Litton C.W., . Reynolds D.C., Collins T.C. Zinc Oxide Materials for Electronic and Optoelectronic Device Applications
Подождите немного. Документ загружается.

energies of 115 and 164 meV would contribute to the measured p-type conduction. In 2004
Jeong et al.
[81]
reported on As-implanted bulk crystals and assigned a peak at 3.3589 eV
to an acceptor b ound exciton, and transitions at 3.3159 and 3.31859 eV to eA
o
and
DAP, respectively.
Results on phosphorous doping in ZnO came from Hwang et al.
[82]
They obtained
a bound exciton recombination at 3.355 eV, a free to bound transition at 3.31 eV, and
a DAP recombination at 3.241 eV, and deduced a binding energy of the P-acceptor in ZnO
of 127 meV in agreement with the results from Ye et al.
[83]
who obtained a value of
123 meV.
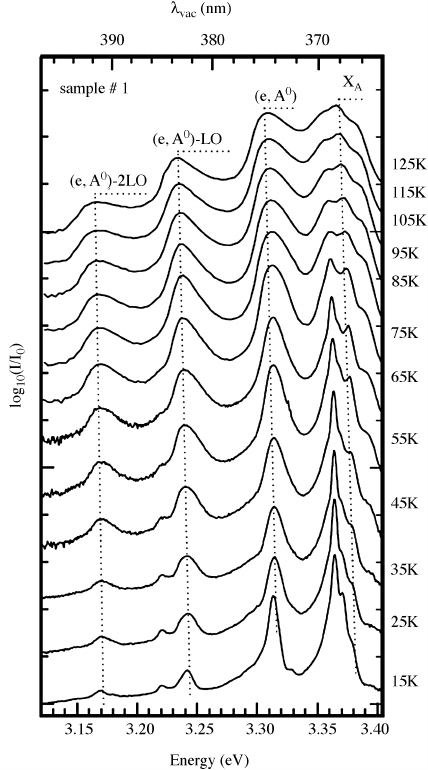
Figure 6.17 Temperature-dependent photoluminescence measurements in an undoped ZnO
epitaxial film grown by vapor phase epitaxy. Reprinted from M. Schirra, et al., Stacking fault
related 3.31-eV luminescence at 130-meV acceptors in zinc oxide, Phys. Rev.B 77, 125215.
Copyright (2008) with permission from American Physical Society
152 Spectral Identification of Impurities and Native Defects in ZnO

Antimony doping was presented in the work of Xiu et al.
[84]
The luminescence line at
3.353 eV was assigned to neutral donor bound exciton recombination. Two emissions at
3.296 and 3.222 eV were attributed to eA
o
and DAP transitions, respectively. The acceptor
binding energy was calculated to be 140 meV.
In the work of Schirra et al.
[80]
the eA
o
transition is correlated with the structural data of
the film as provided by electron microscopy. They concluded that the acceptors are located
in the basal plane stacking faults. Doping with group- V elements could lower the threshold
for stacking fault formation, where the stacking faults act as acceptor s. The observation
and identification of the eA
o
transition at 3.31 eV is not necessarily an indication for
successful p-type doping of ZnO.
The high p-type carrier densities for ZnO:As, ZnO:Sb and ZnO:P require doping
concentrations of the order of 10
20
–10
21
cm
3
. The solubility limits for dilute doping with
the group-V elements are not known. There is experimental evidence
[85]
that secondary
phases within the systems Zn-P-O and Zn-As-O form easily and limit the doping efficiency.
6.3 Magnetic Resonance Investigations
EPR spectroscopy has the ability to resolve hyperfine interactions of electrons or holes
related to doped atoms, impurities, or defects with their own nuclear core and/or the nuclei
of the constituting lattice atoms, e.g. Zn and O. This connection between the nuclear and the
electronic system allows on one hand an unambiguous identification of species via the
isotopes and their abundances, and on the other hand statements on their electrical activity
and wave function distribution. This makes magnetic resonance spectroscopy a valuable tool
for the characterization of materials. The spectra are typically analyzed in the framework of
a Spin-Hamiltonian formalism which we briefly introduce in the following:
[86]
H¼m
B
~
S
~
g
~
H þ
~
S
~
A
~
I ð6:1Þ
The first term on the right-hand side of the equation describes the Zeeman interaction of
an electron characterized by its electron spin S with an external magnetic field H via the g-
tensor, and the second term gives the hyperfine interaction (A-tensor) with the spin I of the
nuclei. For elect ronic centers with a single unpaired spin (S ¼
1
=
2
) in axial symmetry the
Hamiltonian is given by:
H¼m
B
½g
k
S
z
H
z
þ g
?
ðS
x
H
x
þ S
y
H
y
ÞþA
k
S
z
I
z
þ A
?
ðS
x
I
x
þ S
y
I
y
Þ ð6:2Þ
For S H
1
=
2
an additional fine structure interaction has to be taken into consideration:
H¼
~
S
~
D
~
S ð6:3Þ
which in the principal axis system leads to
H¼DS
2
z
1
3
SSþ1ðÞ
þES
2
x
S
2
y
ð6:4Þ
where
~
S;
~
I are the electron spin nuclear spin, respectively and their components S
x
,S
y
,S
z
,
I
x
,I
y,
I
z
,
~
A is the hyperfine interaction tensor and its components A
k
, A
?
, A
xx
, A
yy
, A
zz
,
~
g is
Magnetic Resonance Investigations 153
the g-tensor and its components g
k
, g
?
, g
xx
, g
yy
, g
zz
,
~
D is the fine structure tensor and its
components, D is the axially symmetric part and E is the asymmetry parameter.
It is helpful to recall that the hyperfine interaction A can be split into an isotropic part (a)
and an anisotropic fraction (b):
a ¼ 1=3ðA
xx
þA
yy
þA
zz
Þ; b ¼ 1=2ðA
zz
aÞð6:5Þ
The isotropic part which is a measure of the spin density on the position of the nucleus
(Fermi contact term)is given by:
a ¼ 16p=3m
e
m
N
g
e
g
N
h
2
a
2
Y
2
ð6:6Þ
and the anisotropic part which is due to dipole–dipole interactions is given by:
b ¼ 4=5m
e
m
N
g
e
g
N
h
2
b
2
hr
3
ið6:7Þ
We will use these equations to show that Na
Zn
is a deep acceptor rather than a shallow
one (see below).
To structure the magnetic resonance data on ZnO we have organized the rest of this
section as follows: we start with the results on shallow donors, than describe deep center
properties, especially the oxygen vacancy, and finally present the results on acceptors.
Table 6.1 summarizes the data on the individual centers.
6.3.1 Shallow Donors
In ZnO shallow donors are characterized by a single line spectrum at a field position
corresponding to a g-value of g 1.96. In ZnO single crystals the resonance is slightly
anisotropic due to the wurzite symmetry. The deviation of the shallow donor g-value from
the free electron value of g ¼2.0023 is in first order a result of the admixture of valence
band states (p-type) to the s-type conduction band.
[87–89]
In most cases the EPR linewidth of shallow donors varies between 1 G and 10 G, which
depends on the concentration of the shallow donors and the details of the measurement
conditions. A hyperfine interaction is rarely resolved, thus direct information on the
chemical nature of the donors cannot be given. The reason is that the donor ele ctron is in
an effective mass-like state with a Bohr radius of about 1.5 nm, thus it is distributed over
many lattice sites. Consequently the electron density on the nucleus of the donor atom is
low, typically less than a percent compared with the atomic values, thus the hyperfine
interaction is often smaller than the instrumental resolution in the EPR experiment.
Two fortunate cases have been found where the hyperfine interaction was large enough to
be resolved. In the case of the In-donors a 10 line spectrum was observed reflecting the I ¼9/
2 nuclear spin of the
115
In isotope (95% abundance)
[26,90]
and Ga-donors gave a four line
spectrum due to the nuclear spin I ¼3/2.
[26,91]
For the third common group-III donor in ZnO,
namely Al, the enhanced resolution of the ENDOR had to be used to resolve the inter-
action.
[92]
Experiments of the same kind were successful to identify H as a shallow donor.
[24]
To act as a shallow donor in semiconductors is quite unusual behavior for H. We just recall
here the passivating properties of H on dangling bonds in amorphous Si.
[93,94]
However,
meanwhile also complex centres were observed in which H plays a role as a passivating
154 Spectral Identification of Impurities and Native Defects in ZnO

Table 6.1 Spin Hamilton parameters (spin state, g-values, hyperfine interactions) and defect assignments
Spin g-values Hyperfine interaction Fine structure Defect assignment Reference
S ¼
1
=
2
g
k
¼1.955 Not observed Shallow donor [87]
g
?
¼1.953
S ¼
1
=
2
g
k
¼1.957 Not observed Shallow donor [88]
g
?
¼1.956
S ¼
1
=
2
g ¼1.956 Not observed Shallow halogen donors [88]
S ¼
1
=
2
g
k
¼1.957 A(
115
In) ¼36.6 G Shallow In donor [26,90]
g
?
¼1.956 A(
69
Ga) ¼4.2 G Shallow Ga donor [26]
A(
69
Ga,
72
Ga) ¼6.7 G Shallow Ga donor [91]
Shallow Ga donor [89]
S ¼
1
=
2
g ¼1.9595 A(
27
Al) ¼1.45 MHz Shallow Al donor [92]
S ¼
1
=
2
g
k
¼1.9569 A(
1
H)¼1.4 MHz Shallow H donor [24]
g
?
¼1.9552
S ¼1/2 g
k
¼1.9605 Shallow Zn interstitial [97]
g
?
¼1.9595
S ¼
1
=
2
g
k
¼1.9945 Axial center: Oxygen vacancy, F
þa
[101]
g
?
¼1.9960 A
k
¼57.34 MHz [99,100]
A
?
¼42.3 MHz [102,103]
Nonaxial center:
A
xx
¼76.6 MHz
A
yy
¼75.9 MHz
A
zz
¼94.8 MHz
(continued )
Magnetic Resonance Investigations 155
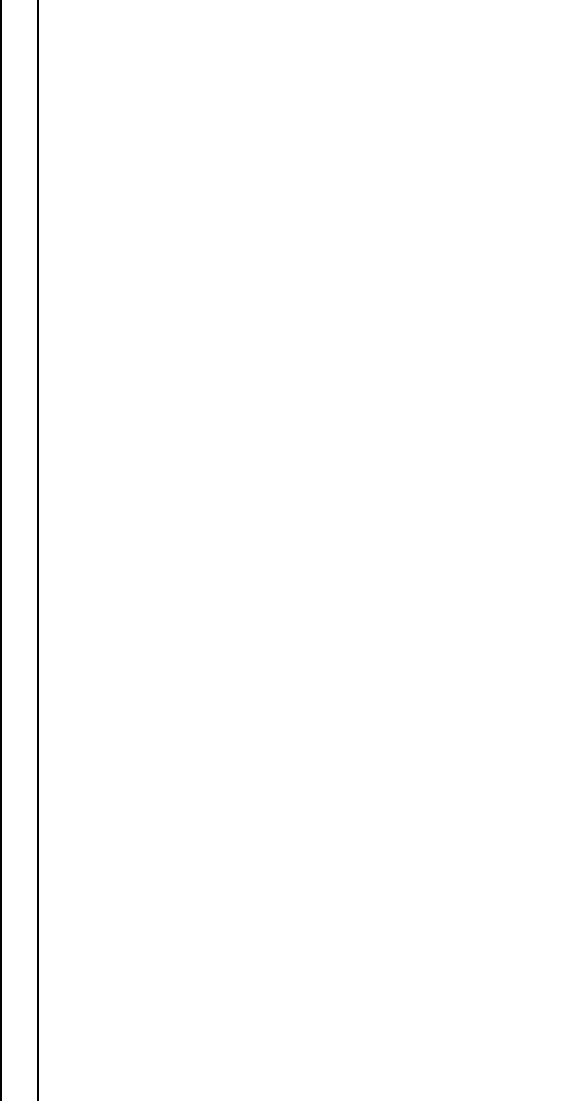
Table 6.1 (Continued)
Spin g-values Hyperfine interaction Fine structure Defect assignment Reference
Axial at room temperature: P-center in ref. [131] [132]
S ¼
1
=
2
g
zz
¼2.0038 Zinc-vacancy related [131]
g
yy
¼2.018 Di- or trivacancy centers
a
[133]
g
xx
¼2.0191 [102,103]
g
k
¼2.0149
g
?
¼2.0134
S ¼
1
=
2
g
zz
¼2.0038 Zinc-vacancy related
a
[131]
g
yy
¼2.0018
g
xx
¼2.0217
S ¼1 g
zz
¼2.0095 |D| ¼1465 MHz Zinc-vacancy related
a
[131]
g
yy
¼2.0141 |E| ¼58 MHz [133]
g
zz
¼2.019 [102,103]
S ¼
1
=
2
Axial center: Axial center: Hole on an oxygen atom adjacent to
a Li atom; deep Li acceptor
[88]
g
k
¼2.0028 A
k
¼0.22 G [114]
g
?
¼2.0253 A
?
¼1.81 G [115]
Nonaxial center: Nonaxial center:
g
zz
¼2.004 A
zz
¼0.29 G
g
yy
¼2.0254 A
yy
¼1.8 G
g
xx
¼2.0223 A
xx
¼1.8 G
156 Spectral Identification of Impurities and Native Defects in ZnO

S ¼
1
=
2
Axial center: Axial center: Hole on an oxygen atom adjacent to
a Na atom; deep Na acceptor
[116]
g
k
¼2.0024 A
k
¼2.89 G
g
?
¼2.0298 A
?
¼1.58 G
Nonaxial center: Nonaxial center:
g
zz
¼2.0032 A
zz
¼4.15 G
g
yy
¼2.0302 A
yy
¼1.42 G
g
xx
¼2.0241 A
xx
¼1.61 G
S ¼
1
=
2
g
k
¼1.9953 A
k
¼81.3 MHz Deep nitrogen acceptor [108]
g
?
¼1.9633 A
?
¼9.5 MHz [91,127]
S ¼
1
=
2
g
k
¼2.0036 A
k
¼9.8 MHz N
2
acceptor [127,128]
g
?
¼1.9935 A
?
¼20.1 MHz
S ¼1 g
k
¼1.971 D ¼0.763 GHz None [108]
g
?
¼2.0224 [106]
g
k
¼1.984
g
?
¼2.025 D ¼260 10
4
cm
1
Oxygen vacancy; F-center [106]
S ¼
1
=
2
g
k
¼2.020 (Zn
i
þ
N
0
) [129]
g
?
¼2.006
a
After e
or n
o
irradiation.
Magnetic Resonance Investigations 157
defect (see below). While the understanding of hydrogen and the group-III donors in ZnO
has greatly developed over recent years much less is known about the halogen donors.
Not only the extrinsic donors may contribute to the n-type conductivity of nominally
undoped ZnO, but also intrinsic defects are often of great importance and relevance.
Very often the exact stoichiometry of the ZnO samples is not known, and defects
accommodating nonstoichiometry such as the donor Zn interstitials and oxygen vacancies
have to be considered with equal importance. In order to study the role of intrinsic defects
Gorelkinskii and Watkins
[95]
and Vlasenko and Watkins
[96,97]
used optically detected
magnetic resonance (ODMR) in combination with low-temperature, high-energy electron
irradiation to create and possibly identify the relevant defects. By choosing the appropriate
electron energy they created and characterized the primary radiation defects and were able
to show convincingly that Zn interstitial atoms are shallow donors. Due to the low
abundance of Zn with nuclear spin (5%) hyperfine interactions were not observed, but
the g-value for the Zn interstitials is clearly that of a shallow donor. The oxygen vacancy
turned out to be a deep level defe ct. Son et al. als o performed ODMR experiments on high
energy electron irradiated samples – this time at room temperature – and discovered
similar defects but with different thermal stabilities.
[98]
6.3.2 Deep Level Defects
By the 1970s the paramagnetic charge state of the oxygen vacancy was observed by
EPR.
[99–103]
Very recently the deep level behavior was confirmed, and the V
O
þ
level
position was determined to be about 0.9 eV above the valence band.
[104]
This diverges
somewhat from theoretical calculations which locate the level closer to the conduction
band.
[105]
Also the negative correlation energy predicted for the oxygen vacancy demands
an experimental confirmation. Another open question is the relationship of the oxygen
vacancy to the “green” luminescence band in ZnO. At least three models are under
discussion: (i) it is related to the presence of Cu impurities
[91]
– a less likely model – the
pronounced phonon structured luminescence band can easily be distinguished; (ii) a
shallow donor (D
0
) to oxygen vacancy (V
0
O
) recombination;
[96,104]
and (iii) it originates
from an internal triplet recombination of the neutral oxygen vacancy (S ¼1), similar to
color center emissions in ionic crystals.
[106]
New aspects in the discussion of the
properties of the oxygen vacancies came recently from the theoretical calculations.
[107]
Their calculations predict that oxygen vacancies have high formation energies in n-type
material, hence have negligible abundance, and play no role in the optical recombinations
in ZnO. They further predict that Zn vacancies are the most abundant intrinsic defects and
recombinations in the green spectral range are caused by Zn vacancies and associates.
They assigned the spin triplet (S ¼1) recombination investigated in the work of Leiter
et al.,
[106]
Carlos et al.
[108]
and Vlasenko and Watkins
[109]
to a pair defect consisting of a
singly negatively charged Zn vacancy (S ¼1/2) and a neut ral shallow donor (S ¼1/2)
which by exchange interaction couple to a S ¼1 state (EM
0
þV
Zn
! EM
þ
þV
Zn
2
).
While such a possibility cannot be ruled out, it is in conflict with the available
experimental data and arguments are given in the following.
In the model of Leiter et al.
[106]
the S ¼1 signal originates from the two-electron state of
the oxygen vacancy (V
O
). In its neutral charge state two electrons are paired with
antiparallel spins, a singlet ground state. Photo-excitation does not involve band states
but occurs as an internal atomic excitation into a singlet excited state. From there
158 Spectral Identification of Impurities and Native Defects in ZnO
inter-system crossing occurs to the emitting states, a spin- and orbital-triplet state. Here the
radiative recombination into the singlet ground state takes place (“green emission”). In an
atomic model this is a
3
Pto
1
S type recombination, the band states of ZnO are not
involved. For a comparison see the results on the two-electron centres in CaO (color
centers).
[110–112]
The parameters of the S ¼1 center are g
k
¼1.984, g
?
¼2.025, and the fine structure
splitting constant is D ¼þ0.78 GHz [see Table 6.1 and Equation (6.3)].
Janotti and Van de Walle
[107]
suggest as a model an exchange coupled pair consisting
of a singly negatively charged Zn vacancy (S ¼1/2) and an effective mass type donor
(S ¼1/2) for the recombinatio n. Coupling the two spins to a total S ¼1 system and
assuming the fine structure splitting D to be zero, we expect the g-value of this center to
be:
[113]
g
pair
¼ðg
V
Zn
þg
donor
Þ=2 ð6:8Þ
Shallow donors have g-values of g
k
¼1.957 and g
?
¼1.955 and the g-values for V
Zn
are g
k
¼2.0024 and g
?
¼2.0193, thus the g-values should be g
k
¼1.979 and g
?
¼1.987.
Such g-values are typical for the centers observed by Vlasenko and Watkins
[109]
but they
are considerably different from the experimental values of g
k
¼1.971 and g
?
¼2.022 (see
Table 6.1). Vlasenko and Watkins
[109]
indeed observed several Zn vacancy related centers
which show an effective mass donor to Zn vacancy acceptor type of recombination
(EM
0
þV
Zn
! EM
þ
þV
Zn
2
), however, none of these had this particular g- and D-value.
Furthermore, the trigonal symmetry of V
Zn
should show up in the angular dependence of the
magnetic resonance signals.
The S ¼1 center detected in the green band has a positive fine structure or zero field
splitting D. The D of spin-exchange coupled pairs is mediated by the dipole–d ipole
interaction D
ss
and is negative.
[112]
D
ss
¼ðm
o
=4pÞð3g
1
g
2
m
B
=2r
3
Þð6:9Þ
where g
1
and g
2
are the g-values of the two spin s and r is the distance between the spins.
For anion vacancies the zero field splitting became positive because there are two
contributions to D ¼D
ss
þD
so
, the dipole–dipole interaction as given in Equation (6.3)
and D
so
is the spin-orbit interaction in the triplet states which are not spin-only states but
have angular momentum.
[112]
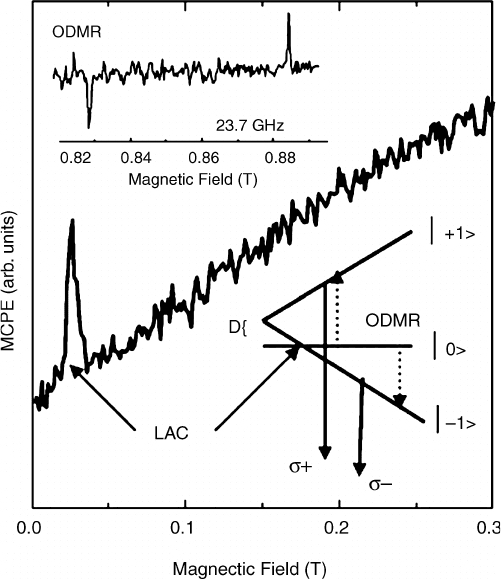
In the ODMR experiments Leiter et al.
[106]
were in the fortunate situation to observe the
level anticrossing (LAC) in the circular polarization of the emission (Figure 6.18). The
LAC allows an unambiguous determination of the sign of D. The intensity of the circular
polarization of the emission is measured as the difference of the left- and right-circular
polarized emission components:
DIðB; TÞ¼I
s
I
s þ
ð6:10Þ
In case of a positive zero field splitting D the |0H sub-state is located below the |1H
sub-states of the S ¼1 triplet. With increasing magnetic field B the MCPE intensity
increases due to partial thermalization in the sub-states. At the field position of LAC the
level mixing of the |1H radiative state and the more populated nonradiative |0H results
in an increase of the s
emission, i.e. a positive signal DI. For a negative D the opposite
holds, the intensity of the MCPE would decrease.
Magnetic Resonance Investigations 159
A typical situation in many ZnO samples is that the emission in the visible spectral range
is a superposition of various recombinations. A broad vibronic coupled band with almost no
structure is the consequence. Therefore it was of importance to investigate the spectral
dependence of the LAC signal and the ODMR resonances responsible for our S ¼1 center
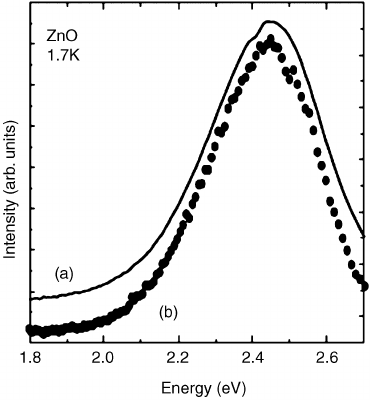
(see Figure 6.19). The dependence of the ODMR (LAC) signals on the emission wavelength
reproduces the photoluminescence spectrum (see Figure 6.19), thus the recombination band
centered at 2.45 eV is caused by this particular spin triplet recombination.
This does not mean that other recombinations and hence other defects cannot participate
in the emission in the visible spectral range but for the samples used by Carlos et al.,
[108]
Leiter et al.
[106]
and Vlasenko and Watkins
[109]
the broad unstructured green luminescence
band was caused by a defect with a S ¼1 configuration in the excited state. However, the
relationship between the “green” emission and the oxygen vacancies is still under discus-
sion. Rather more settled is the identification of the oxygen vacancy in its paramagnetic
single ionized charge state.
[99–103]
Further experiments are needed to get solid information
on the neutral charge state and if possible also on the 2þ charge state. Furthermore, the
predicted negative correlation energy for the oxygen vacancy needs confirmation.
[105,107]
Figure 6.18 Observation of the level-anti-crossing in the magnetic circular polarized emission
(MCPE) of the S ¼1 center in ZnO. The insets show the magnetic resonance as signal decrease
and increase, and a schematic model of the zero-field splitting of the S ¼1 states. Reprinted
with permission from F.H. Leiter, Thesis, Gieben, 2002
160 Spectral Identification of Impurities and Native Defects in ZnO
6.3.3 Extrinsic Acceptors: Li, Na and N
For the group-I elements it is well established that they introduce deep acceptor states.
With binding energies of 800 meV (Li) and 600 meV (Na) they compensate the back-
ground shallow donors and produce high resistive material.
[114–116]
For “shallow” effective
mass like acceptors in ZnO one would expect in an atomic limit g-values around g 1(a
hole localized in a p-orbital) whi ch is clearly not the case for the deep Li and Na centers
(see Table 6.1).
The hyperfine constants of the centers can be interpreted in a linear combination of
atomic orbitals (LCAO) approach where one assumes that the electron wavefunction is
made up from Na 3s and 3p orbitals:
Y ¼ aYð3sÞþbYð3pÞð6:11Þ
a
2
þb
2
¼h
2
G 1 allows for admixtures of non-sodium orbitals for example from
surrounding oxygen atoms. The term b
2
/a
2
reveals the hybridization of the investigated
atom which is also reflected in the bond angle w. Using the relationship:
[117]
tanðwÞ¼½2ð1 þb
2
=a
2
Þ
1=2
ð6:12Þ
and the measured angle w we can estimate the term to be 4.7 times more p-like than in an
ideal sp
3
hybrid.
Inserting the measured values of a and b and the wavefunction values of the free atoms
given by Morton and Preston
[118]
one finds a
2
¼0.12, b
2
¼0.59, and h
2
¼0.71 for the
nonaxial Na centers. For the axial Na centers the values become: a
2
¼0.11, b
2
¼0.29, and
h
2
¼0.4. The h
2
value shows different admixtures of oxygen states for the axial type and
Figure 6.19 (a) Photoluminescence spectrum and (b) ODMR excitation spectrum of the S ¼1
defect in ZnO. Reprinted with permission from F.H. Leiter, Thesis, Gieben, 2002
Magnetic Resonance Investigations 161
