Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.


31.10 CHAPTER THIRTY-ONE
FIGURE 31.5 Scanning electron microphotograph of roll-bonded zinc electrode after 500 cycles.
(Courtesy of Evercel Corp.)
Calcium zincate is much less soluble in alkaline solution than zinc oxide. This forms the
basis for the improvement in cycle life obtained with this technology.
Cells containing calcium zincate electrodes can be manufactured in at least two different
ways. Calcium hydroxide can be added to the zinc oxide electrode mixture. In this case, the
calcium zincate is formed in situ as the electrode is cycled in the cell during the electro-
chemical formation process. Another method is to form calcium zincate in a separate step
and then use this material in the electrode fabrication process.
14
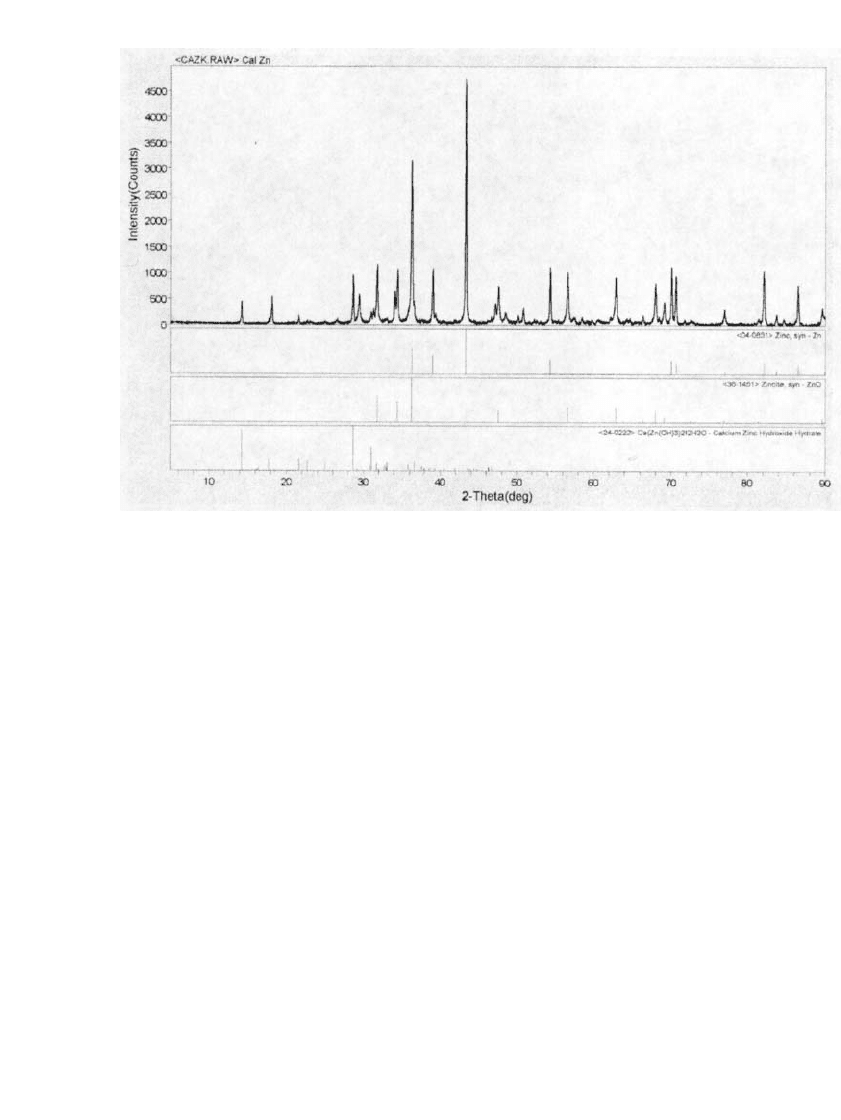
Calcium zincate can be
prepared, purified and identified by its X-ray diffraction pattern, shown in Fig. 31.6. This
process produces electrodes with a more uniform distribution of calcium zincate and over-
comes the problem of zinc dissolution that occurs during the first few cycles before the
calcium-zincate has fully formed in the in situ method. This serves to increase the overall
cycle life and performance of the battery. In either case, an important advantage of the
plastic-bonded calcium zincate structure is the reduction of the tendency to form zinc den-
drites. The zinc active materials are embedded in the three-dimensional structure of the PTFE
nanofibers. This increases the stability of the electrode. Although it is possible for some zinc
dissolution and migration to occur in the cell, dendritic deposits which penetrate the separator
are rarely seen.
NICKEL-ZINC BATTERIES 31.11
FIGURE 31.6 X-ray diffraction pattern showing calcium zincate in zinc electrode after cycling. (Courtesy
of Evercel Corp.)
31.3.3 Separator
Nickel-zinc batteries typically use a multicomponent separator system. This is due to the
requirements for providing both an electrolyte reservoir and for retarding zinc migration. No
single material has been identified as yet that will adequately provide both functions. Even
if a reduced solubility zinc electrode is used in the cell design, such as the calcium-zincate
electrode described above, zinc still has a finite solubility in the alkaline electrolyte. A zinc
migration barrier must be provided in order to prevent shorting of the zinc to the nickel
electrode. The tendency towards dendrite formation is significantly reduced through the use
of calcium zincate electrode technology. This lessens the requirements imposed on the sep-
arator material, particularly that of mechanical strength and penetration resistance.
Traditional nickel-zinc and silver-zinc cell designs used multiple layers of cellulose-based
membrane materials. Many different materials were tried, but the most common material
used was cellophane which is a cellulose film which acts as a membrane and is capable of
resisting zinc penetration. The material has a high molecular weight and varying degrees of
crystallinity, which gives it a wide range of distributions of order (pore size). The cycle life
of cell designs using this material is severely limited due to hydrolysis of the cellophane in
alkaline solution. Various methods have been tried to stabilize cellulose materials, such as
chemical treatment and radiation grafting to other polymers, but none have as yet proved
economically feasible. Considerable work has been done in the past to develop a suitable
separator for nickel- and silver-zinc batteries.
15
An excellent discussion of separator devel-
opment is contained in a comprehensive review.
2
The most successful zinc migration barrier material yet developed for the nickel-zinc
battery is Celgard
䉸
. This is a microporous polypropylene film which has a typical thickness
of 0.025 mm. Polypropylene is inherently hydrophobic so the material is typically treated
with a wetting agent for aqueous applications. One disadvantage of microporous materials,

31.12 CHAPTER THIRTY-ONE
as compared to membrane materials, is the lack of tortuosity. There is not a tortuous path
that the zincate ions must migrate through in order to form an electrical short to the nickel
electrode. As a result, multiple layers of the material must be used. Since the holes in the
layers are typically not ‘‘lined up,’’ some artificial tortuosity is introduced.
31.3.4 Electrolyte
Traditional nickel-zinc cells utilize 31% to 35% concentration aqueous potassium-hydroxide.
Typically 1% lithium hydroxide is also added to the electrolyte. Higher electrolyte concen-
trations reduced the hydrolysis rate of the cellulose-based zinc migration barrier materials.
However, the solubility of zinc also increases as electrolyte concentration is increased. This
increases the likelihood of zinc penetration through the separator. A variety of electrolyte
formulations and additives have been tried with varying degrees of success.
16–17
These in-
cluded potassium fluoride, potassium carbonate and a variety of other additives. The goal
was to reduce the solubility of the zinc to improve cycle life but this goal can also be
achieved through zinc electrode additives, as discussed above.
More recently it has been shown that electrolyte formulations in the potassium hydroxide
concentration range of 20% to 25% can increase the cycle life obtained from the battery.
This is particularly true when used in conjunction with the reduced solubility calcium zincate
electrode. The solubility of zinc is substantially reduced by decreasing the concentration of
the electrolyte below 25%. This reduces zinc dissolution and therefore zinc migration, sta-
bilizing the zinc electrode and increasing the cycle life of the battery.
Newly manufactured cells are typically vacuum filled with the electrolyte. Commercially
manufactured equipment is available for this purpose. In this method, a high vacuum is
applied to the cell and the electrolyte is then drawn in by the vacuum. The purpose of the
vacuum is to remove air from the microporous cell components, such as the electrodes and
separators. This facilitates wetting of these components to make the cell immediately sus-
ceptible to charging. The cell can also be electrolyte activated by simply filling the cell and
allowing it to soak. However, this takes much longer for the cell components to adequately
wet which reduces throughput in manufacturing.
31.4 CONSTRUCTION
Various types of cell and battery design and construction can be used in the nickel-zinc
battery system. Cells have been built in both prismatic and cylindrical designs and both
vented and sealed designs. However, most current commercial applications require the use
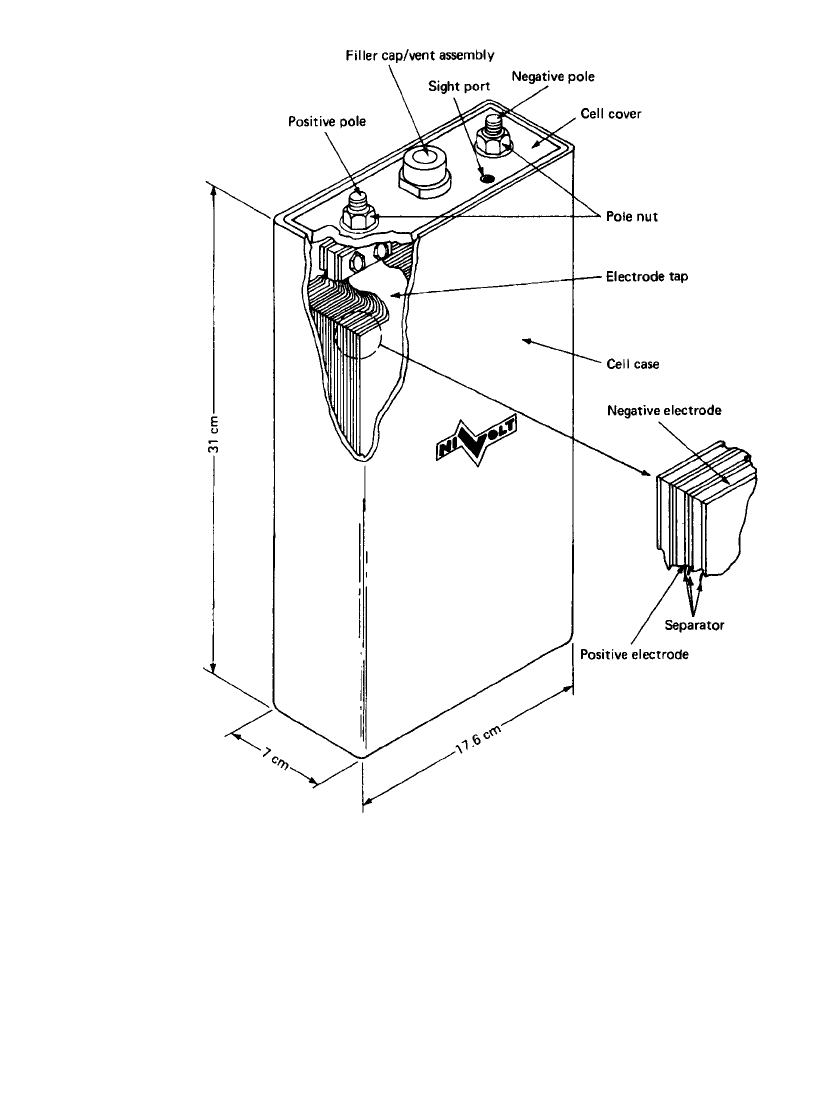
of a sealed, maintenance-free design. A typical sealed prismatic battery is shown in Fig.
31.7. This type of construction can be used for a wide range of cell sizes and is particularly
suited to larger capacity batteries (e.g. greater than 10 Ampere-hours).
NICKEL-ZINC BATTERIES 31.13
FIGURE 31.7 Sealed nickel-zinc cell. (Courtesy of Evercel Corp.)
31.4.1 Sealed Cell Design
Nickel-zinc prismatic cell designs have been developed ranging from 2 Ah to over 100 Ah.
18
The technology is readily scaleable to prismatic cells, cylindrical cells, or monobloc batteries.
In a typical cell design, the cell is stacked with alternating nickel and zinc electrodes, with
the separator material between. Usually a zinc ‘‘half-plate’’ terminates the electrode stack on
either end. Prismatic cell and battery cases and covers are typically plastic. Cylindrical cell
and battery cases and covers are typically nickel-plated steel. Metallic conductors (typically
nickel or copper) are used for the electrode tabs and the cell and battery terminals. Sealed
nickel-zinc cell and battery designs incorporate a resealable safety vent which prevents ex-
cessive pressure from building up inside the cell container. This resealable vent provides for
an operating pressure in the cell which aids in forcing gas recombination. Plastic cased

31.14 CHAPTER THIRTY-ONE
batteries typically vent at a few atmospheres while cylindrical metal-cased batteries may vent
at up to 20 atmospheres or higher. Some nickel-zinc cell designs also use an auxiliary
catalytic gas recombination electrode to facilitate gas management.
Mechanical Design. Mechanical cell design follows the traditional patterns established by
other battery chemistries. Nickel-zinc is best suited for larger form-factor, deep-cycle appli-
cations and these mostly require rectangular prismatic designs. Some work has been done
with cylindrical designs as well but these have not yet been commercialized. Mechanical
design primarily involves the physical configuration of the cell along with non-active material
issues such as the case and cover, structural integrity, internal electrical connections, other
cell parts and components and the external interface. The two major types of cells are briefly
described below.
Prismatic Cell Designs. Nickel-zinc prismatic cell and battery cases and covers are typ-
ically molded from commercial grade resins such as Noryl
䉸
, depending on the application.
Polysulfone has been used in ruggedized designs, but is more expensive than some other
plastics. The cover usually incorporates a resealable safety valve. This valve remains closed
under normal operating conditions, providing sealed operation, yet provides a necessary
safety margin in the event of a catastrophic event such as fire or severe overcharge or
overdischarge abuse. The safety relief valve provides a fail-safe leak-before-burst mode of
operation. Prismatic cells are very easy to manufacture and automated equipment exists for
this purpose. For larger capacity cells (for example greater than 5 to 10 Ah) prismatic cells
are more efficient for most applications.
Cylindrical Cell Designs. Cylindrical nickel-zinc cells have been built in the past.
19
This
design incorporated several interesting features such as the addition of bismuth oxide to the
zinc electrode mixture and an approach for controlling the internal pressure of the cell.
However, cells were never commercialized due to the relatively short cycle life obtained
with traditional zinc electrode technology. With the advent of newer, reduced solubility zinc
electrode designs, the cycle life of cylindrical cells could be extended in the same manner
that has been demonstrated in prismatic cells. Evercel Corporation has developed a sub-C
cylindrical cell in its laboratory. Additional work was performed on AA and C size cylin-
drical cells.
20
Prototype cells were constructed which delivered up to 1.5 Ah depending on
load and cut-off voltage. The cells were designed using the RAM battery concept in which
the zinc anode was made from a gelled mixture of zinc and potassium-hydroxide (see Sec.
36.3).
Electrochemical Design. The electrochemical design of the cell consists primarily of bal-
ancing the active materials present in the electrodes. This previously has been discussed for
each of the two electrodes separately, the nickel positive electrode and the zinc negative
electrode. When combined in the cell, the two active materials must be present in some ratio
with respect to each other. As with most other alkaline nickel batteries, the nickel-zinc system
is typically positive (nickel electrode) limited. This means that the cell contains more zinc
active material, on an Ampere-hour basis, than nickel active material. This must take into
account the active materials present in the cell in addition to the active material utilization
of each.
Excess zinc is included in the cell for a number of reasons. Since zinc has some finite
solubility in the electrolyte, additional zinc must be manufactured into the cell in order to
compensate for the quantity of zinc that dissolves when electrolyte is added to the cell.
Having excess uncharged zinc oxide also minimizes hydrogen evolution during charging
because the zinc electrode normally doesn’t achieve a full state of charge. In addition, excess
metallic zinc serves to react with oxygen produced at the nickel electrode during overcharge
as discussed below. Adequate electrochemical design allows sealed operation of the cell by
managing the gases produced during charging and discharging.

NICKEL-ZINC BATTERIES 31.15
An interesting effect in sealed cells has been noted by Alekseeva.
21
Detailed studies were
performed on the active material balance in vented and sealed nickel-zinc cells as a function
of cycling. It was discovered that zinc metal tends to accumulate as the cell is cycled, such
that the cell may actually become zinc active material limited at some point in its life. This
may be influenced by a number of factors including current distribution, zinc redistribution
and variable utilization effects in the zinc electrode as well as other factors. Typically the
zinc electrode charges more efficiently than the nickel electrode, which also tends towards
the accumulation of zinc metal in the cell. Also, current utilization in the zinc electrode
becomes more efficient as the metallic zinc content of the electrode increases. A similar
effect has been observed at Evercel Corporation. The zinc electrode typically takes longer
to be fully formed than does the nickel electrode and zinc metal tends to accumulate with
cycling. This is offset in the sealed cell design by direct oxygen recombination which may
consume some of the excess zinc metal in forming zinc oxide. The stoichiometric ratio of
zinc to nickel is typically 3-to-1 in long cycle life applications at 100% DOD. This ratio
may be as low as 2.5-to-1 for other applications as long as the KOH concentration is kept
below 23%.
Gas & Electrolyte Management. Vented nickel-zinc cell designs have an advantage over
sealed designs because gas and electrolyte management issues are minimized due to the
flooded electrolyte and vented nature of the battery. Unfortunately vented designs also have
a number of significant disadvantages. These include the requirement that the electrolyte be
periodically replenished and the hazards associated with venting gases such as fire and ex-
plosion or damaging the equipment in which the battery is installed by the entrainment
of corrosive electrolyte in the gases vented. Therefore most applications demand a sealed,
maintenance-free battery.
A delicate balance must be achieved in cell and battery design in order to maintain sealed
operation while providing optimal cycle life. Insufficient electrolyte leads to premature dry-
out of the electrodes and separators which results in failure of the battery. Excess electrolyte
can introduce gas management issues by reducing the rate of oxygen recombination with
the zinc electrode surface. This can result in the venting of gases through the safety vent
which also leads to premature dry-out. Most sealed cell designs are either starved or semi-
starved with electrolyte. Since cell dry-out is a common long-term failure mode, it is desir-
able to put as much electrolyte as possible into the battery without adversely affecting the
gas recombination characteristics. It is important to have as much excess electrolyte as pos-
sible at the beginning of life. This allows a design margin to accommodate the swelling
normally observed when the battery electrodes and other components are first wetted and
subsequently cycled.
Gas recombination can be managed by several means and a split-negative electrode stack
design has been patented.
22
A hydrophobic gas diffusion membrane is used to separate the
back-to-back zinc half-plates in the split-negative design. This allows oxygen gas to diffuse
into the cell stack and increases the surface area of zinc electrode available to recombine
oxygen. A similar stack arrangement has been used in the nickel-hydrogen battery for the
same purpose.
An auxiliary catalytic gas recombination electrode can also be used to facilitate gas man-
agement. This concept has been patented.
23
The auxiliary gas recombination electrode typ-
ically consists of a catalyst, such as platinum or palladium, supported on a high surface area
carbon. The reaction of hydrogen, produced at the zinc electrode during charge, and oxygen,
produced at the nickel electrode, is exothermic. A heat sink is normally provided to dissipate
the heat produced. The recombination of hydrogen and oxygen on the catalyst is a chemical
reaction, not an electrochemical one.

31.16 CHAPTER THIRTY-ONE
31.4.2 Battery Design and Packaging
Single cells can be assembled into a multicell battery by a variety of conventional means or
in a monobloc type of battery construction. Single cells provide a 1.65 VDC building block
from which any desired battery voltage can be achieved. The monobloc is typically more
cost effective and is usually built as a 12 VDC module containing seven or eight cells,
depending on the application. Multiple monoblocs can be grouped for systems requiring
higher voltages.
Electrical Design. Battery electrical design is dictated by the system interface require-
ments. Battery design can be simple, as in the case of several cells strapped together into a
module, or quite complex such as in a hybrid or electric vehicle. The fundamental require-
ment for the electrical design of the battery is the output voltage required. This determines
the number of cells in the battery and all other design aspects flow from this requirement.
Battery electrical design also includes safety and protective devices and components which
can be designed to protect the battery, personnel and systems in which the battery is installed.
These can include protective components such as thermal cut-off (TCO), fuses, protective
diodes and other safety devices. The electrical interface to the battery is also important and
should include safety and operational features such as polarized and /or keyed battery con-
nectors that impede the improper use of the battery. Also, it should be ensured that the
battery polarity is properly labeled and marked on the battery.
Nickel/zinc cells and batteries should not be charged or discharged in parallel configu-
rations. The battery should be sized to provide the Ampere-hour energy storage requirement
of the application rather than operating smaller batteries in an electrically parallel configu-
ration. Operation in parallel presents current sharing and efficiency imbalances which may
adversely affect Ni /Zn battery performance and cycle life. Multi-voltage ‘‘taps’’ should not
be used on the battery for similar cell balance and safety issues.
Monobloc Design. Multi-cell batteries can be configured either as single cells connected
in series or can be constructed as a monobloc similar to the standard lead-acid automotive-
type battery. In monobloc batteries, the entire multi-cell battery case is molded as a single
component as opposed to each cell being in an individually molded case. The cells can be
interconnected either through the cell wall or the terminals can protrude through the top of
each cell and be connected with standard intercell connectors. Normally if cell terminals are
exposed on the top of the battery, a protective cover is fitted to prevent electrical hazards
from the exposed terminals. In the monobloc design, each cell is individually sealed from
the other cells in order to prevent electrolyte bridging. Relief valves are included for safety
to prevent excessive pressure from building inside the battery case.
Thermal Design. As with most batteries, nickel-zinc performance and cycle life are
strongly dependent on the thermal environment in which the battery is operated. System
level design should minimize the temperature differential (
⌬T) experienced by the cells of
the battery. Heat is reversibly consumed and generated during charge and discharge, respec-
tively, as a result of endothermic and exothermic chemical reactions. However, heat is also
generated irreversibly during both charge and discharge as a result of I
2
R losses. Thus, the
net thermal result of discharge is heat evolution, but the net thermal result of charge is
variable. The bulk of the charging process is slightly endothermic but this is compensated
for by I
2
R heating, resulting in a net rise in battery temperature. Near the end of charge, gas
evolution becomes significant and this can generate significant quantities of heat. This is one
reason that overcharge should be avoided. The heat produced near the end of charge may
be carried over into discharge causing a higher than normal temperature increase during
discharge. Whenever possible, heat should be removed from the battery via convection.
Severe overcharge or over-discharge can result in runaway exothermic reactions, and should
be avoided.
NICKEL-ZINC BATTERIES 31.17
31.5 PERFORMANCE CHARACTERISTICS
31.5.1 General Discharge Characteristics
The general characteristics of the nickel-zinc system are presented in Table 31.2. Nickel-
zinc batteries are capable of delivering up to about 50 to 60 Wh /kg and 80 to 120 Wh /L
depending on the specific design characteristics. The batteries have good high rate and high
power discharge capability and very good charge retention characteristics. As with all bat-
teries, cycle life is strongly dependent on the application, environmental conditions, the
depth-of-discharge and the charge/discharge regimen experienced by the battery during use.
In controlled laboratory testing, nickel-zinc batteries yield about 500 cycles when operated
at 100% depth-of-discharge and more than 10,000 cycles at depths as low as 10% DOD.
Nickel-zinc’s specific energy of 60 Wh/ kg is intermediate between nickel-cadmium and
nickel-metal hydride.
TABLE 31.2 Characteristics of Nickel-Zinc Batteries
Parameter Nickel-zinc
Cathode electrochemistry Ni(OH)
2
/NiOOH
Anode electrochemistry ZnO / Zn
Theoretical specific energy (Watt-hours per kilogram) 334
Electrolyte (% potassium hydroxide) 20 to 25
Nominal cell voltage (Volts) 1.65
Operating temperature range (
⬚C) ⫺20 to 50
Specific energy (Watt-hours per kilogram) 50–60
Energy density (Watt-hours per liter) 80–120
Specific power (Watts per kilogram) 280
Power density (Watts per liter) 420
Charge retention (percent loss per month @ 25
⬚C) ⬍20
Cycle life (cycles @ 100% DOD)
⬃500
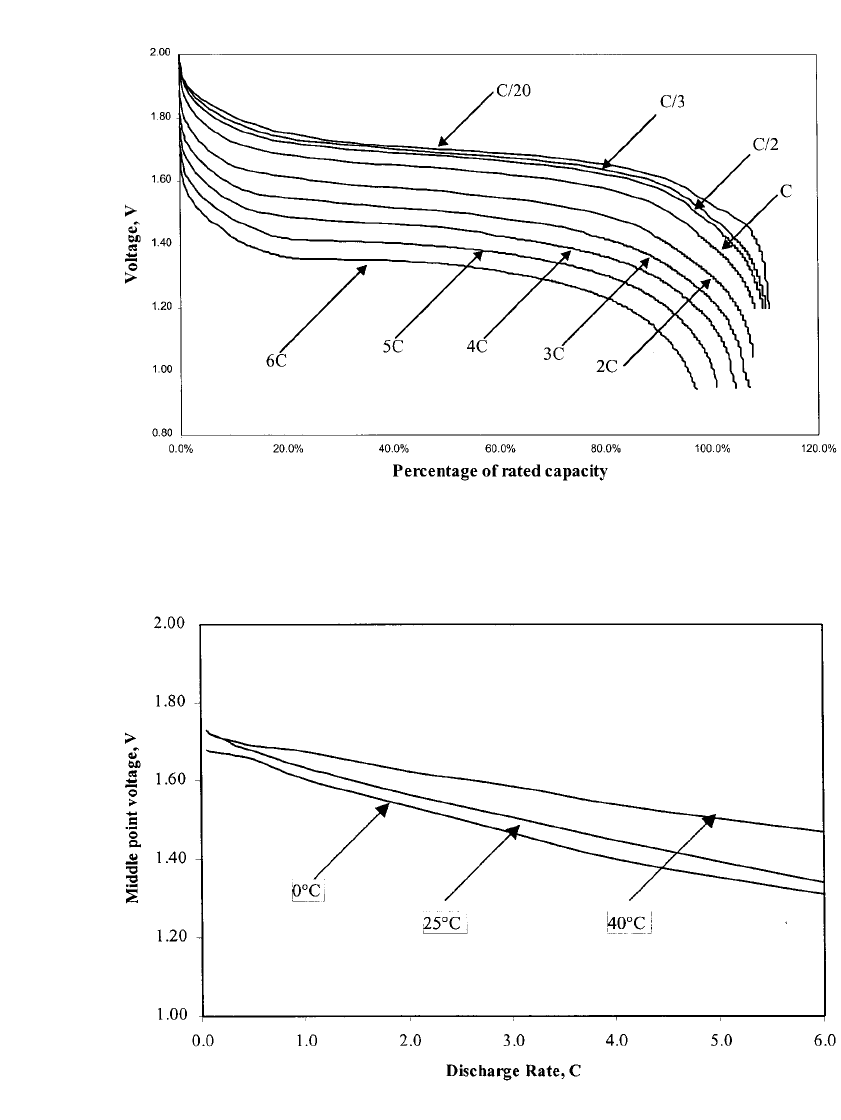
Rate Capability. Figure 31.8 shows a series of discharges on a fresh standard design 30
Ah nickel-zinc battery. The cell was discharged at nine different rates ranging from C /20
up to 6C. The capacity on the initial low-rate discharge is greater than 35 Ah. (This battery
is a prismatic design, incorporating a lightweight plastic cell case and resealable pressure
vent.) The battery was discharged at ambient temperature (about 23
⬚C) with no active cool-
ing. The battery performed extremely well up to the C rate and only started to drop signif-
icantly in loaded mid-point voltage above the 2C rate. Data such as this can be used to
estimate the load voltage of a battery at a given current. System level designers should
consult the manufacturer’s data for the specific design that will be used.
Figure 31.9 shows the typical mid-point discharge voltage under load, as a function of
discharge rate and at three different temperatures. This data allows an estimate of the battery
discharge voltage at different rates and temperatures. Below the 2C rate, the battery voltage
is nearly independent of temperature over the range of 0
⬚Cto40⬚C. As the discharge current
is increased, temperature has an increased effect on battery voltage, primarily due to the
increasing impedance (and polarization) of the battery at colder temperatures. At high rates
(6C) and very cold temperatures (0
⬚C), the battery voltage is 1.32 V per cell. At 40⬚C and
at the 6C rate the average battery voltage is about 1.50 V. This difference is entirely due to
the effect of operating temperature. The data also show that the effect of temperature is not
linear. For example at the 6C rate, decreasing the temperature from 40
⬚Cto25⬚C produces
31.18 CHAPTER THIRTY-ONE
FIGURE 31.8 Multi-rate discharge curves for 1.65 V nickel-zinc battery. Battery discharged at room tem-
perature. C / 20, C /3, C / 2 and C rate: battery was discharged to 1.2 V cutoff; 2C rate: battery was discharged
to 1.05 V; 3C, 4C, 5C and 6C rate: battery was discharged to 0.95 V. (Courtesy of Evercel Corp.)
FIGURE 31.9 Typical nickel-zinc battery MPDV as a function of discharge rate and temperature. (Courtesy
of Evercel Corp.)
NICKEL-ZINC BATTERIES 31.19
a relatively large decrease in battery voltage (approximately 160 mV) while decreasing from
25
⬚Cto0⬚C only reduces the battery voltage 40 mV. This nonlinearity is due to a variety of
effects including the nonlinearity of electrolyte impedance, electrode kinetics and polarization
as a function of temperature.
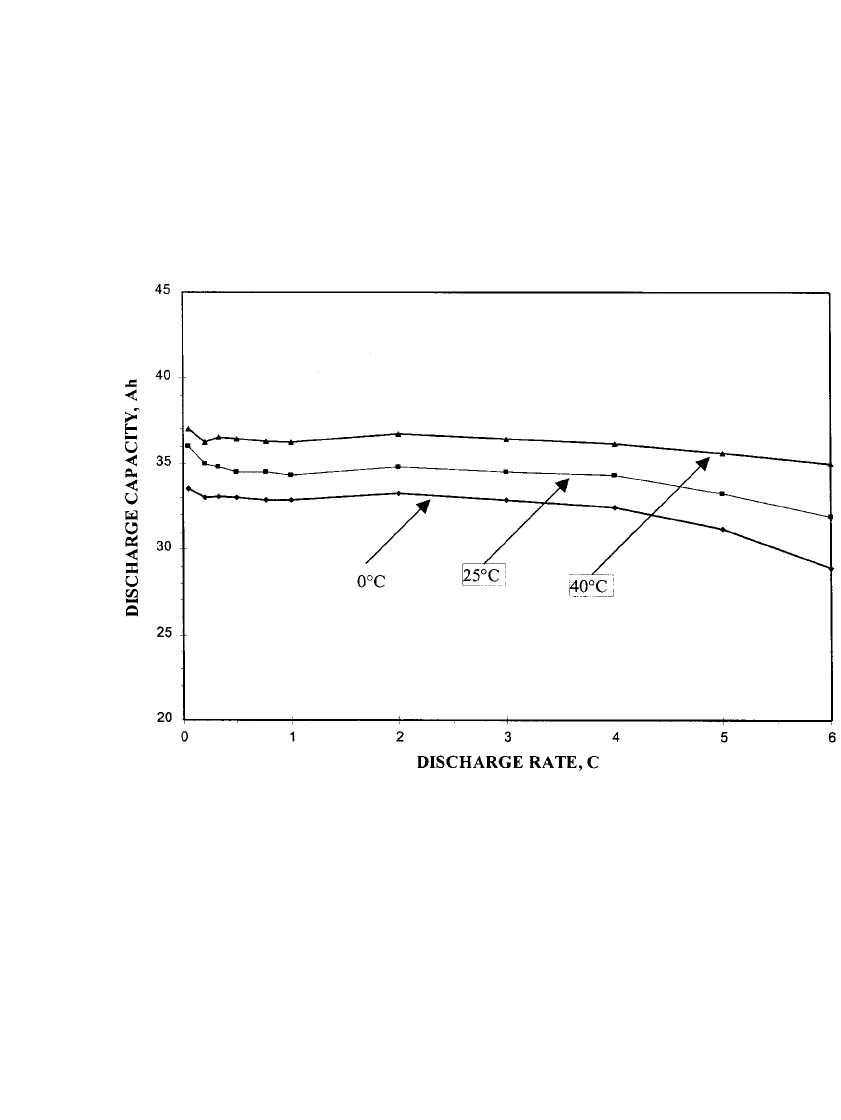
Figure 31.10 indicates the capacity delivered by the nickel-zinc battery as a function of
discharge rate and temperature. As the discharge current is increased, the battery output
voltage decreases due to impedance and polarization losses. The data show that the capacity
is nearly independent of discharge rate, up to the 6C rate, but that the capacity is somewhat
dependent on temperature. The discharge capacity drops about 12% when the temperature
decreases from 40
⬚Cto0⬚C.
FIGURE 31.10 Discharge capacity of a nominal 30 Ah nickel-zinc battery as a function of discharge rate
and temperature. (Courtesy of Evercel Corp.)
Figure 31.11 shows the specific energy (Watt-hours per kilogram) obtained from a typical
nickel-zinc battery which is a direct function of the battery discharge capacity. Therefore,
the specific energy curve versus discharge rate behaves similar to the discharge capacity
versus discharge rate data shown above. Specific energy is highly dependent on specific
battery design and can vary within the nickel-zinc chemistry depending on the design used.
Battery design can be optimized for energy storage capacity, rate capability, power density,
cycle life or other specific performance factors. These data show a standard type of battery
which has not been specifically designed for energy, rate or power.
