Litton C.W., . Reynolds D.C., Collins T.C. Zinc Oxide Materials for Electronic and Optoelectronic Device Applications
Подождите немного. Документ загружается.

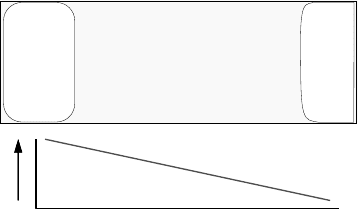
CVT growth of ZnO is illustrated in Figure 7.1. In this configuration, a quartz ampoule is
used to hold a polycrystalline charge of ZnO at one end with a seed crystal at the other end. By
placing the ampoule in a temperature gradient such that the polycrystalline charge is at a
higher temperature than the seed, the charge will be vaporized and transported to the cooler
end where it recrystallizes on the seed crystal. To form a single crystal, the seed would ideally
be of the same material as the growing crystal. However, in some cases, single crystal seeds
composed of other materials have been used.
[6]
“Self-seeding” (no seed is used; the ampoule
is usually configured to localize the initial growth to a few small single crystals) is also
possible but usually requires a tapered ampoule such that the first deposited material will form
at the tip in such a small size that it will naturally form a very small single crystal. The rest of
the deposit then grows epitaxially on this seed and, as it gets thicker, expands in diameter as
the ampoule tapers out. This can require an extended growth time for the growing crystal to
expand to a usable diameter. A more economical method has been developed by ZN
Technology in which a full diameter seed is used with a straight wall ampoule. This method
has been shown to produce high quality, large area, single crystals of CdS, CdTe,
[7]
ZnTe,
ZnSe,
[8,9]
and many ternaries. These crystals are typically twin free without low angle grain
boundaries and have been grown at 2- and 3-in. diameters by the PVT method. The same
methodology was adapted for use in the CVTof ZnO and 2-in. diameter single crystals have
been grown. The addition of a full diameter seed to the PVT method results in the process
being named the Seeded Physical Vapor Transport (SPVT) method and for CVT, the Seeded
Chemical Vapor Transport (SCVT) method.
7.2 Transport Theory and Comparison with Growth Data
While PVT of ZnO is possible, the sublimation vapor pressures of zinc and oxygen over
ZnO are very low at practical growth temperatures: 3 10
6
atm at 1000
C and
1 10
4
atm at 1200
C for the Zn partial pressure.
[10]
Typical Zn vapor partial pressures
in the SCVT system are calculated
[10]
to be 1.5 10
2
atm at 1000
C and 1.9 10
2
atm at 1200
C. Thus the use of the chemical transporting agent should increase the
(g)
↔ Zn
(v)
+H
2
O
(v)
2
ZnO(s) + H
→
Zn (v)
→
H
2
O (v)
H
2
(g)
←
Single
Crystal
Poly
Charge
T
Figure 7.1 Schematic illustration of the chemical vapor transport of ZnO in an enclosed
ampoule
172 Vapor Transport Growth of ZnO Substrates and Homoepitaxy of ZnO Device Layers

transport and growth rates by a factor of approximately 10
4
at 1000
C and 10
2
at 1200
C.
Most vapor growth is done in quartz ampoules and the typical upper limit for use of quartz
is in the 1200–1300
C range. Experimental measurements by ZN Technology of the
transport rate of ZnO using PVT have typically resulted in rates lower than 1 10
6
mol
cm
2
h
1
which is too low to be practical for crystal growth. In these experiments, a
buffer gas of helium at a pressure of 370 Torr was used with a temperature differential
between the charge and the seed of 50
C. Even this low rate may have been
enhanced by residua l moisture or other impurities in the growth charge and system that
may have acted as transporting agents and increased the transport rate beyond that of
sublimation only.
The SCVT method provides the potential for much higher transport rates of ZnO at
significantly lower temperatures. In this method, the transporting agent reacts with the
source material causing a decrease in the partial pressure of the transporting agent and
production of high vapor pressure products. The vapor products formed by the reaction
then flow towards the growing crystal where the reverse reaction takes place. At the growth
interface, the reverse reaction releases the transporting agent causing an increase in the
transporting agent’s partial pressure at that point. Th erefore, the pressure differentials of
the transporting agent and the crystal forming products force the net fluxes of the zinc and
oxygen cont aining vapors to be from source to crystal while that of the transporting agent
is in the opposite direction. This counter flow of gases does not normally exist in PVT and
results in a slightly more complex flow system inside the ampoule.
Although not the only possibility, the transporting agent chosen for ZnO is hydrogen.
The reversible chemical reactio n of hydrogen with ZnO proceeds as:
ZnOðsÞþH
2
ðgÞ$ZnðvÞþH
2
OðvÞð7:1Þ
At the source (polycrystalline charge) end, the reaction proceeds from left to right (see
Figure 7.1) resulting in vaporization of the solid ZnO through usage of the hydrogen. At
the growth end, the reaction proceeds in the opposite direction with solid ZnO formed and
molecular hydrogen released. Transport rates of 5 10
4
mol cm
2
h
1
were achieved by
ZN Technology using a 20% mixture of hydrogen in helium.
While it would be possible to use pure hydrogen to increase the transport rate, growth of
high quality crys tals often requires reduced growth rates. To control the growth rate, the
SCVT growth of ZnO has been conducted with a mixture of inert gas and hydrogen. This
not only reduces the hydrogen partial pressure, a controlling factor in the transport rate, but
also provides a medium through which the vapors must diffuse to reach the growing
crystal. If conditions are set such that the transport rate is limited by the diffusion of the
vapors from one end of the ampoule to the other, independent control of the growth rate
and growth temperature can be achieved resulting in improved reproducibility and long
term stability of the system.
Faktor et al . developed a one-dimensional model for vapor transport and applied it to
crystal growth of CdS by PVT.
[11]
In their analysis, they used a combination of drift and
diffusion to describe the flux of a vapor per unit cross-sectional area given by:
J
vap
¼
U
RT
p
vap
D
RT
d
dx
p
vap
ð7:2Þ
Transport Theory and Comparison with Growth Data 173
where the term
U
RT
p
vap
represents the drift of the vapor and
D
RT
d
dx
p
vap
represents the
diffusion term. Boone
[12]
originally applied these equations to the SCVT system to
determine the transport and growth rates as functions of the growth parameters. This work
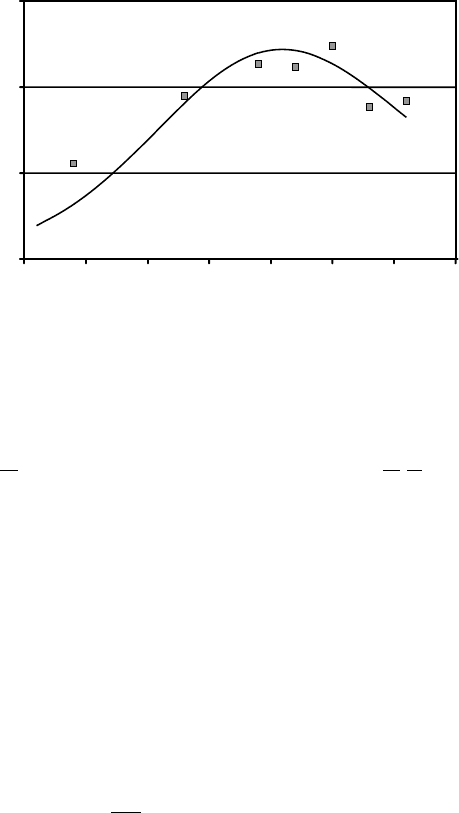
was extended by ZN Technology to more closely model the current growth system. The
results of these calculations along with experimental data are shown in Figure 7.2 where the
growth rate of ZnO is plotted vs furnace temperature. The experimental values are averages
over all growth runs conducted at each temperature. Though the fit of the calculated curve is
not exact, it does exhibit the maximum in growth rate seen in the data.
In addition to growth rate, it is important to maintain an optimum stoichiometry in
the vapor at the crystal growth interface for vapor grow th of high quality II–VI
compound crystals. In the case of the SCVT ZnO growth process, this means
controlling the ratio of water, the oxygen bearing compound, to zinc vapor. A set
of experiments by Reisma n et al.
[13]
involving the gas phase reaction of Zn and H
2
O
vapors in an helium carrier gas indicated the conditions for producing smooth and
transparent layers involved a
P
H
2
O
P
Zn
ratio of 10–50. Similar observations have been made
for metal organic chem ical vapor deposition (MOCVD) growth processes
[14,15]
except the
ratios were in the 4–8 range. Toaccomplish vaporstoichiometry control, an external water
vaporsourcewas addedtotheSCVTZnOgrowthsystem.Thissourcep ermitscontrolofthe
water vapor pressure in the g rowth ampoule. The one-dimensional growth model utilized
for this growth system includes the external water vapor source. In conjunction with the
crystal growth temperature the influence of the external water vapor source can be used to
change the ratio of water vapor to zinc vapor parti al pressures a t the g rowing crystal
interface from 1.5athighgrowthtemperaturesandlow external water vapor pressure to
80 at low growth temperatures and high external water vapor pressure. This is illustrated
in Figure 7.3 by the two curves representing the lowest and highest temperatures used for
ZnO crystal growth.
0.00
0.10
0.20
0.30
-250 -200 -150 -100 -50
0
50 100
Furnace Temperature (Deviation from Standard Temperature) (ºC)
Growth Rate (a.u.)
Figure 7.2 Comparison of experimental growth rates (points) with the growth rate (solid line)
as calculated by the thermodynamic model described in the text. Each point is the average
growth rate for that growth temperature
174 Vapor Transport Growth of ZnO Substrates and Homoepitaxy of ZnO Device Layers

7.3 Characterization
7.3.1 Crystallinity
High resolution X-ray omega rocking curves were used to check the crystallinity of the
SCVT ZnO. In this procedure, the crystal is rocked along the omega axis through the
Bragg peak and the detector is unmasked such that all of the diffracted radiation is
collected. The values measured are the full width at half-maximum (FWHM), the peak
height relative to the incident beam intensity, and the omega position of the Bragg peak.
The rocking curves resulting from this procedure are similar to those in Figure 7.4. The
1
10
100
0.0450.0350.0250.015
0.005
External H
2
O partial pressure (atm)
H
2
O/Zn vapor pressure ratio
Figure 7.3 The ratio of water vapor to zinc vapor partial pressures at the growth interface vs
the applied external water vapor pressure for two different growth temperatures. Upper curve:
240
C; lower curve: þ60
C
Omega
0
0.2
0.4
0.6
0.8
1
I/Io
24"
23"24"
23"
23"
26"24"27"24"24"
22"
25"
Figure 7.4 X-ray rocking curves (omega) for one of the best SCVT ZnO crystals. The
measurements shown are for different spots along the diameter of the wafer and cover a
length of approximately 40 mm
Characterization 175
FWHM (shown in Figure 7.4 as the number above each peak in arc-sec) is measured as the
width of the peak at a vertical position half the total height of the peak. The rocking curves
shown in Figure 7.4 are from several positions across a single 40 mm diameter SCVT ZnO
wafer. The uniformity of the FWHM values and the peak heights are indicative of both the
high crystalline quality and excellent uniformity of the materia l. Although lower (better)
FWHM values have been measured in individual areas on a wafer, the crystalline quality
indicated by the FWHM values in Figure 7.4 should be sufficient for excellent homo-
epitaxial results and subsequent device fabrication.
7.3.2 Purity
One characteristic of the SCVT ZnO growth process is the extremely high purity of the
resulting ZnO crystals. This can be very important for homoepitaxial growth of ZnO since
any impurities in the substrate may diffuse into the homoepitaxial film and alter its
electrical characteristics. Glow discharge mass spectrometry (GDMS) was used to
characterize the impurities in the SCVT ZnO crystals. Table 7.1 shows the results for
the impurities detected in three samples from different growth runs. All other elements
were either not detectable down to a few parts per billion atomic (ppba) or were a part of
the sample matrix or sample mount such as zinc, oxygen, and tantalum. The majority of
the impurity content is silicon and nitrogen. Silicon is expected since the crystals are
grown in quartz ampoules. There has been no indication this level of silicon doping
changes the electrical properties of SCVT ZnO. The amount of nitrogen present in the
three samples shows significant variation, but is consistently present. This nitrogen
presence may be related to imperfect sealing of the growth apparatus resulting in a minor
component of nitrogen in the growth atmosphere. Of the remaining detected impurities,
only aluminum and boron are known to be n-type dopants and their total concentration of
20–50 ppba is not likely to impact the doping of any epitaxial films grown on these
substrates by outdiffusion from the substrate to the film. This high purity is also evidenced
in the optical data discussed in a later section.
Table 7.1 Results of GDMS of one of the SCVT ZnO crystals. Only detected elements are
shown. Note that the data are shown in parts per million atomic (ppma)
Sample A Sample B Sample C
(ppma) (ppma) (ppma)
B 0.012 0.012 0.028
C 0.040 0.004 0.091
N 0.028 0.180 1.200
Na 0.015 ND ND
Al 0.009 0.007 0.020
Si 0.330 0.280 0.660
Ti 0.001 ND ND
Sn ND 0.077 ND
Pb 0.002 ND ND
ND, not detected.
176 Vapor Transport Growth of ZnO Substrates and Homoepitaxy of ZnO Device Layers
7.3.3 Electrical
All undoped ZnO grown by the SCVT method has been n-type conductive. Typical room
temperature electrical characteristics are 0.5 ohm cm with a mobility of 200 cm
2
V
1
s
1
and a net donor concentration of 6 10
16
cm
3
. Growth parameters have been found
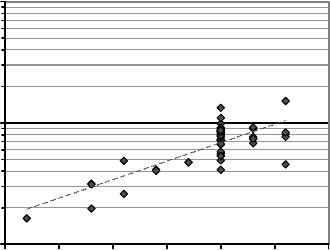
to cause the net donor concentration to change, though not drastically. Figure 7.5
illustrates the decrease observed in the net donor concentration as the growth temperature
decreases. Although the direct cause of this has not been investigated, as was shown in an
earlier section, the thermodynamic model describing the transport process predicts that the
H
2
O/Zn ratio at the growth interface will increase with decreasing growth temperature.
Thus, changes in the stoichiometry at the growth interface for different growth tempera-
tures may be the cause of this trend. Changes in mobility are also observed, but they appear
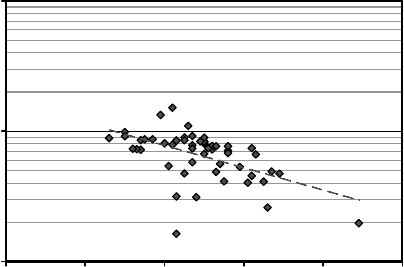
to be associated with the decrease in the net donor concentration. Figure 7.6 plots the
mobilities and net donor concentrations for all samples measured showing the apparent
correlation.
The conductivity of the bulk SCVT ZnO has been attributed to three donors by Look
et al.:
[16]
one with a 30 meV activation energy proposed to be due to a native defect
complex donor, the second at 44 meV activation energy associated with hydrogen
interstitials, and the third at 75 meV that is probably associated with a group III
impurity (most likely Ga or Al) on a Zn site. From Hall vs temperature measurements,
the density of each of these was estimated to be 4.5 10
15
cm
3
for the 30 meV donor
level, 3.0 10
16
cm
3
for the 44 meV donor level, and 2.0 10
16
cm
3
for the 75 meV
level. The acceptor density was calcul ated to be 1.3 10
15
cm
3
. Therefore, the room
temperature n-type conductivity of the SCVT ZnO is likely due to a combinatio n of the
three sources and the compensation level is low with acceptor densities more than a factor
of 20 lower than the donor densities. In addition, the peak electron mobility has been
measured at H2000 cm
2
V
1
s
1
at low temperatures after a thermal anneal that is thought
to remove hydrogen.
[17]
1 x10
16
1 x10
17
1 x10
18
100500-50-100-150
-200
Furnace Temperature
(Deviation from Standard Temperature) in (ºC)
Net Carrier Concentration (cm
−3
)
Figure 7.5 Plot of the net carrier concentrations for SCVT ZnO samples grown over a range of
furnace set point temperatures
Characterization 177
The donor level associated with hydrogen has been eliminated by annealing the SCVT
ZnO in air at temperatures aroun d 800
C. Look et al.
[16]
report elimination of this donor
level using a 715
C anneal. This resulted in a reduction of the room temperature net donor
concentration by a factor of 1.7. The results of annealing in air at 1000
C have been found
to reduce the room temperature net donor concentration by a factor of two to three.
[10]
7.3.4 Optical
SCVT grown ZnO has very good optical properties as measured by photoluminescence
and cathodoluminescence. At room temperature, the typical photoluminescence response
shows a strong UV peak at 378.6 nm along with a very low intensity visible (green)
luminescence.
[10]
Cathodoluminescence also shows the extremely low value of the visible
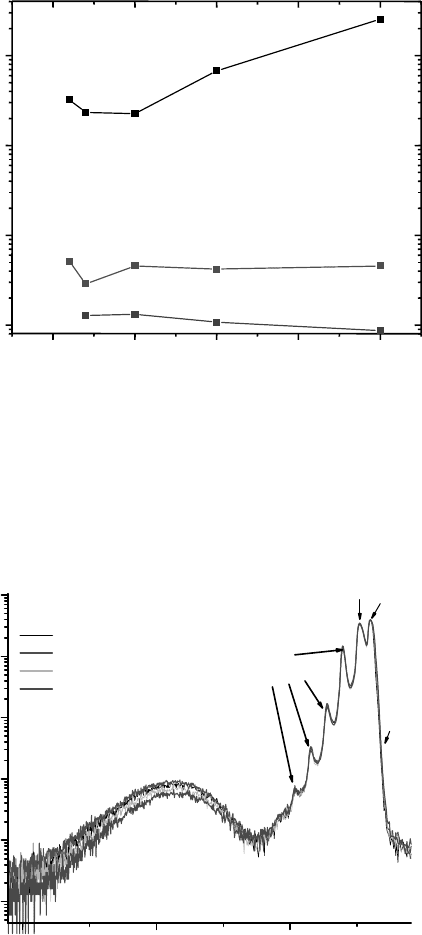
as compared with the UV luminescence. This is illustrated in Figure 7.7
[18]
where, in this
experiment, the cathodoluminescence visible peak height is less than 1% of the UV peak
height. Figure 7.7 also includes a comparison of the cathodoluminescence of SCVT ZnO,
hydrothermal ZnO, and melt grown ZnO showing that the SCVT ZnO ratio of visible/UV
luminescence is much better than the samples grown by the other two techniques.
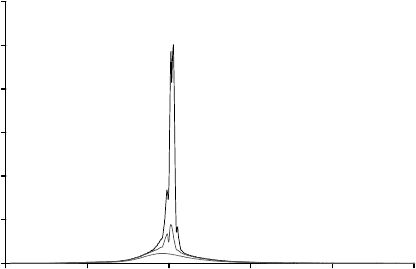
Using low temperature cathodoluminescence, the large number of observable phonon
replicas in Figure 7.8
[18]
also indicates the extremely high optical quality of the SCVT
ZnO sample. The uniformity of the optical properties was also tested on this sample,
showing less than 0.1% variation in the cathodoluminescence when map ped across the
face of the wafer.
[18]
Similar luminescence spectra exhibiting phonon replicas are
observed using low temperature photoluminescence.
[19]
Room temperature, optically
pumped stimulated emission has also been observed in SCVT ZnO samples.
[10]
Figure 7.9
shows the spectra of the stimulated emission. The luminescence spectra are shown for
various pump densities from below to above the stimulated emission threshold. This
observation of room temperature stimulated emission in an SCVT ZnO substrate with no
special optical cavity preparati on demonstrates the exceptional optical quality of the
SCVT ZnO and the efficiency of ZnO’s radiative processes.
1 x 10
16
1 x 10
17
1 x 10
18
250230210190170150
Hall Mobility (cm
2
V
–1
s
–1
)
Net Carrier Concentration (cm
–3
)
Figure 7.6 Plot of average net carrier concentrations of SCVT ZnO grown over a range of
furnace set point temperatures
178 Vapor Transport Growth of ZnO Substrates and Homoepitaxy of ZnO Device Layers
0 5 10 15 20
0.01
0.1
1
10
ZnO, SCVT, Zn
P
b
= 2.0 μW
ZnO, Hydrothermal, Zn
P
b
= 2.0 μW
ZnO, Melt Grown, Zn
P
b
= 2.0 μW
P
b
= 2.0 μW
Green/NBE
Voltage (kv)
Figure 7.7 Ratio of green luminescence to near band edge (NBE) luminescence as a function
of electron beam energy for SCVT grown bulk ZnO (lower plot) and those of two other methods
showing that the SCVT ZnO has very low green luminescence. Reprinted from L. J. Brillson
et al., Nanoscale depth-resolved cathodoluminescence spectroscopy of ZnO surfaces and
metal interfaces, Superlattices and Microstructures 45, 206–213. Copyright (2009) with
permission from Elsevier
3.02.4
1.8
10
100
1000
10000
100000
1000000
3.3605 eV
3.3111 eV
I
4,6,8,9
FE
x
BE
x
BE
x
Phonon Replicas
(0.071eV)
2.48 eV
Constant Power SEM CL Intensity at 12K
CL Intensity (a.u.)
Photon Energy (eV)
Electron Beam Energy
2keV
5keV
10keV
20keV
Figure 7.8 Cathodoluminescence (conducted at Ohio State University) of an (0001) SCVT
ZnO substrate. The sample shows no discernible dependence on electron beam energy
indicating that surface damage was not detectable. Reprinted from H. L. Mosbacker, et al.,
Role of subsurface defects in metal-ZnO(000) Schottky barrier formation, J. Vac. Sci. Technol.
25, 1405. Copyright (2007) with permission from American Vacuum Society
Characterization 179
7.4 In-situ Doping
Even though the SCVT ZnO is electrically conductive as grown, a higher conductivity
would be beneficial for its use as a substrate for light-emitting diodes (LEDs) and lasers.
By adding n-type dopants to the growth system, it is possible to incorporate them during
growth. The key is to find dopants or dopant compounds that have appropriate volatility in
the growth system with normal growth parameters. In addition, a method of control of the
doping rate must be devised to keep its vapor concentration constant for the entire growth
run to assure uniform doping. Group VII dopants such as fluorine, chlorine and bromine
are potential candidates for use. However, they may also act as transporting agents,
complicating the growth system; not to mention that they are hazar dous and can severely
deteriorate the growth equipment during an accidental leak. Possible group III dopants are
boron, aluminum, gallium and indium. These are more benign but elemental forms are not
volatile at the growth temperatures typically employed in the SCVT syst em. Most reports
of highly doped, high conductivity ZnO utilize gallium as the dopant.
[20–22]
However,
calculation of the probable dopant compound vapor pressures in the SCVT growth system
showed that those most suited to maintaining a desirable and controllable vapor pressure
were compounds of boron and indium. Both have previously been reported for use in thin
film deposition methods to increas e the conductivity of ZnO films.
[23–25]
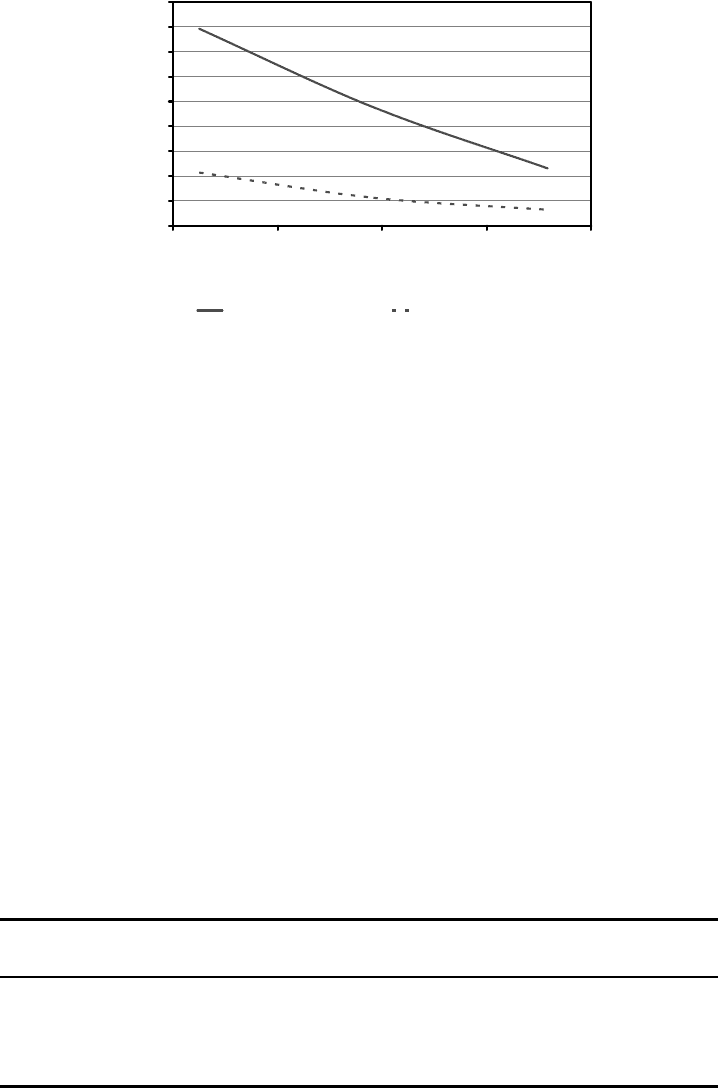
Figure 7.10
shows plots of the calculated ratios of In/Zn and B/Zn in the vapor for a range of growth
temperatures. As the ratio affects the degree of doping, there will be an optimum dopant to
Zn vapor pressure ratio. This is usually determined experimentally but one could estimate
that the optimum ratio should be in the 10
5
–10
3
range as a starting point. The optimum
value will also depend upon how effectively the dopant is incorporated into the growing
interface. The In/Zn ratio is higher than wha t is considered optimum but can be lowered
somewhat by maintaining a high growth temperature. The B/Zn vapor ratio is much lower
than the In/Zn ratio for the sam e temperatures. This ratio is also temperature dependent
with the lowest ratio being at the highest growth temperatures.
0
200
400
600
800
1000
1200
460
440
420
400
380
360
Wavelength (nm)
Intensity (a.u.)
Figure 7.9 Room temperature, optically pumped, stimulated emission from a centimeter
square, standard chemo-mechanically polished SCVT ZnO wafer. At low pumping power, the
emission peaks are broad
180 Vapor Transport Growth of ZnO Substrates and Homoepitaxy of ZnO Device Layers
ZnO crystals were doped in situ by this method with indium and boron. Both dopants
were successfully incorporated into ZnO ingots and the room temperature Hall measure-
ment data for the resulting substrates are shown in Table 7.2. While the indium seems to be
the more effective dopant of the two, giving a resistivity of the order of 10
3
ohm cm with a
mobility of the order of 40 cm
2
V
1
s
1
, the indium-doped ZnO was completely opaque for
this dopin g level. The boron-doped ZnO crystals showed a much larger decrease in the
room temperature Hall mobility (6–10 cm
2
V
1
s
1
) than did the indium-doped crystals but
the transparency of the ZnO was much better. Further work is needed to find the correct
dopant and concentr ation to give the increased conductivity without having a dramatic
impact on the transparency of the ZnO.
7.5 ZnO Homoepitaxy
The primary use of SCVT ZnO is projected to be substrates for homoepitaxy of ZnO-based
optoelectronic devices, particularly LEDs and laser diode s (LDs). Therefore, the ultimate
test of the quality of the substrates is their ability to be used to produce high quality
epitaxial layers. The results of the homo epitaxy (defined here as growth of ZnO-based
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1200-120-240
-360
Furnace Temperature
(Deviation from Standard Temperature) (ºC)
Dopant/Zn Ratio
In/Zn Ratio in the Vapor B/Zn Ratio in the Vapor
Figure 7.10 Calculated ratios of dopant atoms to Zn atoms in the vapor of the SCVT growth
system for boron and indium
Table 7.2 Room temperature Hall measurement results for boron- and indium-doped SCVT
ZnO. A typical undoped result is included for comparison
Resistivity
(ohm-cm)
Mobility
(cm
2
/Vs)
Net carrier
concentration (cm
3
)
In Doped 7.9 10
3
41.5 1.9 10
19
B Doped
1.2 10
1
9.5 5.4 10
18
2.6 10
1
5.9 4.1 10
18
Typical Undoped 5.0 10
1
200 6.0 10
16
ZnO Homoepitaxy 181
