Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

31.20 CHAPTER THIRTY-ONE
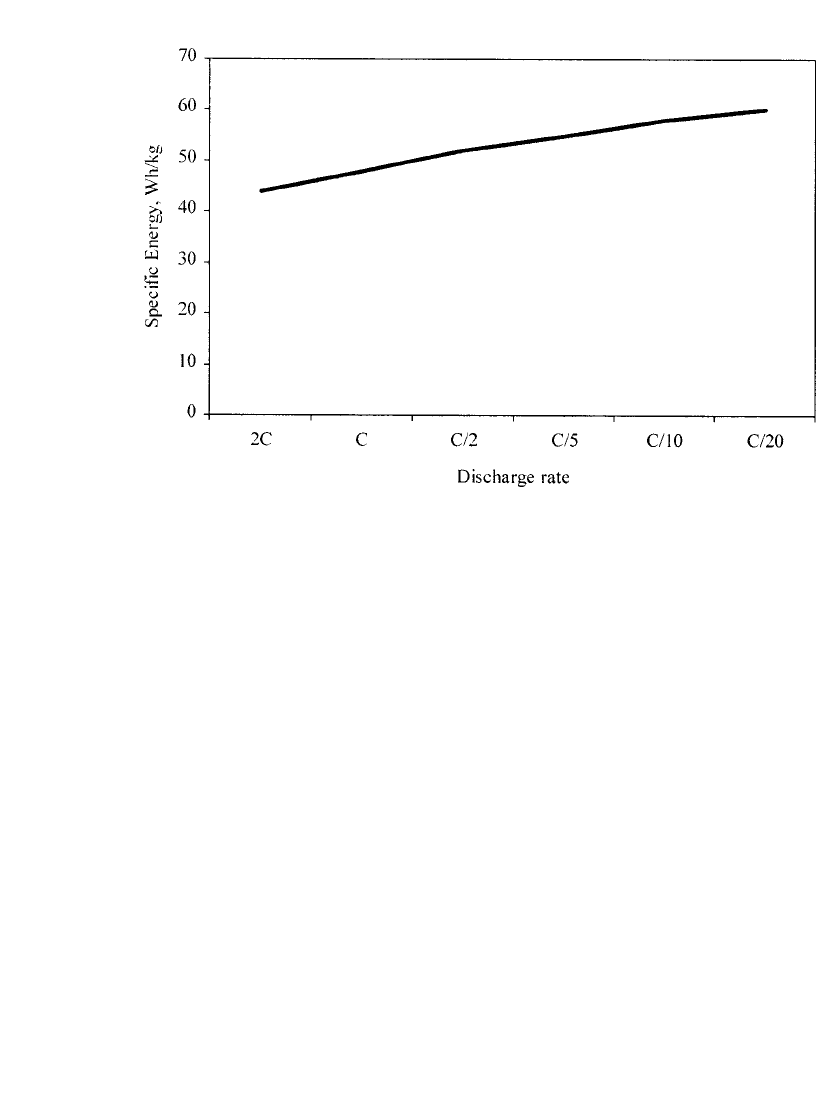
FIGURE 31.11 Nickel-zinc specific energy as a function of discharge rate. Batteries were discharged
at room temperature. 2C: battery was discharged to 1.05 V cutoff; others: battery was discharged to
1.2 V. (Courtesy of Evercel Corp.)
Temperature Dependence of Performance. Temperature has a strong influence on battery
performance. As a general rule of thumb, nickel-based batteries achieve optimal performance
when charged cold and discharged warm. Unfortunately, in many applications, temperature
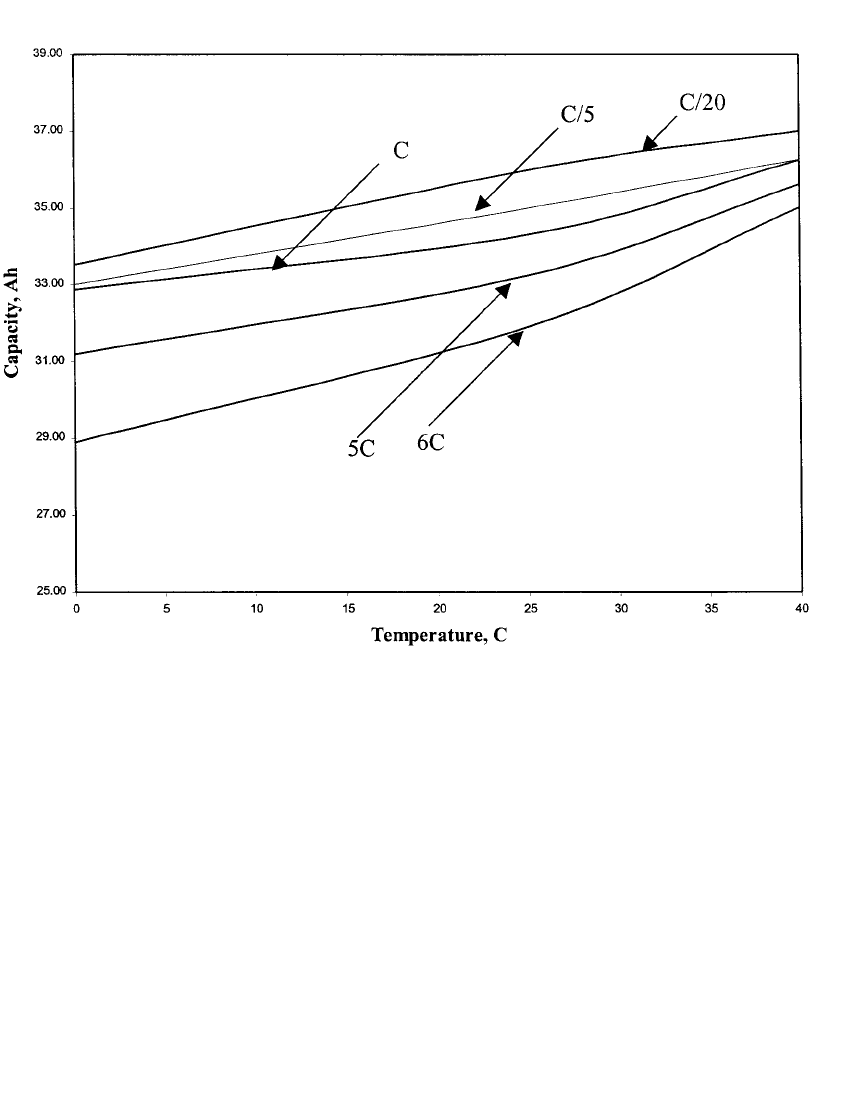
is ambient and uncontrolled. Figure 31.12 shows the effect of temperature on the discharge
capacity of the nickel-zinc battery at four different rates. At lower rates, the discharge ca-
pacity is a linear function of temperature. At the 6C rate, the relationship starts to become
nonlinear, primarily due to the increased conductivity of the electrolyte above 30
⬚C.
Cell Balance on Discharge. Cell balance becomes an important issue in the discharge
performance of multicell batteries. The capacity of the battery will be reduced by weaker
(lower capacity) cells. Newly manufactured cells typically fall within a narrow range of
capacity (for example, plus or minus two or three percent over a manufacturing lot). As a
battery is cycled, the individual cells may diverge in performance. In practical terms this
means that the cells in the battery exist at different states-of-charge. This affects battery
performance because the discharge capacity of the battery is dominated by the weaker cells,
which tend to depress the overall battery voltage. If the cells in a battery become extremely
unbalanced, battery performance and cycle life may be adversely affected. This is strongly
influenced by system level design, particularly thermal design. Temperature differentials in
the battery will cause cells to diverge in performance and thereby reduce the overall per-
formance of the battery. It is therefore recommended that systems be designed such that a
minimum temperature difference (
⌬T) exists among the cells in the battery.
Reconditioning. Many alkaline rechargeable nickel-based batteries, such as nickel-
cadmium, nickel-hydrogen and nickel-metal hydride, are capable of being reconditioned.
Typically this means that the battery is taken to a very low state-of-charge and then recharged
at a moderate rate. Sometimes the reconditioning effect is only seen after several such cycles.
NICKEL-ZINC BATTERIES 31.21
FIGURE 31.12 Discharge capacity of a nominal 30 Ah nickel-zinc battery as a function of temperature at different
discharge rate. C / 20, C/ 5, C rate: battery was discharged to 1.2 V, 5C, 6C: battery was discharged to 0.95 V. (Courtesy of
Evercel Corp.)
Reconditioning is not currently recommended for nickel-zinc batteries. Should more data
become available in the future, reconditioning may become an option for the nickel-zinc
system. In the meantime, the nickel-zinc battery should not be treated as a generic nickel-
based rechargeable battery with respect to reconditioning.
31.5.2 Charge Retention
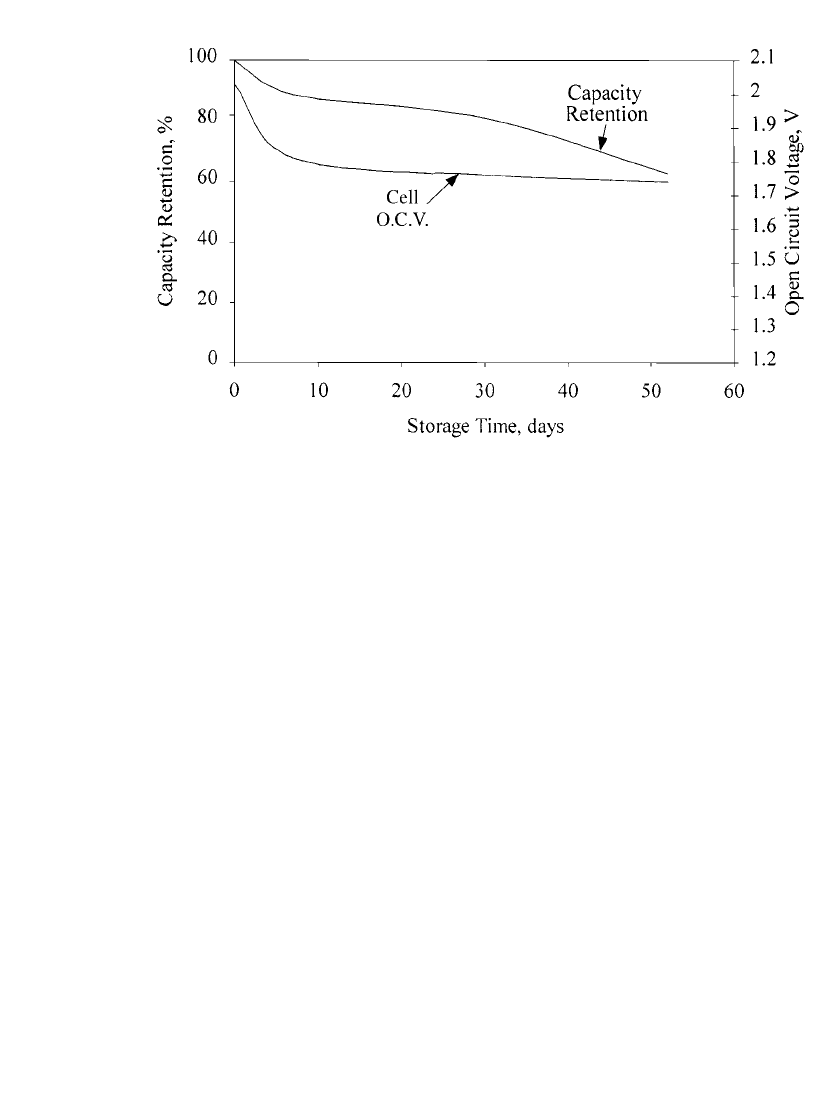
Fully charged nickel-zinc batteries lose only about 20% of their original capacity within a
month on open-circuit stand at 25
⬚C. The self-discharge rate of nickel-zinc, like that of most
batteries, increases with temperature.
Typical charge retention data for the nickel-zinc battery is shown in Fig. 31.13. As with
most batteries, the voltage decays exponentially the first few days and then levels out to a
very slow rate of decline. Capacity decay during open-circuit stand amounts to less than 1%
per day after the initial higher loss rate that occurs during the first 4 or 5 days. If the battery
is allowed to stand for very long periods of time, the battery voltage may reach zero volts.
If this happens the battery usually requires 3 or 4 cycles to fully recover capacity when the
battery is brought into service again.
31.22 CHAPTER THIRTY-ONE
FIGURE 31.13 Self-discharge of a nickel-zinc battery at room temperature. (Courtesy of Evercel
Corp.)
31.5.3 Cycle Life
The nickel-zinc battery is capable of delivering more than 500 cycles at 100% depth-of-
discharge. Cycle life is largely a function of the application, including factors such as duty
cycle, depth-of-discharge, the charging regime used, the cumulative amount of overcharge,
the level of abuse the battery receives and the thermal and mechanical environment. As the
battery is cycled, the capacity gradually declines due to physical changes and degradation
processes in the battery. This gradual decline in capacity is both normal and predictable for
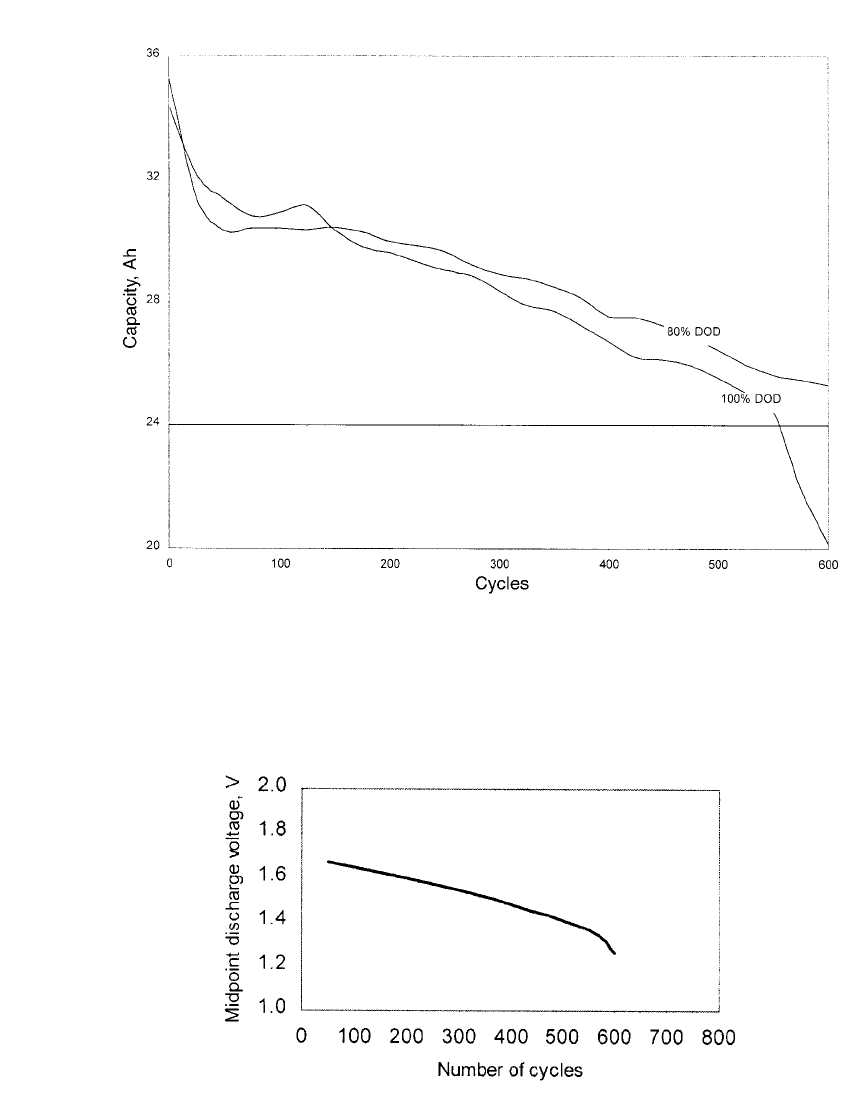
a given battery design. Fig. 31.14 shows the discharge capacity for a 12 VDC nickel-zinc
battery (30 Ah nominal capacity) as a function of cycling. The battery under test is a standard
design built by Evercel Corporation using a plastic-bonded graphite composite nickel elec-
trode and a plastic-bonded calcium zincate zinc electrode. The battery achieved more than
600 cycles at 80% DOD while retaining in excess of 80% of its designed capacity. During
each cycle, the battery was discharged to 80% of its rated capacity at the C/ 5 rate (6
Amperes) and was charged using a two-step CC /CV method, described in Sec. 29.6. The
discharge capacity of the battery was checked at 25 cycle intervals, which adds approximately
24 additional cycles performed at 100% DOD. The data shows a gradual and predictable
decline in performance which is important from a system level. This allows the system
designer to account for battery aging in the overall product specification and design. At
100% DOD, the battery achieved 550 cycles before the capacity dropped to 80% of its rated
value. The rate of decline of capacity tends to increase near the end-of-life which provides
an early indication that failure is imminent.
Figure 31.15 shows voltage performance as a function of cycle life for a single-cell nickel-
zinc battery. The chart plots the mid-point discharge voltage (MPDV) as a function of the
number of cycles. The MPDV is defined as the loaded cell voltage at the mid-point of
discharge as calculated based on the capacity removed from the cell (i.e. half of the actual
capacity of the cell). The battery was cycled at 100% depth-of-discharge, both charging and
discharging at the C /2 rate (15 Amperes). This represents an accelerated test in which
slightly more than 3 cycles per day can be accumulated, whereas in most applications only
a single cycle per day is performed. The loaded discharge voltage of the battery gradually
NICKEL-ZINC BATTERIES 31.23
FIGURE 31.14 Cycle life of 12 V, 30 Ah nickel-zinc battery. 80% DOD testing performed by JBI. (Courtesy
of JBI and Evercel Corp.)
FIGURE 31.15 Nickel-zinc battery MPDV, measured at the C / 2 rate, as a
function of cycle number. (Courtesy of Evercel Corp.)
31.24 CHAPTER THIRTY-ONE
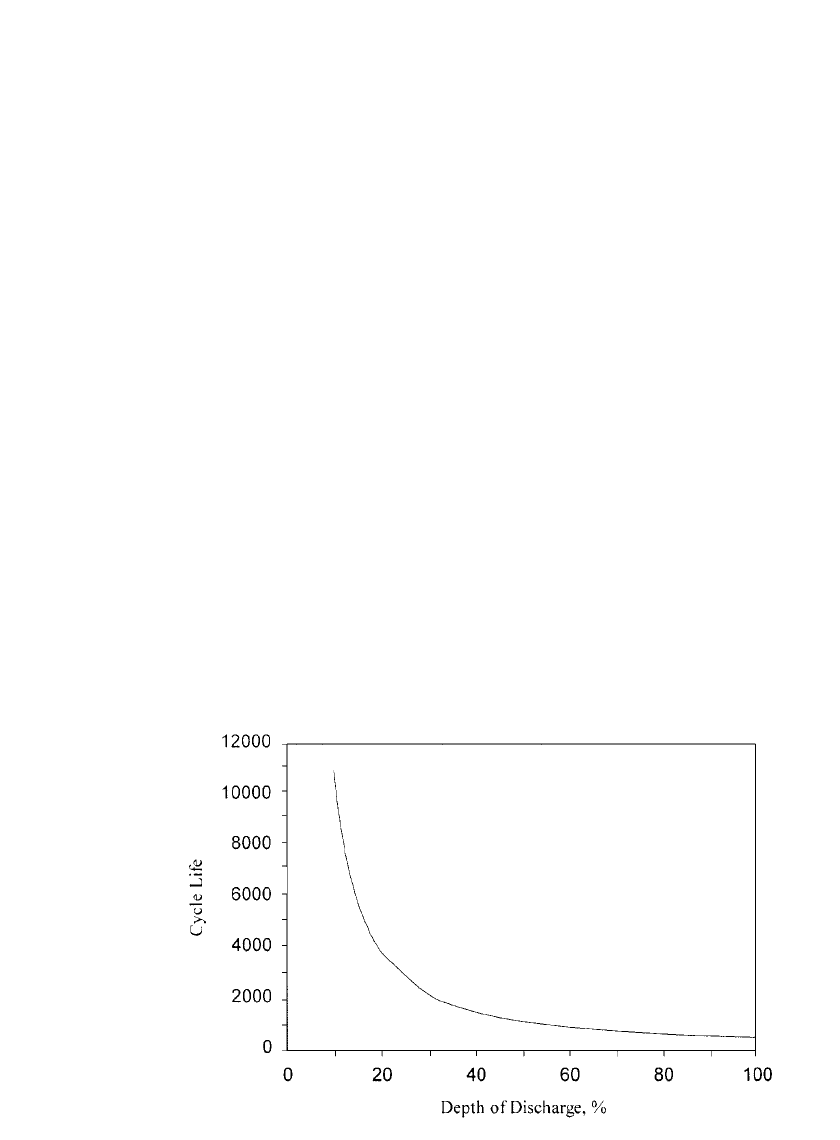
FIGURE 31.16 Nickel-zinc cycle life as a function of DOD. (Courtesy of Evercel Corp.)
decreases with cycling. The effect is similar to that observed with the gradual decline in
capacity. The voltage declines primarily due to the gradually increasing impedance of the
battery as the electrodes slowly degrade as it gradually dries-out. The decay in voltage is
linear and predictable until a point is reached at which the battery can no longer support the
imposed discharge current. When this happens the battery has essentially ‘‘failed’’ from the
standpoint that the required load voltage can no longer be supplied. If the discharge current
is reduced, it will continue to function and will still deliver greater than 80% of its rated
capacity. The battery shown in Fig. 31.15 delivered nearly 600 cycles under these test con-
ditions.
In each set of data, the batteries were cycled at 100% depth-of discharge (DOD).
Depth of Discharge. Cycle life is a direct function of depth-of-discharge, as well as being
a function of many other factors discussed elsewhere in this chapter. Generally, cycling the
battery at deeper depths-of-discharge results in reduced cycle life. The general relationship
between cycle life and depth-of-discharge for the nickel-zinc battery is shown in Fig. 31.16.
This empirical data can be used as a guide in system level design to achieve the required
cycle life by limiting the depth-of-discharge of the battery. Battery life is reduced when
cycled at deeper depths-of-discharge because of the higher stress levels induced in the elec-
trodes. Mechanical expansion and contraction, zinc electrode solubility issues and electro-
chemical issues are all involved in the process.
Temperature Dependence. Temperature is also an important factor in relation to cycle life.
Temperature affects all aspects of battery performance. In general, nickel-based alkaline
batteries perform best at moderate temperatures in the range of 10
⬚Cto30⬚C. Outside of
this temperature range, performance and cycle life may be less than optimum. If the system
design and the application environment are able to maintain the battery within the optimum
range, improved battery performance and cycle life will result.
Failure Mechanisms. Failure mechanisms for earlier nickel-zinc batteries include zinc mi-
gration, shape change, dendritic shorting and hydrolysis of the cellulose-based separator.
These have been substantially eliminated in modern nickel-zinc battery technology. Dendritic
shorting and shape change have been virtually eliminated through the use of reduced solu-

NICKEL-ZINC BATTERIES 31.25
bility calcium zincate electrode technology. Zinc migration has been substantially reduced.
Separator systems have also improved substantially. Stable polymeric zinc migration barrier
materials are used in place of cellulose-based separators. There remain two primary failure
mechanisms in sealed nickel-zinc batteries, failure of the zinc electrode and cell dry-out.
Even with the use of reduced solubility calcium-zincate technology, the zinc electrode
still has some finite solubility in the alkaline electrolyte. Zinc can form complex zincate
anions in the electrolyte and diffuse throughout the battery. Some of this zincate is deposited
within the pores of the nickel electrode. This may adversely affect the performance of the
nickel electrode and thus the performance of the battery. It is possible that the gradual
decrease in capacity observed is partially for this reason.
In plastic bonded electrodes, the fibrillated Teflon
䉸
structure minimizes mechanical fatigue
in the electrode and provides dimensionally stable long-term performance by allowing the
electrode active materials to expand and contract during charge and discharge. Conventional
sintered or pasted electrodes provide no mechanism for this expansion and contraction. In
addition, a fibrillated Teflon
䉸
structure prevents zinc migration and shape change by locking
the active material in place within a stable three-dimensional structure. This effect also
reduces the extrusion of active material from the nickel electrode into the separator.
31.5.4 Memory Effect
Nickel-zinc batteries may exhibit only a very mild memory effect that is associated with the
nickel electrode. Nickel-cadmium batteries commonly exhibit what is termed ‘‘memory ef-
fect’’ or ‘‘fading.’’ This is a reversible phenomenon usually caused by repetitive cycling
at less than full depth-of-discharge. The observed effect is a depression in the discharge
voltage (as much as 120 millivolts) when the battery is discharged below the depth at which
it was previously cycled. Nickel-zinc batteries are only slightly affected by a similar
phenomenon.
31.6 CHARGING CHARACTERISTICS
Proper charging of the nickel-zinc battery is a critical factor in achieving maximum per-
formance and cycle life. The goal of recharging any battery is to input the correct quantity
of charge to deliver the optimum discharge capacity. Charging beyond this point is not
productive and in many cases may cause degradation in battery performance and cycle life.
This is particularly true in the nickel-zinc battery because the zinc electrode is susceptible
to increased zinc dissolution/ migration during extreme overcharge. The critical factor in
charging system design is how to detect when the battery has achieved a full state-of-charge.
Several methods can be used for charge termination including temperature compensated
voltage, the rate of change of voltage with respect to time, increased battery temperature or
a variety of other commonly used techniques.
Development of the commercial nickel-zinc battery necessitated the development of
charging methods and algorithms in order to supply charging systems for commercial ap-
plications. Extensive testing has been performed to fully characterize the nickel-zinc system
as a function of both charge rate and temperature. Several charging algorithms and methods
of charge termination have been evaluated. The defining characteristic for a charging system
is cost, as the cost of the charger must be proportional to the cost of the battery and the
system in which the battery is used.
It should be stressed that the manufacturer’s recommendations should be strictly adhered
to in charging any battery. Excessive overcharge, current which is too high or too low, or
the use of an inappropriate charging algorithm may result in reduced performance, reduced
cycle life and potential safety hazards. Use only a charger specifically designed for the nickel-
zinc battery.

31.26 CHAPTER THIRTY-ONE
31.6.1 Charging Regimes
The most common charging method is a two-stage constant current/constant voltage (CC/
CV) regime, with the CC phase cutting off at a temperature-compensated termination voltage.
The CV phase can be terminated when the current falls below a predetermined value or after
a specified time. Other charging schemes can also be used with the nickel-zinc system, but
it is important to follow the manufacturer’s recommendations. The primary charging criterion
is the required time for recharging the battery. This affects battery and system design and
also determines the cost of the charger.
31.6.2 Fast Charging
For applications where frequent, repeated use is anticipated, a high-rate fast charging system
can be used, with more sophisticated charging algorithms and charge termination methods.
Fast charge systems are capable of achieving a fully charged battery in as little as 2.5 h.
This method works particularly well for smaller systems such as bicycles and small battery
applications where extremely high currents are not required. At higher charging currents,
charge termination becomes much more critical as Ampere-hours accumulate much faster.
Higher currents also may induce higher temperatures in the battery which may reduce its
overall charge efficiency. A balanced approach to charging at high rates is required in order
to achieve maximum performance and cycle life.
In a typical high-rate charging cycle, the battery is charged at the C /2 rate up to nearly
95% state-of-charge. A five-minute rest is included at the end of the higher rate C/2 portion
of the charge to allow depolarization of the battery and voltage relaxation. When the current
is reapplied, the battery is clamped at a constant voltage and the current is allowed to taper,
topping off the battery to full capacity. The electrochemistry of the battery is such that large
quantities of charge can be accepted at a lower state-of-charge. As the state-of-charge in-
creases, parasitic gas evolution reactions become significant and charge efficiency decreases.
This two-step method greatly reduces oxygen evolution at the nickel electrode during over-
charge and improves overall charge efficiency. Fig. 31.17 shows a standard two-step charging
method used for nickel-zinc batteries. The voltage rise near the end of charge signals that
the battery is approaching full charge. The voltage rise occurs because the electrochemistry
of the cell is transitioning from the normal charge reactions to oxygen evolution, which
occurs at a different characteristic voltage. This phenomenon provides an easily implemented
method of charge termination. Once this point is reached, the charge current is reduced to
compensate for the reduced charge efficiency of the battery and the lower current thereby
reduces the gas evolution rate. Typically only about 10% of the rated capacity of the battery
is input during the second step. A back-up charge termination time limit should also be
provided to prevent thermal runaway.
Charge termination voltage is a function of both temperature and charge rate. At higher
charge rates, the end-of-charge voltage increases as a function of cell impedance and polar-
ization. The charge termination voltage also increases at colder temperatures, primarily due
to the decreased conductivity of the electrolyte. Because of this strong temperature depend-
ence, the battery charger must be temperature compensated, requiring the use of a thermistor
to detect battery temperature. This slightly increases the cost of the charging system but
provides much more efficient charging of the battery.
NICKEL-ZINC BATTERIES 31.27
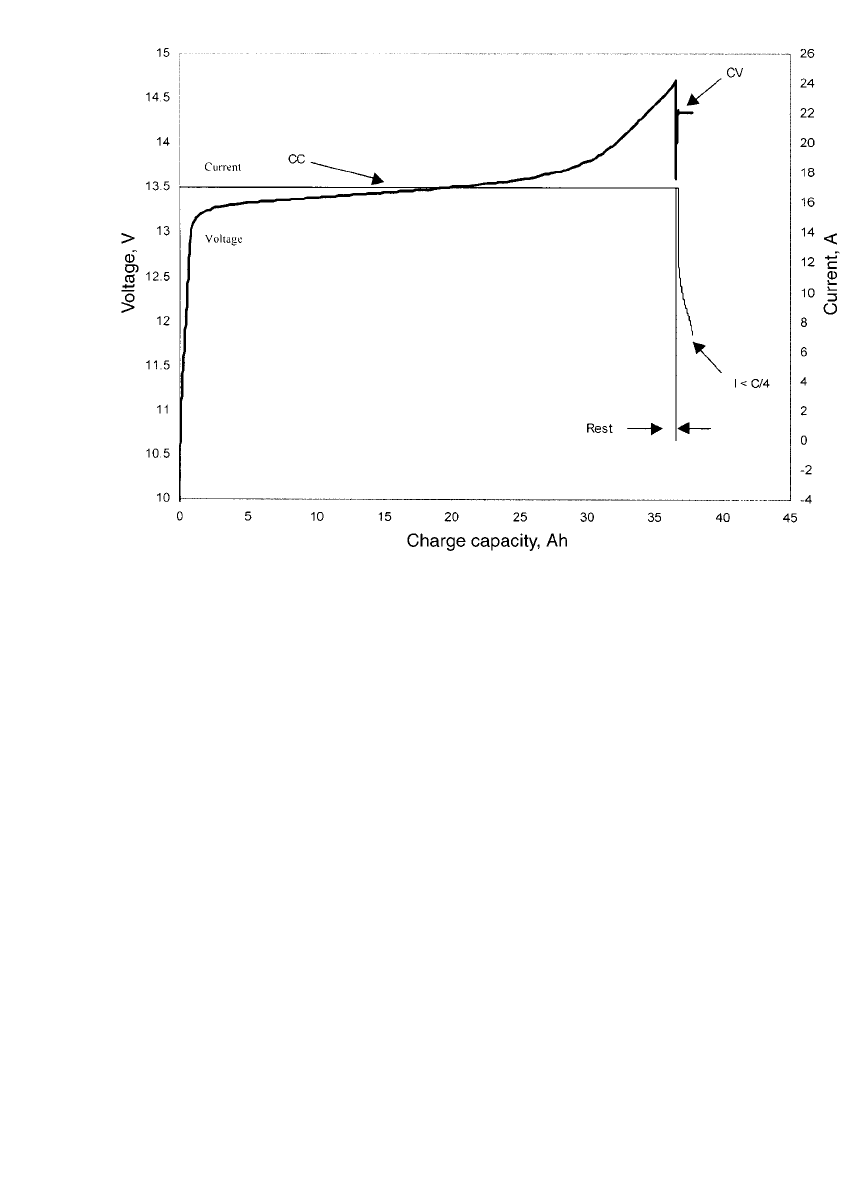
FIGURE 31.17 Seven-cell, 30 Ah nickel-zinc battery charge profile at room temperature. C/ 1.75 charge to
14.7 V then 14.35 V charge to I ⬍ C/4. (Courtesy of Evercel Corp.)
31.6.3 Slow Charging
A slow charge method can be used for applications where rapid recharging is not required
or where the cost of the charger must be reduced. This might include commercial applications
where only one battery discharge is required per day. Slow charging in these types of ap-
plications is typically done overnight. Charge termination is less critical when charging at
low rate. After charge termination, the battery can be left on a low rate trickle charge,
however this is typically not necessary as the self-discharge rate for the battery is low. Trickle
charging is generally not recommended for the nickel-zinc system.
31.6.4 Charge Termination
Charge termination is the critical issue for all types of charging methods, either fast or slow
charging. It is necessary for the charger to detect when the battery has achieved a full state-
of-charge and either stop charging or reduce the charging current. There are a number of
charge termination methods which are used in nickel-based alkaline rechargeable batteries
such as nickel-cadmium and nickel-metal hydride. These include voltage,
⫺dV/ dt (the neg-
ative derivative of voltage with respect to time), temperature rise and a variety of other
methods based on the current /voltage profile of the battery as it nears a full state-of-charge
or other properties of the battery. Similar techniques can be used with the nickel-zinc system.
As seen in Fig. 31.17, the reproducible rise in voltage at the end of charge can be used
as a charge termination method. This voltage is a function of several factors including cur-
rent, temperature and the specific battery design. Fig. 31.18 shows the relationship between
the end-of-charge (EOC) termination voltage and charging current at three different temper-
atures which bracket many normal operating conditions. The charge termination voltage is
nearly a linear function of charging current at each temperature. It is apparent that the
temperature has a very large effect over the range of 0
⬚Cto40⬚C. Figure 31.19 shows the
31.28 CHAPTER THIRTY-ONE
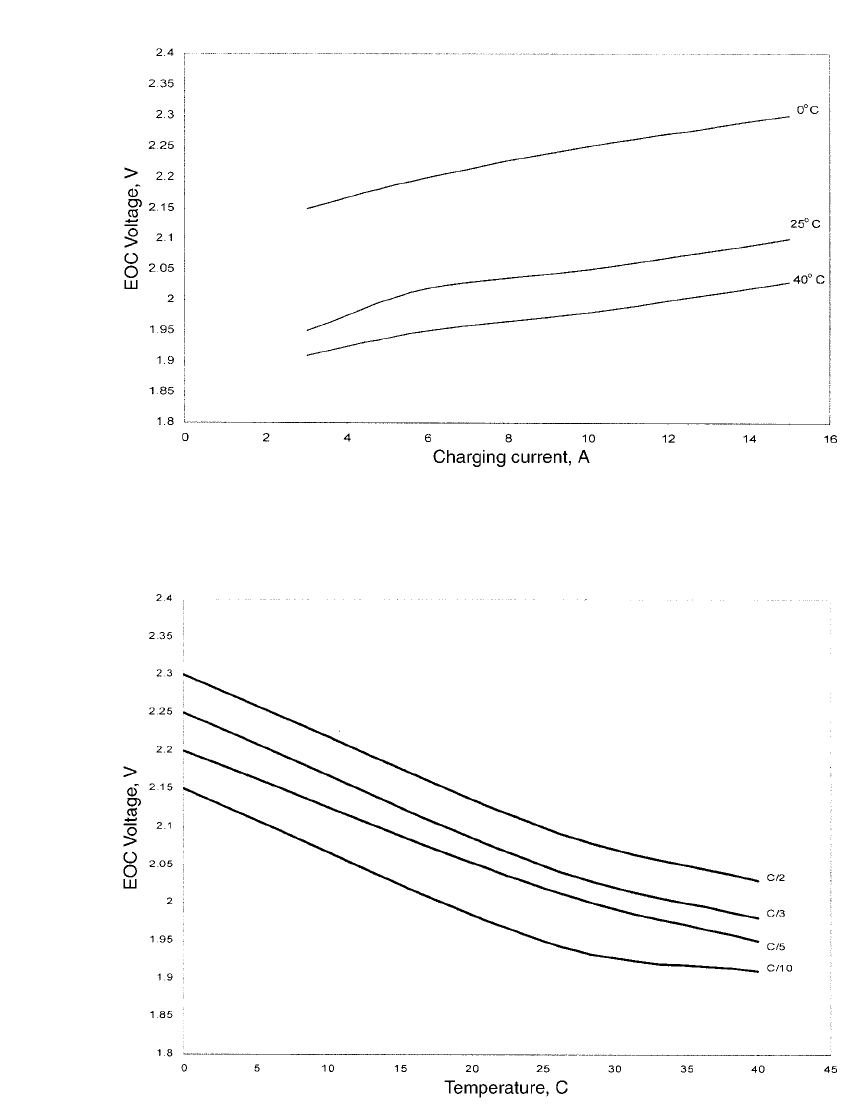
FIGURE 31.18 EOC voltage as a function of charging current and temperature for a 30 Ah single-cell
nickel-zinc battery. (Courtesy of Evercel Corp.)
FIGURE 31.19 EOC voltage as a function of temperature and charge rate. (Courtesy of Evercel Corp.)

NICKEL-ZINC BATTERIES 31.29
relationship between charge termination voltage and temperature graphically as a function
of charging current. The data show that the charge termination voltage is linear as a function
of temperature (at rates between C/10 and C/ 2) up to about 25
⬚C. Above this temperature,
the slope of the end-of-charge voltage versus temperature relationship declines significantly.
At temperatures above 25
⬚C, the EOC termination voltage varies very little with increasing
temperature.
31.6.5 Overcharging
Overcharging is detrimental to most batteries, including nickel-zinc. Overcharge is based on
the nominal (or actual) capacity of the battery being defined as 100% state-of-charge. Charge
input above the 100% state-of-charge level is defined as overcharge. Overcharge simply
means that more Ampere-hours of charge were put into the battery than was removed from
the battery on the previous discharge. It is typically expressed as a percentage of the nominal
(or actual) capacity. Batteries are more tolerant to overcharge at lower charge rates. But even
low current can damage a battery if enough total Ampere-hours are input. As a battery
approaches full state-of-charge, parasitic gas evolution reactions occur which generate heat
and can cause a battery to vent. Excessive overcharge may also degrade the electrodes and
reduce battery performance and cycle life. Therefore, it is highly recommended to prevent
severe overcharging of the battery.
31.6.6 Cell Balance During Charging
Cell balance also becomes an issue in multicell batteries from the standpoint of charging.
As a battery is cycled, the individual cells may diverge in performance. Many of the concepts
from the discussion of the effect of cell balance in discharge performance also apply here.
Cell balance affects charging because cells may receive differing amounts of overcharge
depending on the state-of-charge of the individual cell. Cell balance may also affect the
efficiency of various charge termination methods, making charging the battery less uniform
and reliable. If the cells in a battery become extremely unbalanced, battery performance and
cycle life may become adversely affected. Thermal imbalance can cause electrical unbalance
in the battery. It is therefore recommended that systems be designed such that a minimum
temperature difference (
⌬T) exists among the cells in the battery.
31.7 APPLICATIONS
Nickel-zinc provides the lowest-cost option for a long-cycle-life alkaline-rechargeable
system. The nickel-zinc system is suited for mobile applications such as electric bicycles,
electric scooters and electric and hybrid vehicles or other deep cycle applications. Nickel-
zinc may also replace other nickel based batteries with a less expensive system.
