the process gas to the reformer results in
the formation of carbon dioxide by the water gas shift reaction. Thus the gas
leaving the steam reformer also contains between 7 and 15% carbon dioxide:
CO + H
2
O ↔ CO
2
+ H
2
(9.9)
Typical operating conditions for a modern steam reformer are given in Ta-
ble 9.9 although these can be slightly different depending on the overall plant
design. The table also includes typical conditions for the steam reformers in
methanol and hydrogen plants which use the same catalysts. These examples
illustrate the wide variations in gas composition which can be achieved by
changing the operating steam ratio, temperature and pressure, to increase me-
thane conversion.
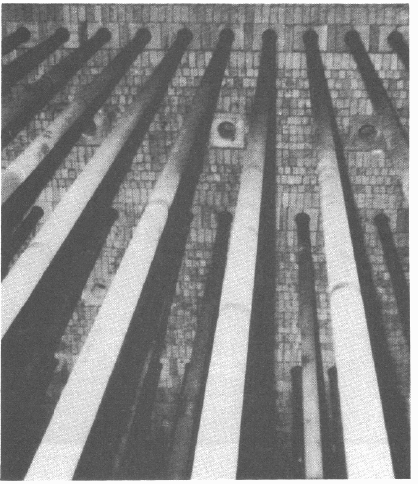
The long catalyst tubes are suspended vertically in rows within the furnace
(Figure
9.3). The hydrocarbon/steam mixture, preheated to about 500°C, passes
into the reformer through a header pipe connected to the tops of each tube by
pigtails or flexible joints. Following reaction, the products leave the reformer
via a manifold pipe to the secondary reformer. Heat can be supplied to the fur-
nace from burners at the top, sides or bottom of the insulated box. The intensity
of the heat, or heat flux, in the furnace can be varied depending on the demands
of the reaction but is generally highest at about one third from the top of the
tube, where most of the endothermic reaction takes place.
Furnace design is complex and is fully described in specialized publica-
tions.
12
Catalyst operation at extremely high temperatures is critical in the pro-
duction of ammonia and full instructions on handling and operation are issued
by all catalyst suppliers.
TABLE 9.9. Steam Reforming Operating Conditions with Natural Gas.
Process Ammonia
1000 t/day
Methanol
1000 t/day
Hydrogen
30 mm cfd
Catalyst volume (m
3
) 17–20 20–25 20
Steam/carbon 3–5 5 6
Inlet pressure (atm) 35 20 20
Temperature inlet (°C)
525 515 520
Temperature outlet (°C)
790 850 780
Outlet gas composition (vol%):
Nitrogen <2 Trace Trace
Hydrogen 70 73 75
b
Carbon monoxide 8 14
a
10
Carbon dioxide 12 8 12
Methane 10 4–5 2–3
a
Addition of CO
2
required before reforming or synthesis.
b
CO removed by catalysts up to methanation or pressure swing absorption after HTS converter.
9.4.2
Early
r
magne
s
p
ound
s
Cimen
t
strengt
h
mixtur
e
The co
I. G.
F
Oil in
reform
i
p
ressu
r
the ref
o
zation
a
Figure
top-fir
e
gas pr
o
ed., by
R
eformin
g
C
a
r
eforming cata
l
s
ia and kaoli
n
s
and olefins i
n
t
fondu was t
h
h
and giving
b
e
of precipita
t
mposition of
a
F
arben catalyst
1932 was pr
o
i
ng catalysts
w
r
e drop. Durin
o
rming proces
a
nd zinc oxid
e
9.3. Arrangeme
n
e
d furnace for t
h
o
duction. Repri
n
kind permissio
n
a
tal
y
sts
l
ysts were ma
d
n
. These were
n
the feed gas
h
en found to b
e
b
etter resistanc
e
t
ed nickel oxi
d
a
number of ea
r
to be used i
n
o
bably produc
e
w
ere found b
y
g the 1930s,
I
s
. This led to
t
e
absorption pr
o
n
t of burner an
d
h
e primary ref
o
n
ted from Catal
y
n
of M. Twigg.
d
e by mixing
p
active but e
a
obtained fro
m
e
a useful add
e
to sulfur poi
s
d
e with magn
e
r
ly catalysts is
n
the large-sca
l
e
d as three-qu
a
y
ICI to give
I
CI also bega
n
t
he modern co
b
o
cedures.
Synt
d
tubes in a typi
c
o
rmer in synthe
y
st Handboo
k
,
2
p
recipitated n
i
a
sily
p
oisone
d
m
coal hydroge
n
d
itive by incre
a
s
oning. The b
e
e
sia, kaolin a
n
s
shown in Ta
b
l
e plants oper
a
arter inch cu
b
better perfor
m
n
to desulfuriz
e
b
alt molybdat
e
t
hesis Gas
3
c
al
sis
2
nd
i
ckel oxides
w
d
by sulfur co
m
n
ation process
a
sing the catal
y
e
st catalyst wa
n
d ciment fon
d
b
le 9.10. The f
i
a
ted by Stand
a
b
es. Raschig r
i
m
ance and lo
w
e
the feed gas
e
hydrodesulf
u
3
69
w
ith
m
-
es.
y
st
s a
d
u.
i
rst
a
rd
i
ng
w
er
to
ur
i-
370 Chapter 9
TABLE 9.10. Examples of Early Reforming Catalyst Composition.
First Catalysts Raschig Ring
Catalyst
Wt% A B C D
Nickel 20 10–16 20 15
Magnesia 20 0–5 15 12
Kaolin 60 – 35 40
Alumina – 10–16 – –
Ciment Fondu – 68–75 30 35
Early catalysts were slowly developed to improve resistance to carbon formation.
A Operated well with pure methane but when poisoned by sulfur and unsaturated hydrocarbons led
to carbon deposition.
B Ciment fondu acted as a filler and partly inhibited carbon formation.
C Gave activity as high as A and less carbon than B but still sensitive to unsaturated hydrocarbons.
D Could be used with most natural gases and off gas, providing that poisons were removed. Some
early charges operated for 10–20 years with occasional steaming to clean catalyst.
The early catalyst formulations, with only minor changes, were still being
used until about 1965 when methane was first reformed at pressures above about
15atm. Silica is volatile in steam at the reforming temperature in high-pressure
plants and it is then deposited at a lower temperature in the waste heat boilers, or
on to the high-temperature shift catalyst. This led to the development of silica-
free reforming catalysts made from alumina, rather than kaolin or magnesia, and
alumina cement, rather than ciment fondu. Although alumina based catalysts
were active, it was found that they lost strength at high temperature. This led to
some breakage and caused hot or patchy tubes. Attempts were made to improve
strength by incorporating titanium dioxide into the catalyst recipe rather than
alumina although these were not really successful. The best solution was found
to be the use of silica free preformed rings, impregnated with nickel nitrate,
which could be decomposed to give nickel oxide. Problems in the early days in
the selection of a support that was stable in the severe operating conditions led
to the introduction of several different catalysts. The supports ranged from pure
alpha alumina to calcium aluminate and magnesium aluminate. Operational
problems were experienced in the early days, arising from poor thermal stability
and loss of activity when using α–alumina supports. Carbon was deposited about
one third of the way down from the top of the tube and hot bands formed in
high-heat-flux converters.
A partial solution to the low activity problem was to use shorter rings to
improve heat transfer although this did, of course, this did increase the pressure
drop through the tubes. Hot bands could also be avoided by using alkalized cata-
lysts in the top part of tubes to control carbon deposition. It was clear that im-
proved reforming catalysts were required, not only with a higher, stable activity
but also, as it turned out, with a different shape.
By the time most ammonia plants were being operated beyond the limits of
their design capacity, new catalyst shapes were eventually developed. The origi-
Synthesis Gas
371
nal intention was to increase the geometric surface area of the ring and thus to
increase activity. A further benefit was, however, found to be a reduction of the
pressure drop through the tubes and improved heat transfer from the tube wall to
the reacting gases. Once again a variety of different shapes were introduced
ranging from rings, spoked wagon wheels and cylinders with either four or sev-
en holes. Part of the range of catalyst compositions and shapes available is
shown in Table 9.11.
9.4.3. Reformer Operation
The catalyst should be loaded carefully into the reformer tubes to avoid
breaking the rings. The normal procedure is to fill socks or soft tubes with a
bottom flap which can be lowered into each of the tubes before use. The sock is
then withdrawn from the tube and the flap opens to leave the catalyst in place.
To ensure even packing, the same weight of catalyst is placed in each tube and
the pressure drop measured. Gentle tapping, at the bottom of the tubes, may be
needed to pack the catalyst properly. In common with all other tubular reactors
charging catalyst into the tubes of a steam reformer is a laborious process.
The catalyst must be activated by reduction with a hydrogen-rich gas added
to the steam passing through the tubes. A steam to hydrogen ratio of about six is
used during reduction at operating temperature. The procedure can be completed
in about six to eight hours. Feed may then be gradually introduced and operation
begins. Catalyst breakage can result from shortage of steam or thermal shock
during operation. This leads to an uneven gas flow with hot tubes and possible
TABLE 9.11. Reforming Catalysts: Composition and Shapes.
Calcium
aluminate
α
-aluminate
Magnesium
aluminate
Composition (wt%):
NiO 15–20 15–20 15–20
Al
2
O
3
65–70 80–85 60–65
CaO 10–15 – –
MgO – – 15–20
Bulk density (kg liter
-1
) ~1.0 ~0.8–0.9 ~1.0
Surface area (m
2
g
-1
) 10–20 2–5 10–20
Shapes Size: diameter x length x wall
Raschig Rings mm 16x16x6
16x16x6
Rings 16mm 7 or 9 ribbed rings
Rings 16mm 4 to 9 holes
372 Chapter 9
carbon deposition. In badly affected tubes the catalyst must be replaced. The
leakage of sulfur or chlorine from the purification stage also results in poisoning
and deactivation of the catalyst. Any decrease in activity leads to a high methane
content in the reformed gas from the reformer tubes which results in hot spots in
the tube and the deposition of more carbon. A mild occurrence of sulfur or chlo-
rine poisoning can generally be reversed by increasing the steam ratio for a short
period. Arsenic is a permanent poison to the catalyst. If the arsenic concentration
in the catalyst at the top of the tubes exceeds about 150 ppm, then the catalyst
must be changed, and the tubes cleaned very carefully. The arsenic usually
found its way into the reformers in the early days either as a component of the
feedstock, or through some maloperation of the Vetrocoke system, used for the
absorption of carbon dioxide. A typical early warning of catalyst poisoning is a
gradual increase in the methane content of gas leaving the reformer and, in seri-
ous cases, an increase in pressure drop.
The introduction of the first 1000 tons per day high-pressure steam reform-
ers in single stream plants led to some operating difficulties. For example, the
new high heat flux reformers developed overheated bands, about one third of the
way down the reformer tubes. Hot bands, as they were called, resulted from the
deposition of carbon from the thermal cracking reaction as the catalyst lost ac-
tivity. Carbon can most easily form from methane cracking:
CH
4
↔ C + 2 H
2
(9.10)
This normally takes place when flow of steam stops for any reason but is also
thermodynamically possible during full-scale operation of a reformer before the
gas temperature reaches about 650°C.
Fortunately, two other reactions lead to the removal of carbon under normal
reforming conditions:
C + CO
2
↔ 2 CO Boudouard reaction (9.11)
C + H
2
O ↔ CO + H
2
Water-gas reaction (9.12)
Thus, any carbon formation arising from the cracking reaction at the tube inlet is
controlled to some extent by the two carbon removal reactions, which tend to be
rather faster than the cracking reaction. Unfortunately, the cracking reaction
becomes the faster reaction at temperatures above about 650ºC and this tempera-
ture corresponds to the position in the reactor tube where the hot bands tend to
form. The rate of carbon deposition is greater than its rate of removal, unless the
activity of the catalyst is sufficient to provide sufficient hydrogen by the reform-
ing reaction to prevent the deposition of carbon in the first place.
It was clear that a more active and thermally stable catalyst was needed. For
some time the situation was controlled by the use of shorter rings at the top of
Synthesis Gas
373
the tube. This provided more geometric surface area and increased reforming
activity. Even more successful operation was possible if the shorter rings were
alkalized. The addition of potash to catalysts under development for the reform-
ing of naphtha was introduced by ICI during the 1960s and was a major factor in
the control of the formation of hot bands. The potash had the effect of accelerat-
ing the steam carbon reaction and inhibiting cracking and polymerization on the
catalyst surface.
During the 1970s and 1980s the cost of oil had increased substantially and
production of ammonia in the older plants was becoming uneconomical. This
led to the revamping of many units and the use of more energy efficient process-
es. A steam reformer uses about one third of the total energy required by a large
ammonia plant. It was therefore suggested that the steam ratio could be de-
creased, from 3.5–4.0 to less than 3.0, even though this could mean that more
carbon would deposit on the catalyst and that higher tube wall temperature
would lead to shorter tube life. A penalty of operating a reformer with hot tubes,
for any reason, is that a temperature only 10°C above the design level can re-
duce the tube life by up to 50%. The cost of tube failures resulting from the use
of low activity catalysts or decreasing the steam ratio was very high.
The new catalyst shapes that were under development gave some compen-
sation for these disadvantages. Higher activity, particularly at lower temperature,
could help to avoid carbon formation. Carefully optimized shapes also gave a
lower pressure drop and provided better heat transfer within the tubes to de-
crease the temperature of the tube wall. The use of manaurite tubes also helped
to improve operation.
Some operators decided to use a small pre-reformer able to ease the load on
the primary reformer and to give further energy savings. The small adiabatic
reactor was loaded with a stable high activity catalyst operating at 530°C. The
alkalized nickel alumina catalyst, introduced by British Gas in their CRG Pro-
cess during the 1960s, can operate continuously for about two years. All traces
of high hydrocarbons were removed in the prereformer and methane was partial-
ly reformed to give about 20% hydrogen in the feed gas entering the primary
reformer. This allowed the minimum practicable steam ratio to be used.
An overall fuel saving of 7–10% was possible, and the tube wall tempera-
ture of the reformer tubes can be reduced by about 20°C by decreasing the firing
rate and heat flux in the reformer. This leads to a longer tube life. Alternatively,
up to 5–10% extra ammonia could be produced by increasing the feed rate.
When mixed feeds were used, as in some hydrogen plants, steadier operation
was possible. Brief details of the CRG pre-reforming process operation are giv-
en in Table 9.12.

374 Chapter 9
TABLE 9.12. Prereforming of Natural Gas or Refinery Feeds
Process Composition (dry outlet gas)
Feed Natural Gas Butane Naphtha
Steam ratio 0.3 1 1.5
H
2
22–24 30–34 20–22
CH
4
66–68 50–55 60
C
2
H
6
+ <10 ppm <10 ppm –
Balance CO/CO
2
CO/CO
2
CO/CO
2
Temperature inlet (°C)
530 530 450–530
ΔT (°C)
–64 +11 +40
Pressure Reformer design
9.4.4. Secondary Reforming
When coke was the basic raw material for the production of ammonia synthesis
gas, the hydrogen and nitrogen required was supplied by mixing water gas and
producer gas. Several small ammonia plants were, in complete contrast, built in
the United States during the 1920s to use electrolytic hydrogen.
8
The operators
found it relatively easy to introduce the necessary nitrogen by burning some of
the hydrogen in air. When the war time methane steam reformers were built by
the US Government, the same procedure was modified by burning primary re-
former outlet gas in a high temperature adiabatic reactor containing more prima-
ry reforming catalyst. The additional reactor became known as the secondary
reformer. Eventually a catalyst with lower nickel content, and an even higher
thermal stability, was developed to withstand the very high temperature reached
at the top of the bed.
In modern ammonia plants, air containing the design volume of nitrogen
needed for ammonia synthesis is added to the secondary reformer. Two im-
portant reactions can take place over the heat-resistant nickel catalyst. Two im-
portant reactions take place over the heat resistant nickel catalyst in the second-
ary reformer. Firstly, the oxygen component of the air is consumed by combus-
tion with hydrogen and possibly carbon monoxide. Secondly, some of the resid-
ual methane from the primary reformer undergoes further reforming. Both reac-
tions reach equilibrium at a bed outlet temperature of about 1000°C. The me-
thane content of gas leaving the secondary reformer is usually in the range 0.2–
0.5% while oxygen is completely removed.
The main problems in the operation of a secondary reformer are associated
with the high temperatures generated by the combustion reactions. The gas dis-
tributor must be well designed so that the process gas and air are mixed as rapid-
ly and thoroughly as possible. The catalyst bed itself is protected from the very
high temperatures generated by the homogeneous combustion of air, by a layer
of refractory material that is placed on top of the large, temperature-resistant
catalyst particles. The rest of the bed is filled with secondary reforming catalyst.
Synthesis Gas
375
TABLE 9.13. Secondary Reforming Catalyst Operation.
Gas Composition Secondary Reformer
Inlet Outlet
CH
4
(dry vol%) 9.4 0.2
CO
2
(vol%) 11.6 8.8
CO (vol%) 8.3 11.5
N
2
(vol%) 0.5 22.1
H
2
(vol%) 70.2 57.1
Air (vol% of dry reformed gas) ~38
Pressure (atm) 30 29
Temperature (°C)
790 971
Catalyst volume (m
3
)(1000 tonnes/day plant) 30
The catalyst can operate for several years provided that the reacting gases
are well mixed and that no hot spots (which in extreme cases could reach
1500
0
C) are allowed to develop in the catalyst bed. The gas distributor plays an
important part in protecting the catalyst. The temperature of the catalyst bed in
the secondary reformer falls as gas passes through the bed. At the inlet to the
bed, the temperature is extremely high due to the highly exothermic combustion
reaction in which all of the oxygen is consumed. The temperature of the bed
towards the outlet then falls as the endothermic reforming reaction takes place.
It is interesting to note that when the temperature of the primary reformer outlet
falls and the slippage of methane increases, the temperature of the outlet of the
secondary reformer also falls. Typical operating conditions are shown in Table
9.13.
Secondary reforming catalyst contains about 7% nickel oxide, supported on
temperature-resistant α–alumina or calcium aluminate, in the form of raschig
rings. Additional catalyst used at the top of the bed as a heat guard is in the form
of large solid cylinders of α-alumina containing 5% nickel oxide.
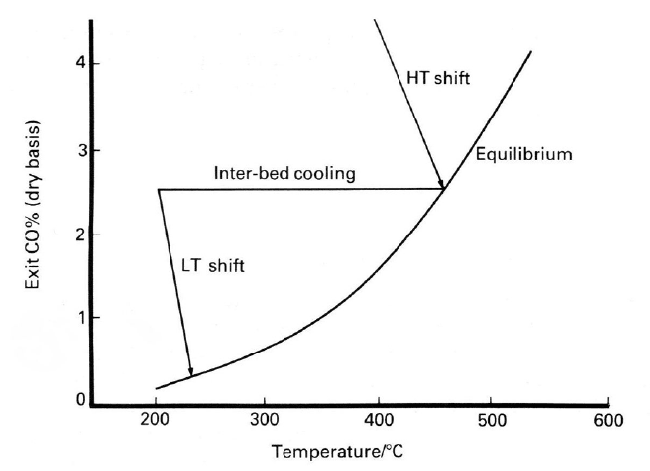
9.5. CARBON MONOXIDE REMOVAL
Carbon monoxide leaving the secondary reformer is converted to useful hydro-
gen by the water gas shift reaction in a two-stage recovery section. The original
high temperature shift reactor is now combined with a low temperature reactor
filled with a copper catalyst. The removal of carbon monoxide is shown in
Fig-
ure 9.4. Since copper catalysts are extremely prone to poisoning by sulfur and
chlorin
e compounds, it is therefore essential that the concentration of these con-
taminants is reduced to an absolute minimum.
376
Figu
r
lytic
Twi
g
9.5.1.
In 191
5
b
y rea
c
the fir
s
b
ased
o
work
d
Si
n
has be
e
stabili
z
operat
i
T
h
togeth
e
use, th
e
and at
state.
U
mody
n
the me
t
Chapter 9
r
e 9.4. Typical
v
beds. Reprinte
d
g
g.
H
i
g
h Temper
5
,
t
he water-g
a
c
tion with ste
a
s
t commercial
o
n catalysts d
i
d
ating from the
n
ce then the
b
e
n iron oxide,
z
ing the cataly
s
ng conditions
h
h
e catalyst as
s
e
r with a vari
a
e
catalyst mus
the same tim
U
nder the typi
c
n
amically-stabl
e
t
allic state. So
m
v
ariations of ca
r
d
from Catalyst
ature Carbo
n
a
s shift reactio
n
a
m and to incr
e
ammonia pla
n
i
scovered by
W
late 1800s.
b
asic formulat
i
stabilized wi
t
s
ts and produc
h
ave changed
s
upplied consi
s
a
ble amount
o
t
be activated
e, any chrom
i
c
al conditions
e
state for the
m
e HTS catal
y
r
bon monoxide
Handboo
k
, 2
nd
n
Monoxide C
o
n
has been us
e
e
ase the poten
n
t began oper
a
W
ild of BAS
F
i
on of high te
m
t
h chromium
o
ing it in large
s
ts mainly of
h
o
f the hexaval
e
by reduction
t
i
um trioxide i
s
for the shift
r
o
xides of, and
y
sts may cont
a
levels in HT a
n
ed., by kind p
e
o
nversion
e
d to remove c
tial hydrogen
a
tion in 1913.
F
in 1912
2
w
h
m
perature shi
f
o
xide, althoug
h
quantities hav
e
h
ematite and
c
e
nt chromium
t
o magnetite u
s
s
also reduce
d
r
eaction, mag
n
there is no fu
r
a
in traces of s
u
n
d LT shift cata
-
e
rmission of M
arbon monoxi
d
production si
n
The process
w
h
ile following
f
t catalyst (H
T
h
the methods
e been refined
c
hromium oxi
d
trioxide. Bef
o
s
ing process g
d
to the trival
e
n
etite is the th
r
ther reductio
n
u
lfu
r
, particula
r
-
.
d
e,
n
ce
w
as
up
T
S)
of
as
d
e,
o
re
as,
e
nt
er-
n
to
r
ly
Synthesis Gas
377
if the catalyst has been manufactured from ferrous sulfate. When commissioning
such a catalyst, most of the sulfur is reduced to hydrogen sulfide by the process
gas and this amount would be sufficient to destroy the LTS catalyst if it were
allowed to pass into the catalyst bed. It is normal practise, therefore, to flare the
gas product until it is free from sulfur, before allowing the low temperature shift
to come on line.
The forward shift reaction that is the conversion of carbon monoxide into
hydrogen and carbon dioxide is quite strongly exothermic, and in common with
all exothermic reactions, the level of conversion of carbon monoxide to products
at equilibrium is greater at lower temperatures. The equilibrium constant is in-
dependent of pressure, but higher conversions are also obtained at higher steam
ratios. Unfortunately, the preferred iron catalyst is not active at low temperature
and plants operate in the temperature range 350° - 500°C. Furthermore, the more
active catalysts based on copper that are active at low temperatures are not suffi-
ciently stable to be able to withstand the exotherm associated with the shift reac-
tion when the feed gas contains high levels of carbon monoxide. The exact con-
ditions for operation of the high temperature shift converter are therefore deter-
mined by the carbon monoxide content of the gas entering the reactor, and the
steam ratio used in the primary reformer.
At the time when natural gas was introduced as feed to a steam reformer
from about 1940 onwards, most ammonia plants were designed to use single
beds of HTS catalyst and the concentration of carbon monoxide in the outlet gas
was about 2%. The synthesis gas from the older plants, which used coal as feed-
stock, contained higher concentrations of carbon monoxide, and it was often
necessary to control the temperature by splitting the reactor into two or more
separate beds, with inter-bed cooling or by the incorporation of a quench system.
More recently, when the first ammonia plants based on natural gas were first
operated at the higher pressures, a two bed/inter-cooled reactor design was also
used and the concentration of carbon monoxide in the exit gas was lowered to
about 1% It was then practicable to use a methanator to hydrogenate the residual
carbon monoxide to methane, rather than operating with the copper liquor
scrubbing stage which had previously been used for the final removal of carbon
monoxide.
9.5.2 High Temperature Conversion Catalysts
The first HTS catalysts were reported to operate for about two years before re-
placement was required. As production techniques were developed, however,
catalyst lives improved so that by 1940, lives of more than 14 years were regu-
larly achieved. There were few poisons which affected the catalyst performance
although sulfur, which was the most common impurity in early plants, did sul-
fide the magnetite. This reaction was, nevertheless, reversible. If hydrogen sul-
fide levels exceeded about 300 ppm, sulfided catalysts could not be regenerated
