Litton C.W., . Reynolds D.C., Collins T.C. Zinc Oxide Materials for Electronic and Optoelectronic Device Applications
Подождите немного. Документ загружается.

temperature and pressure. Also, the mineralizer must not form stable solid compounds at
the growth interface.
H
€
uttig and Mo
ldner studied the phase equilibrium of the ZnO–H
2
O system to 40
C and
found ZnO to be the stable solid phase at pres sures above 50 Torr and temperatures above
35
C.
[21]
Lu and Yeh experimentally showed that ZnO is the stable product up to
pH ¼12.5 in an aqueous ammonia solution at 100
C.
[10]
Laudise and Ballman grew
large ZnO crystals in alkaline media and found ZnO to be the stable product from 200 to
400
C in 1.0 M NaOH.
[22]
ZnO has been grown in hydroxide solutions up to 10 M. Recent
advances in thermodynamic modeling of aqueou s solution chemistry can aid in choosing
conditions that achieve crystal growth of ZnO and other materials. A thermodynamic
model of aqueous-based chemistry has been developed that computes the stability of ZnO
in different aqueous regimes. The model uses commerci al software (OLI systems Inc.,
Morris Plains, NJ, USA) and is detailed in several publications.
[23–25]
McCandlish and
Uhrin initially modeled ZnO in the hydroxide system to validate the model against
experimental data. Subsequently, a thermodynamic model was created for the g rowth of
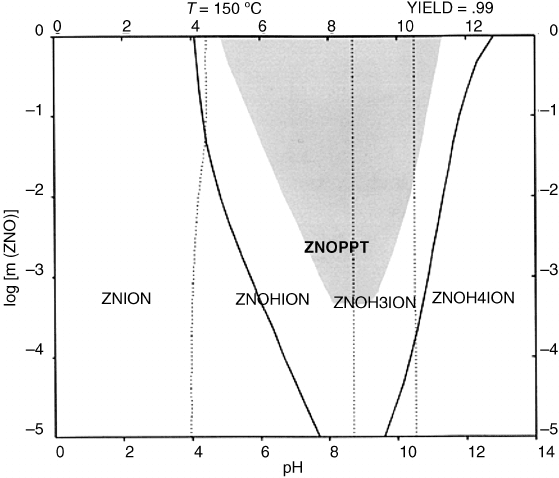
ZnO in acidic environments. Figure 8.3 exhibits the computed stability of ZnO at 150
Cas
a function of pH with HNO
3
as the mineralizer.
[20]
Figure 8.3 Yield diagram for the precipitation of ZnO in 2 molal nitric acid at 150
Casa
function of pH. Reprinted from Handbook of Crystal Growth, Editors Govindhan Dhanaraj,
Kullaiah Brrappa, Vishwanath Prasad, and Michael Dudley, Publisher Springer 2010, ISBN:
978-3-540-74182-4, Chapter 19 Hydrothermal and Ammonothermal Growth of ZnO and GaN
pp 655–689, Figure 19.6
Thermodynamics of Hydrothermal Growth of ZnO 193
8.4 Hydrothermal Growth Techniques
8.4.1 Hydrothermal Growth of ZnO Powder
Hydrothermal growth of microcrystalline ZnO can improve particle size uniformity and
suppress agglomeration of crystallites.
[10]
A desire for higher purity, low cost, microcrys-
talline material for varistors,
[26]
spurred research in microcrystalline ZnO synthesis in
various aqueous media by several groups.
[9,10,27–29]
Recently, an intense interest in
nanocrystalline ZnO for a variety of new applications has resulted in an increase in the
use of the hydrothermal technique for the synthesis of microcrystalline and nanocrystalline
ZnO. Since many experiments can be performed in a short period of time, growth of
microcrystalline ZnO can provide insights that may be helpful in optimizing future
production methods for bulk ZnO crystals. Although an in-depth review of hydrothermal
synthesis of micro- and nano-ZnO structures is not presented, much of the initial work on
ZnO powder synthesis will be reviewed as it provides insight into the growth kinetics of
large ZnO crystals grown by the hydrothermal method.
Various hydrothermal media have been used to prepare ZnO powder. Chen et al.
[9]
used
Zn(OH)
2
colloids in aqueous HCl at pH ¼5–8 and growth temperatures of 100–220
Cto
produce ZnO powder; they also investigated several organic and inorganic solvents. Lu
and Yeh
[10]
used zinc nitrate as a source in aqueous ammonia to investigate product yield
of ZnO powder. Koma reni et al.
[30]
formed ZnO by using microwave irradiation to
decompose zinc nitrate in aqueous sodium hydroxide. Wang et al.
[27,28]
and Li et al.
[29]
used Zn(OH)
2
colloids and Zn(CH
3
COO)
2
as source materials, dissolving them in pure
H
2
O and aqueous KOH, KBr, and NaNO
2
solutions at temperatures up to 350
C and
pressures approaching 400 bar. Their observations of changes in ZnO morphology as a
function of pH will be discussed in Section 8.5.
Recently considerable effort has been made by dozens of research groups on synthe-
sizing nanocrystalline ZnO structures. Zinc acetate, zinc nitrate and zinc chloride have
been used as zinc sources. CTAB (cetyltrimethylammonium), PPA (poly acrylic acid),
SDS (sodium dodecyl sulfate), HMT (hexamethylenetramine), ethanol and PVP (poly-
vinylpyrrolidone) have all been used as chemical additives, surfactants, and modifiers to
alter the morphology of the ZnO nanostructures. The intense effort on controlling ZnO
nanostructures can provide additional insight on altering the kinetics of large ZnO crystals.
8.4.2 Hydrothermal Crystal Growth of ZnO Single Crystals
Hydrothermal growth of single crystals is performed in steel vessels called autoclaves. The
autoclaves are usually made out of some form of stainless steel, or a high strength nickel-
based alloy if higher growth temperatures or pressures are required. The vessel must be
corrosion-resistant and able to withstand the temperature and pressure requirements for
long periods of time. Large ZnO crystals are typically grown in 2–10 molal alkali solutions
at 200–500
C and 500–2000 bar.
[12,13,19,31–34]
Corrosive solutions employing concentrated KOH mineralizer are required to obtain
acceptable growth rates for ZnO crystals. Therefore, to protect the autoclave, a noble metal
liner (e.g. silver,gold orplatinum) must be used. Two techniques can be employed. The first is
to insert a lipped noble metal liner against the wall of a Bridgman autoclave and then put a
194 Growth Mechanisms and Properties of Hydrothermal ZnO
noble metal disk between the lip and the plunger. The second method is to contain the
experimentina completeweldednoblemetal “can” inside theautoclave.In thiscaseaspecific
amount of mineralizer solution is put inside the container (“can”) and a less corrosivesolution
outside the container so that, upon heating to the growth temperature, the pressure caused by
the solution inside the container is approximately balanced by the pressure generated by the
solution outside the container. Such pressure balancing prevents the noble metal container
fromrupturing. Seetheliterature
[7,8]
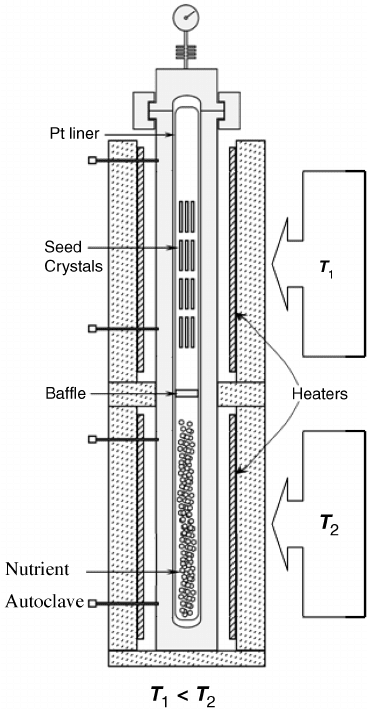
fordetails onautoclavedesigns andapparatus. Figure8.4
schematically shows the cross-section of a large hydrothermal autoclave used by Tokyo
Denpa LLC for the production of ZnO. The autoclave has an inner diameter (ID) of 200 mm
with an inner length (IL) of 3–4 m.
The growth of ZnO crystals is typical of the hydrothermal growth of many other
crystals, e.g., a-ZnO,
[35,36]
Bi
12
SiO
20
[37]
and LiGaO
2
.
[38]
ZnO nutrient, which is either
Figure 8.4 Hydrothermal autoclave used for growth of ZnO. Adapted from Zinc Oxide
Materials and Devices II, edited by Ferechtec Hosseini Teherani, Cole W. Litton, Proc. of SPIE
Vol. 6474, 647412, Fig. 1 on page 647412-2. Copyright (2007) with permission from SPIE
Hydrothermal Growth Techniques 195
crystalline material (free nucleated material from previous hydrothermal runs) or high
purity ZnO powder that has been sintered or hot pressed at high temperature (usually
1000–1300
C) to obtain dense material
[39,40]
is processed into 1 mm
3
–1 cm
3
pieces and
put into the bottom of the liner. Single crystal seeds are cut in the desired orientation, and a
small hole is drilled near the top of the seeds. A wire is inserted through the hole so seeds
can be hung in the upper half of the liner.
The aqueous solvents fill 60–90% of the total volume (the liner, and also the rest of the
autoclave). When heated, the liquid expands to 100% of the total volume; continued
heating generates pressure, which is a function of temperature, degree of fill, and
composition of the liquid. For data on pure water at different percent fills, see the PVT
curves generated by Kennedy.
[41]
Lithium in the solvent produces crystals of greater perfection, as will be d iscussed in
Section 8.6. Before using a new autoclave for ZnO growth, quartz growth is performed to
coat the walls of the autoclave with acmite, Na
2
OFe
2
O
3
SiO
2
. The acmite helps protect
the autoclave from corrosion and subsequent failure in case the platinum container leaks.
Hydrothermal growth of large crystal predominately employs the temperature gradient
method. The upper region T
1
is maintained at a lower temperature than the lower region T
2
as depicted in Figure 8.4. The solvent in the lower region of the vessel dissolves the
nutrient until it reaches saturation. The hotter-lighter-solvated species is transported by
fluid convection to the colder seed region. Because of the lower temperature at the seed the
solvated species becomes supersaturated, comes out of solution, and deposits on the seed
(normal saturation conditions). Fluid convection returns the cooler-heavier-depleted
solution to the hot zone, where additional nutrient is dissolved to regain equilibrium
solubility (saturation). The cycle repe ats as long as there is nutrient in the lower zone. ZnO
growth rates of up to 0.3 mm day
1
perpendicular to the basal plane can be maintained for
months by using the temperature gradient method. For large crystals of ZnO, dissolution
temperatures in the lower region or zone of the autoclave are typically above 350
C; with
a temperature gradient of at least 10
C between the lower region and the upper region
where crystallization takes place. Various conditions for hydrothermal growth of ZnO
single crystals over the past 40 years have been tabulated elsewhere.
[42]
Detailed computational fluid dynamics simulations have been performed in various
hydrothermal systems to analyze the flow and temperature gradients in large autoclaves.
Uniform flow and temperature profiles are critical for the growth of large hydrother-
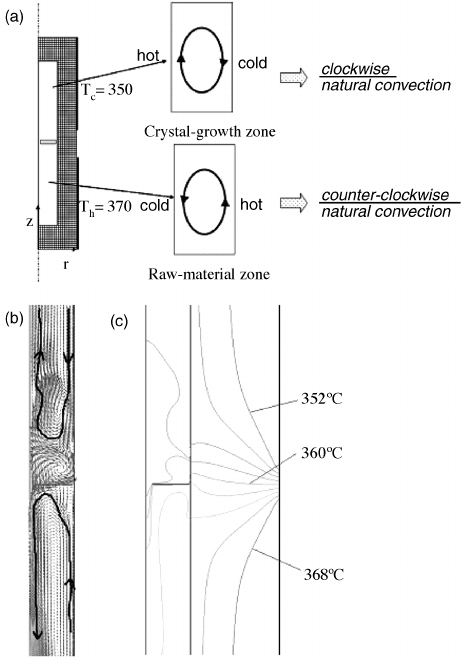
mally grown crystals of high perfection. Figure 8.5 shows a computer simulation of the
fluid flow and thermal profile of an autoclave used to grow ZnO crystals.
[43]
The
simulations were only performed on one half of the autoclave because the system is
assumed to be axisymmetric. The simulation shows that the solu tion in the upper region
primarily flows up through the center and down in close proximity to the autoclave
walls in a clockwise motion. The fluid passes through the baffle primarily through
diffusion and an opposite counter-clockwise motion is seen in the lower region. Several
simulations were done to see the effect a change in viscosity had on the flow in the
system. The authors found that changes in the thermal conductivity of the fluid had only
a small effect on fluid flow, whereas changes in the heat capacity and viscosity of the
fluid had much greater effects. Because of the difficulty in getting accurate fluid
properties such as density, viscosity, specific heat, thermal conductivity, and thermal
expansion coefficient in high pressure closed systems, the models may not accurat ely
196 Growth Mechanisms and Properties of Hydrothermal ZnO
represent real systems. Nonetheless, fluid flow and thermal profile simulation can still
yield viable insights in optimizing autoclave design to increase the yield and uniformity
of hydrothermal ZnO crystals.
8.4.3 Industrial Growth of Large ZnO Crystals
The minim um wafer diameter size typically required by semiconductor device manu-
facturers is 50 mm, therefore ZnO crystals with diameters in excess of 50 mm must be
grown and afterwards processed to ship as epi-ready wafers. The first ZnO crystals
weighing upwards of 200 g were reported in 1997 and 2001.
[44,45]
Fifty milli meter
diameter (0001) ZnO bulk crystals were first reported in 2004 using the hydrothermal
Figure 8.5 (a) Main flow direction of natural convection in a raw-material zone and a crystal-
growth zone. Flow (b) and temperature (c) fields using reference values as follows: thermal
conductivity ¼0.6143 W m
1
K
1
; heat capacity ¼4605 J kg
1
K
1
; and reference viscosity ¼
9.574 10
5
Pa s. Reprinted from Yoshio Masuda et al., Numerical simulation of natural
convection heat transfer in a ZnO single-crystal growth hydrothermal autoclave—Effects of
fluid properties, Journal of Crystal Growth, 311. Copyright (2009) with permission from Elsevier
Hydrothermal Growth Techniques 197

growth technique.
[46]
Furthermore, to yield reasonable prices for 50 mm wafers, large
quantities of ZnO crystals are required, which therefore stipulates growth processes with
high throughput. Despite the slow growth rate of ZnO (about one-fifth the growth rate of
quartz) and the need to use noble metal liners, hydrothermal technology is predestined to
yield large quantities of high-quality ZnO crystals in a single growth run: currently around
100 crystals of ZnO
[47]
or even H1000 crystals as in the case of quartz (SiO
2
).
[48]
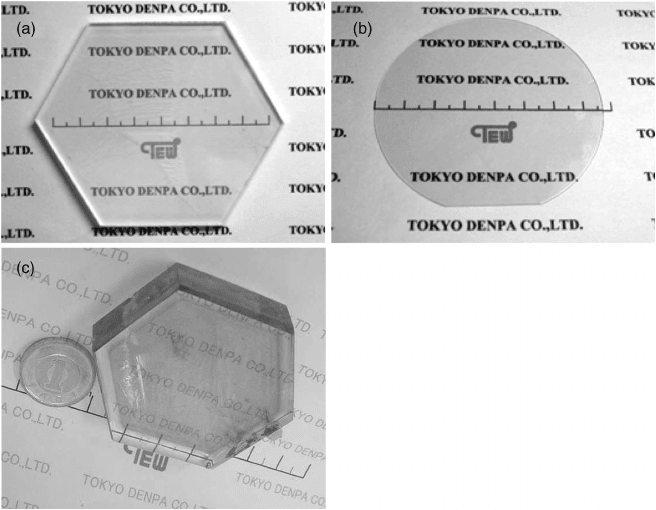
Figure 8.6 illustrates the result from one growth run at Tokyo Denpa.
[47,49]
Almost 100
crystals approximately 50 mm in diameter have been grown on (0001) and (10
10) oriented
seeds. Smooth facets were formed and the average crystal thickness was approximately
1 cm for thos e crystals grown on (0001) ZnO seeds. The aqueous growth solution was
composed of 3 M KOH þ1 M LiOH at pressures of 80–100 MPa with temperatures
approaching 400
C.
A 75 mm diameter (0001) ZnO crystal and a (0001) wafer processe d from it are shown
in Figure 8.7(a) and (b), respectively. The crystal is almost 1 cm in thickness. Note the
homogeneously pale-yellow coloration which points to lower impurity levels than ZnO
crystals grown previously. Figure 8.7(c) shows a typical crystal. Clearly visible are the
newly formed, almost colorless crystal fractions which are surrounding the seed crystal.
Contrary to the crystal growth from the melt with its higher growth rates of the order of
mm–cm h
1
as in the case of silicon, the slow growth rates of 200–300 mm day
1
for the
h0001i direction of ZnO requires time-consuming scale up of crystal size in two steps.
After the size has been increased, the subsequent step is to greatly improve the structural
quality. Next, new seeds are fabricated from this high-quality crystal and the cycle starts
again until the desired size has been obtained.
Recently, several more reports on 75 mm size ZnO single crystals grown by the
hydrothermal method have been published which employed autoclaves with volumes of
up to 500 liters to grow 100–200 crystals per growth cycle.
[50,51]
These developments are
encouraging and enhance the likelihood that large diameter ZnO wafers, i.e. 50 mm will
soon enter the market in high qualities if commercial homoepitaxial ZnO light-emitting
diodes (LEDs) on ZnO substrates can be developed in the near future.
Figure 8.6 Almost 100 ZnO crystals of 50 mm diameter in size displayed after growth at Tokyo
Denpa. Reprinted from D. Ehrentraut et al., Solvothermal growth of ZnO, Prog. Cryst. Growth
Charact. Mater. vol. 52. Copyright (2006) with permission from Elsevier
198 Growth Mechanisms and Properties of Hydrothermal ZnO
The major challenge to be solved for large industrial ZnO crystals is the lowering of
impurity concentrations of the alkali metals such as Li, Na and K, which gives rise to
acceptor compensation, and Al and Fe, both of which are electronic donors. Non-
radiative recombination centers are observed. Growth sectors are typically formed due to
differences in the growth rate and surface chemistries for faces with different crystallo-
graphic o rientations. The thermodynamically stable hexagonal phase of ZnO incorpo-
rates impurities such as Li, Al and Fe with quite different concentrations in the basal,
prismatic and pyramidal growth sectors which will be described in more detail later in
this chapter. Growth in the h0001i direction is preferred since the impurity levels are
strikingly lower and growth rates are higher than the h000
1i direction. The formation of
{10
11} facets should be suppressed for economic reasons Figure 8.7(a) and (c) display
the proper morphology to ensure that all wafers cut from one crystal are of the same size
and have uniform impurity concentrations across the wafer. The preceding discussions
show that under standing and controlling the kinetics of large hydrothermal ZnO crystals
is critical for the production of low cost ZnO wafers with uniform properties. Th erefore,
growth kinetics will be discussed in the next section and impurities and their influence on
kinetics will be discussed in detail in Section 8.6.
Figure 8.7 A 75 mm diameter (0001) ZnO crystal as grown recently (a), a 75 mm diameter
epi-ready (0001) ZnO wafer prepared from such a crystal (b) and a 75 mm diameter (0001)
ZnO crystal obtained from an earlier growth cycle (c). Reprinted from E. Ohshima, et al.,
Growth of the 2-in-size bulk ZnO single crystals by the hydrothermal method, Journal of Crystal
Growth 260, 166–170, Issue 1–2. Copyright (2004) with permission from Elsevier
Hydrothermal Growth Techniques 199
8.5 Growth Kinetics of Hydrothermal ZnO
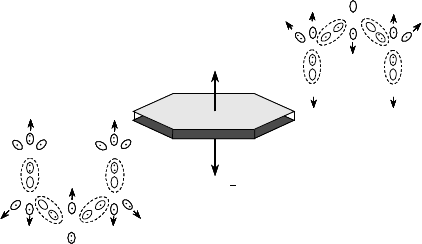
8.5.1 Crystallographic Structure of Hydrothermal ZnO
The stable phase of ZnO has the wurtzite crystal structure, which is hexagonal with a space
group of P6
3
mc. The noncentrosymmetric structure of wurtzite ZnO produces an
anisotropy in which the opposite sides of a basal plane wafer have different atomic
arrangements at their surfaces. The C
þ
side of the basal plane is comprised of a Zn-rich
layer, and the C
is comprised of an O-rich layer, as illustrated in Figure 8.8.
[12,52]
The resulting distribution of electric charge causes disparities, among the various
growth planes, in hydrothermal growth rates, as well as in impurity incorporation,
chemical etching, optical, and elect rical properties.
8.5.2 Growth Rates of the Crystallographic Facets of Hydrothermal ZnO
Figure 8.9 shows the growth planes for hydrothermal ZnO crystals. Hydrothermal crystals
are highly faceted due to the slow growth rates and lack of confinement duri ng growth.
Hydrothermal ZnO in an alkaline medium grows with the following facets: (0001) and
(000
1) monohedra (C
þ
and C
planes, respectively), the six (10
10) prismatic faces (M
planes), and the six (10
11) pyramid faces (P planes). Laudise and Ballman first observed
the anisotropic growth rate on both spontaneous crystallites and crystals grown on seeds in
1 M NaOH.
[22]
Growth on the C
þ
face is always faster than on the C
face in hydroxide solutions above
1 M. Typical growth rates for 6 M KOH and 1 M LiOH are approximately 0.45 mm day
1
in the C
þ
direction, and 0.20 mm day
1
in the C
direction for growth on C-plane seeds.
[31]
Growth rates on M-plane seeds average 0.20 mm day
1
in the direction normal to the
M-plane.
[32]
ZnO crystals grown in KOH solutions are of higher structural quality but
more highly faceted than those grown in NaOH solutions.
[34]
At AFRL-Hanscom AFB, a
Zn
Zn
BULK
O
Zn
Zn
O
Zn
O
Zn
Zn
BULK
“Zn – Surface”
O
BULK
O
Zn
O
Zn
Zn
O
O
O
O
“O – Surface”
BULK
][
0001
+
C
][
0001
−
C
Figure 8.8 Electronic charge distribution of ZnO basal faces. Reprinted from M. Suscavage,
et al., MRS Internet J. Nitride Semiconductor Res. (USA) vol. 4, p. 294., Figure 1 Page 295.
Copyright (1999) with permission from MRS
200 Growth Mechanisms and Properties of Hydrothermal ZnO

3:1 NaOH: KOH solution produc ed crystals with low defect densities and less faceting
than KOH-grown crystals. The mixed NaOH–KOH solvent had the added benefit of being
less corrosive than KOH solutions. ZnO crystals grown on a C-plane seed and a M-plane
seed are shown in Figure 8.9.
[53]
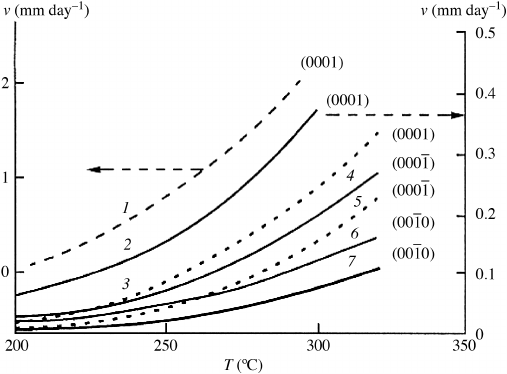
Demianets et al. measured the growth kinetics on the different crystallographic faces by
varying the type and concentration of mineralizer, growth temperature, and temperature
difference between the dissolution and crystallization zones.
[54]
Figure 8.10 shows more
detailed kinetics of ZnO growth as a function of temperature. Note the effect the addition
of lithium, which improves the perfectio n of the ZnO crystal, has in decreasing the
growth rate.
The authors went on to determine the elementary surface layers for the possible growth
facets of ZnO. The elementary surface layers were then used to determine the relative
theoretical growth velocities under ideal conditions (the absence of any additional
components in the crystallization medium) for the different crystallographic faces of
ZnO. The relationships of the velocities are:
vð10
10ÞGvð000
1Þvð0001ÞGvð10
11ÞGvð10
12ÞGvð1
120Þ
The sequence would be reversed to characterize the prevalence of the faces in the
formed crys tal. The ideal velocities above would form simple shapes such as monohedra
Figure 8.9 ZnO crystal grown on M-plane seed (a) and ZnO crystal grown on C-plane seed at
AFRL-Hanscom (b). Image in (a) reproduced from Sekiguchi et al.
[52]
. Image in (b) provided by
M. J. Calahan while at AFRL-Hanscom AFB. Reprinted from T. Sekiguchi, et al., Hydrothermal
growth of ZnO single crystals and their optical characterization, J. Cryst. Growth, vol. 214/215,
Copyright (2000) with permission from Elsevier
Growth Kinetics of Hydrothermal ZnO 201
and prisms but water, a polar solvent, adds a great deal of complexity and anisotropy to the
kinetics of crystal growth.
Growth kinetics in solutions with forced convection can be simplified into two basic
components: supersaturation and surface kinetics.
[55]
Growth rates in solutions are linearly
dependent on supersaturation on all faces for compounds whose supersaturations are large,
e.g. in the percent range.
[56]
Surface kinetics has a much more complicated effect on
growth rates and can be broken down into several molecular processes: surface diffusion,
adsorption, desorption, desolvation, and finally molecular exchanges between various
surface atoms or molecules with each other and in and out of solution.
[56]
The polar nature of water, ZnO, and the intermediate complexes in solution further
complicates growth rates for the various crystal facets. Each family of a crystallo-
graphic plane has a unique set of activation energies in the molecular processes
discussed above. A charge distribution is also associated with each plane which will
interact with the charge distribution and surface composition of the ions in solution.
Thus to gain insight into the growth kinetics of hydrothermal ZnO crystals, one needs
not only to understand the atomic composition and charge of each crystallographic
surface, but also the atomic composition and charge of intermediate species at or near
the growth interface.
Several researchers have studied the solubility and thermodynamic parameters of
aqueous Zn species for hydrothermal systems. Wesolowski et al.
[57]
and B
en
ezeth
et al.
[58]
investigated the solubility of ZnO in 0.03–1.0 M sodium trifluoromethanesulfo-
nate solutions to determine thermodynamic properties of the transport species in dilute
acidic and alkaline solutions. The Gibbs free energy of formation, entropy, and enthalpy at
Figure 8.10 Growth rates of the faces of the monohedra and the {10
10} prism of ZnO single
crystals in alkaline solutions as a function of temperature: (1) 5 M KOH; and (2–7) 5.15 M
KOH þ1.2 M LiOH. Solid lines correspond to DT ¼75
C; dashed lines correspond to DT ¼
50
C. Reprinted from L.N. Demianets, et al., Crystallography Reports vol. 47, Supp 1, S86,
Fig. 4, pg S94. Copyright (2002) with permission from Springer
202 Growth Mechanisms and Properties of Hydrothermal ZnO
