Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.


29.2 CHAPTER TWENTY-NINE
TABLE 29.1 Major Advantages and Disadvantages of Sealed Nickel-Metal
Hydride Batteries
Advantages Disadvantages
Higher capacity than nickel-cadmium
batteries
Sealed construction, no maintenance
required
Cadmium-free, minimal environmental
problems
Rapid recharge capability
Long cycle life
Long shelf life in any state of charge
High-rate performance not as good
as with nickel-cadmium
batteries
Poor charge retention
Moderate memory effect
Higher cost negative electrodes
29.2 CHEMISTRY
The active metal of the positive electrode of the nickel-metal hydride battery, in the charged
state, is nickel oxyhydroxide. This is the same as the positive electrode in the nickel-cadmium
battery.
The negative active material, in the charged state, is hydrogen in the form of a metal
hydride. This metal alloy is capable of undergoing a reversible hydrogen absorbing-desorbing
reaction as the battery is charged and discharged.
An aqueous solution of potassium hydroxide is the major component of the electrolyte.
A minimum amount of electrolyte is used in this sealed cell design, with most of the liquid
absorbed by the separator and the electrodes. This ‘‘starved-electrolyte’’ design, similar to
the one in sealed nickel-cadmium batteries, facilitates the diffusion of oxygen to the negative
electrode at the end of the charge for the oxygen-recombination reaction. This is essentially
a dry-cell construction, and the cell is capable of operating in any position.
During discharge, the nickel oxyhydroxide is reduced to nickel hydroxide.
⫺
NiOOH ⫹ HO⫹ e → Ni(OH) ⫹ OH E ⴖ ⫽ 0.52 V
22
and the metal hydride MH is oxidized to the metal alloy M.
⫺
MH ⫺ OH → M ⫹ HO⫹ eEⴖ ⫽ 0.83 V
2
The overall reaction on discharge is
MH
⫹ NiOOH → M ⫺ Ni(OH) Eⴖ ⫽ 1.35 V
2
The process is reversed during charge.
The sealed nickel-metal hydride cell uses an oxygen-recombination mechanism to prevent
the buildup of pressure that may result from the generation of gases toward the end of the
charge and overcharge. It is based on the use of a negative electrode (the metal hydride
electrode) that has a higher effective capacity than the positive or nickel oxyhydroxide elec-
trode. This is shown schematically in Fig. 29.1. During charge the positive electrode reaches
full charge before the negative and begins to evolve oxygen.
⫺
1
–
2OH → HO⫺ O ⫹ 2e
222
The oxygen gas diffuses through the separator to the negative electrode, the diffusion
facilitated by the starved-electrolyte design and the selection of an appropriate separator
system.

PORTABLE SEALED NICKEL-METAL HYDRIDE BATTERIES 29.3
NiOOH/Ni(OH)
2
FIGURE 29.1 Schematic representation of electrodes of sealed
nickel-metal hydride cell, divided into useful capacity, charge re-
serve, and discharge reserve. (Courtesy of Duracell, Inc.)
At the negative electrode the oxygen reacts with and oxidizes or discharges the hydrogen
electrode to produce water, and the pressure does not build up,
4MH
⫹ O → 4M ⫹ 2H O
22
Furthermore, the negative electrode will not become fully charged, which prevents the gen-
eration of hydrogen.
The charge current, however, must be controlled at the end of the charge and during
overcharge to limit the generation of oxygen to below the rate of recombination to prevent
the buildup of gases and pressure.
The nickel-metal hydride cell also is designed with a discharge reserve in the negative
electrode to minimize gassing and degradation of the cell in the event of overdischarge (see
Sec. 29.4.6). Overall, as shown in Fig. 29.1, the negative electrode has excess capacity
compared to the positive to handle both overcharge and overdischarge. The useful capacity
of the battery is thus determined by the positive electrode.
A key component of the sealed nickel-metal hydride cell is the hydrogen storage metal
alloy. The composition of the alloy is formulated to obtain a material that is stable over a
large number of charge-discharge cycles. Other important properties of the alloy include:
1. Good hydrogen storage to achieve a high-energy density and battery capacity
2. Thermodynamic properties suitable for reversible absorption/desorption
3. Low hydrogen equilibrium pressure
4. High electrochemical reactivity
5. Favorable kinetic properties for high-rate performance
6. High oxidation resistance
7. Stability, with repeated charge/ discharge cycles, in alkaline electrolyte
Two types of metallic alloys are generally used. These are the rare-earth (Misch metal)
alloys based on lanthanum nickel (LaNi
5
), known as the AB
5
class of alloys and alloys
consisting of titanium and zirconium, known as the AB
2
class of alloys. In both cases, some
of the base metals are replaced by other metals to improve performance characteristics.
In the case of the AB
5
class of alloys, substitutions have improved the alloy as follows:
1,2
1. Ce, Nd, Pr, Gd and Y as a mixed or Misch metal (a naturally occurring mixture of rare-
earth metals) are low cost substitutes for La.
2. Ni and Co are major constituents and suppress corrosion resulting in longer cycle life.
3. Al, Ti, Zr and Si are minor constituents and increase corrosion resistance resulting in
longer cycle life.
29.4 CHAPTER TWENTY-NINE
In the case of the AB
2
class of alloys, substitutions have improved the alloy as follows:
3
1. V, Ti, and Zr improve hydrogen storage.
2. Ni and Cr are major constituents which suppress corrosion and provide longer cycle life.
3. Al, Ti, Zr and Si are minor constituents which also suppress corrosion and provide longer
cycle life.
The AB
2
alloy has a higher capacity per unit weight and volume over a moderate oper-
ating range of temperature and discharge rate than the AB
5
alloy as shown in Table 29.2.
However, over a very broad and demanding operating range of temperature and discharge
rate, the lower capacity AB
5
alloys are favored. AB
5
alloys have advantages and better
performance
4
under a number of conditions, including high discharge rates, high rate charge
acceptance, low and high discharge temperatures and superior high temperature stability.
4
To
compensate for some of these advantages of the batteries made with AB
5
alloy, batteries
made with the AB
2
alloy have been constructed with larger conductors, more surface area
and electrolyte, leaving less room for the active material. As a result, the overall energy
density of batteries made with AB
2
alloy may actually be the same or even lower than those
made with AB
5
alloy. Therefore, the use of the AB
5
alloy dominates in portable sealed nickel-
metal hydride batteries because of their overall advantages. (See discussion of metal hydride
alloys in Chap. 30.)
TABLE 29.2 Comparison of Metal Hydride
Alloys—Electrochemical Equivalents
Alloy
Gravimetric
Ah/kg
Volumetric
Ah/L
AB
5
AB
2
270–290
360–400
2200–2400
2500–2800
29.3 CONSTRUCTION
Sealed nickel-metal hydride cells and batteries are constructed in cylindrical, button, and
prismatic configurations, similar to those used for the sealed nickel-cadmium battery.
The electrodes are designed with highly porous structures having a large surface area to
provide a low internal resistance and a capability for high-rate performance. The positive
electrode in the cylindrical nickel-metal hydride cell is a highly porous sintered, or felt nickel
substrate into which the nickel compounds are impregnated or pasted and converted into the
active material by electrodeposition. Felts and foams have generally replaced sintered plaque
electrodes. Expanded metals and perforated sheets are cheaper, but they have poor high rate
capability. Sintered structures are much more expensive. The negative electrode, similarly,
is a highly porous structure using a perforated nickel foil or grid onto which the plastic-
bonded active hydrogen storage alloy is coated. The electrodes are separated with a synthetic
nonwoven material, which serves as an insulator between the two electrodes and as a medium
for absorbing the electrolyte.
29.3.1 Cylindrical Configuration
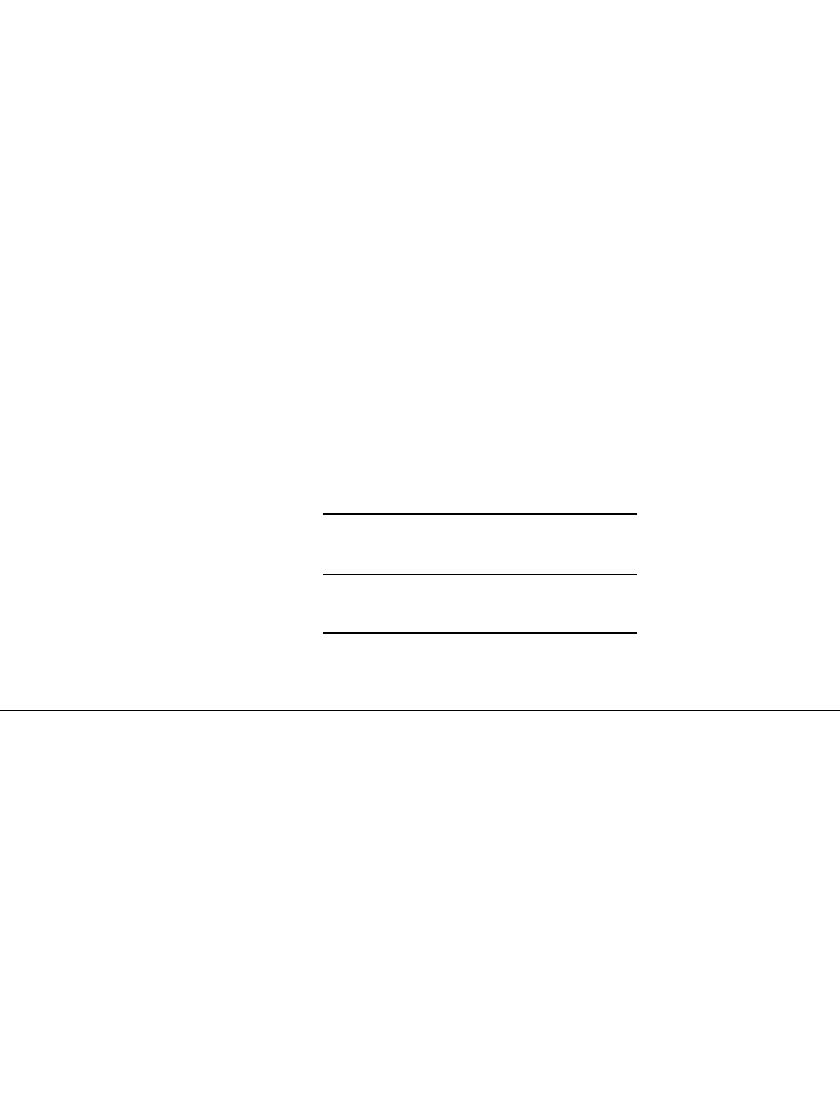
The assembly of the cylindrical unit is shown in Fig. 29.2a. The electrodes are spirally
wound and the assembly is inserted into a cylindrical nickel-plated steel can. The electrolyte
is added and contained within the pores of the electrodes and separator.
PORTABLE SEALED NICKEL-METAL HYDRIDE BATTERIES 29.5
FIGURE 29.2a Construction of a sealed cylindrical
nickel-metal hydride battery. (Courtesy of Duracell, Inc.)
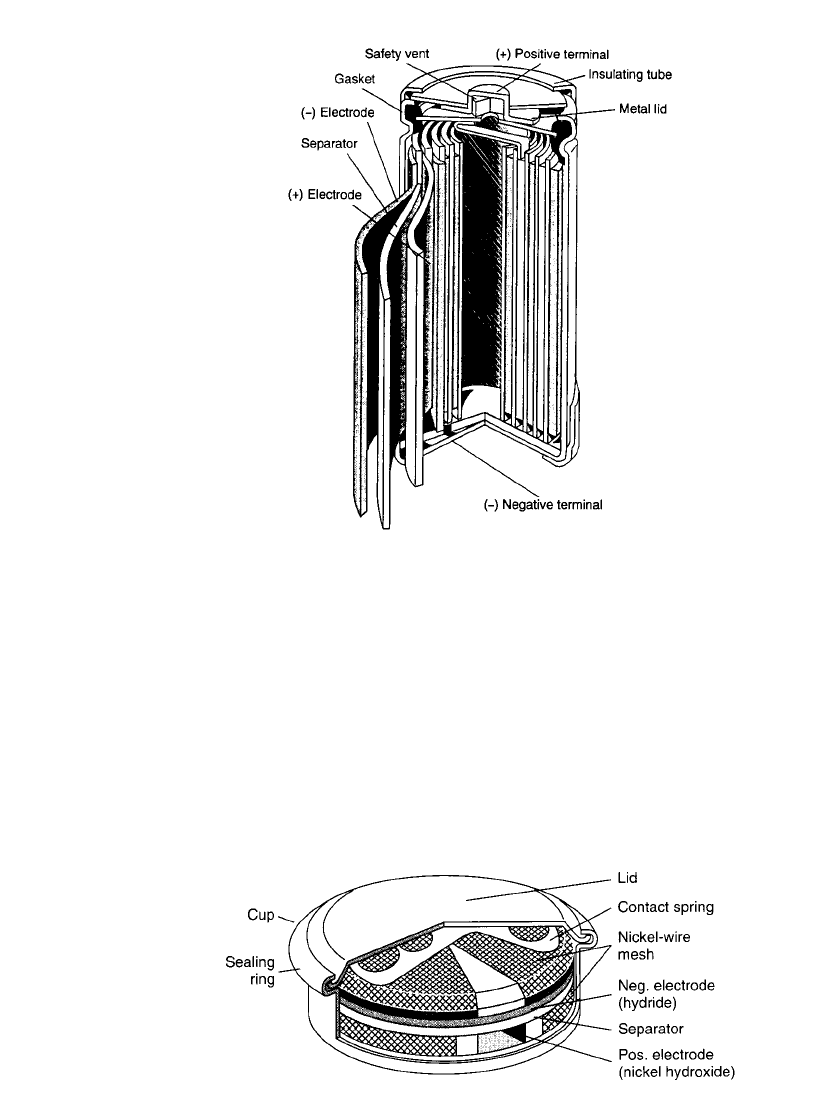
FIGURE 29.2b Construction of a sealed-nickel metal hydride button cell.
(Courtesy of Varta Batteries AG.)
The cell is sealed by crimping the top assembly to the can. The top assembly consists of
a lid, which includes a resealable safety vent, a terminal cap, and a plastic gasket. The can
serves as the negative terminal and the lid as the positive terminal, both insulated from each
other by the gasket. The vent provides additional safety by releasing any excessive pressure
that may build up if the battery is subjected to abuse.
29.3.2 Button Configuration
The button configuration is illustrated in Fig. 29.2b. It is similar in construction to the nickel-
cadmium button cell, except that the cadmium is replaced by the hydrogen storage alloy.
29.6 CHAPTER TWENTY-NINE
29.3.3 Prismatic Configuration
The thin prismatic batteries are designed to meet the needs of compact equipment. The
rectangular shape permits more efficient battery assembly, eliminating the voids that occur
with the assembly of cylindrical cells. The volumetric energy density of the battery can be
increased by a factor of about 20%. The prismatic cells also offer more flexibility in the
design of batteries, as the battery footprint is not controlled by the diameter of the cylindrical
cell.
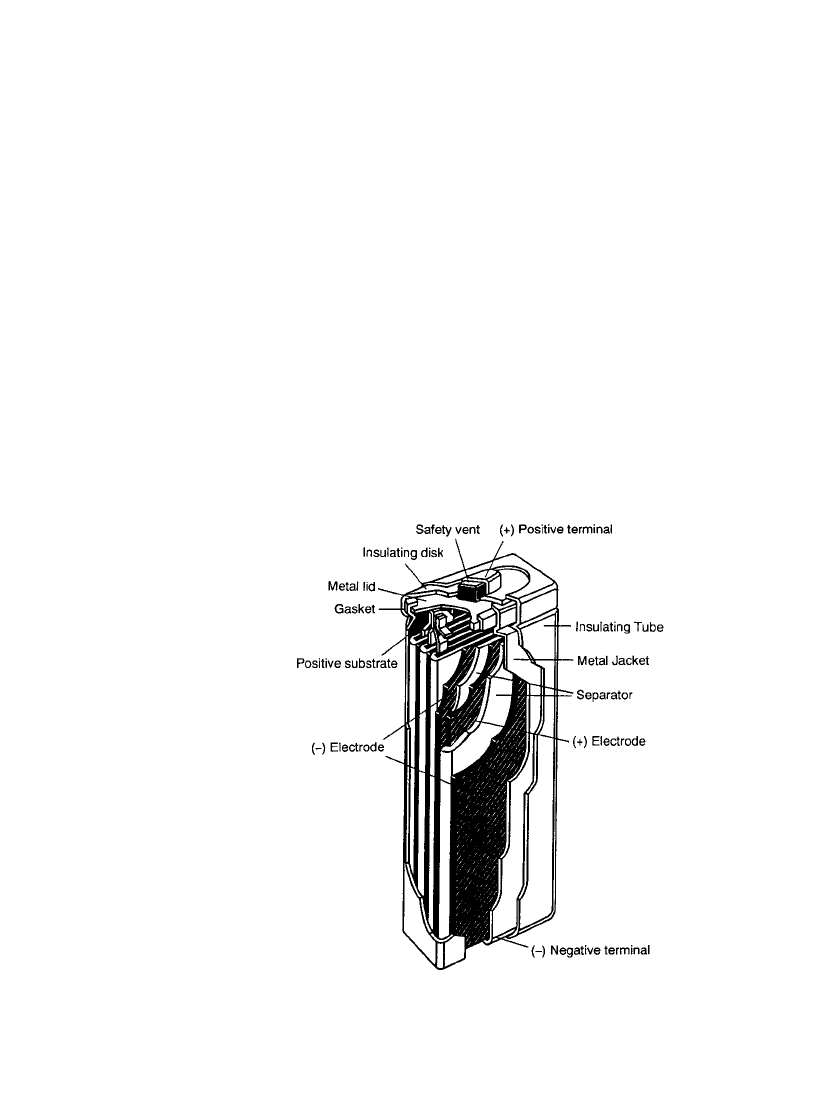
Figure 29.2c shows the structure of the prismatic battery. The electrodes are manufactured
in a similar manner as the electrodes for the cylindrical cell, except that the finished elec-
trodes are flat and rectangular in shape. The flat electrodes are then assembled, with the
positive and negative electrodes interspaced by separator sheets, and welded to the cover
plate. The assembly is then placed in the nickel-plated steel can and the electrolyte is added.
The cell is sealed by crimping the top assembly to the can. The top assembly is a lid which
incorporates a resealable safety vent, a terminal cap, and a plastic gasket, similar to the one
used on the cylindrical cell. An insulating heat-shrink tube is placed over the metal can
(jacket). The bottom of the metal can serves as the negative terminal and the top lid as the
positive terminal. The gasket insulates the terminals from each other.
FIGURE 29.2c Construction of a sealed prismatic
nickel-metal hydride battery.
PORTABLE SEALED NICKEL-METAL HYDRIDE BATTERIES 29.7
29.3.4 9-Volt Multicell Battery
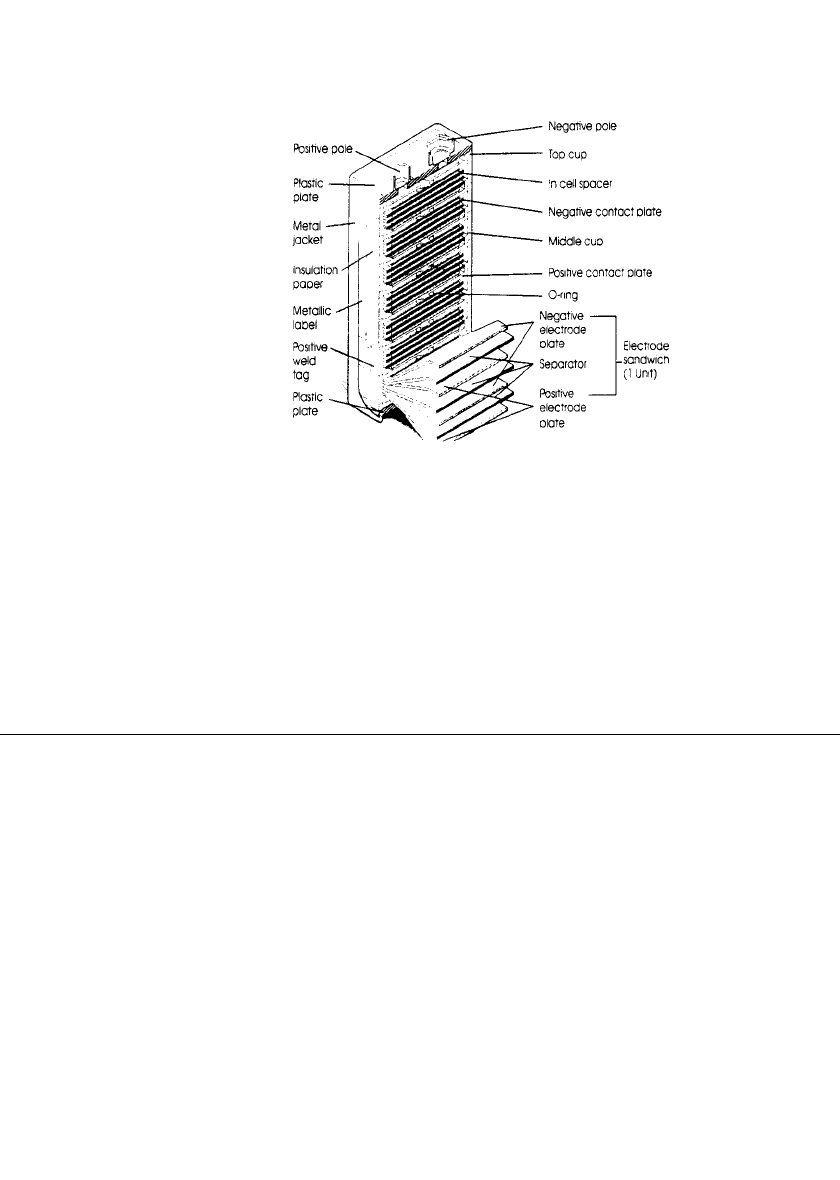
The construction of the 9-volt multicell battery is shown in Fig. 29.2d.
FIGURE 29.2d Construction of a sealed 9-volt nickel-
metal hydride battery.
29.3.5 Larger Prismatic Batteries
Larger prismatic cells with flat plates are being developed for hybrid and all electric vehicles
and other applications requiring larger batteries in sizes up to about 250 Ah. These cells use
the lightweight substrates, such as foams and fibers, for the electrodes in conjunction with
mechanical loading of the active materials, lightweight containers, and so on, to achieve the
higher specific energy required for these applications. These larger nickel-metal hydride cells
and batteries are covered in Chap. 30.
29.4 DISCHARGE CHARACTERISTICS
29.4.1 General Characteristics
The discharge characteristics of the sealed nickel-metal hydride batteries are very similar to
those of the sealed nickel-cadmium battery. Several comparisons are illustrated in Chap. 22.
The open-circuit voltage of the batteries of both systems ranges from 1.25 to 1.35 V, the
nominal voltage is 1.2 V, and the typical end voltage is 1.0 V.
(Note: All batteries were recharged between 20
⬚C and 25⬚C under the conditions shown,
unless specified otherwise.)
29.4.2 Discharge Characteristics
Cylindrical Batteries. Typical discharge curves for the cylindrical sealed nickel-metal hy-
dride battery under various constant-current loads and temperatures are shown in Fig. 29.3.
(The data are based on the rated performance at 20
⬚C at the 0.2C discharge rate to 1.0 V.)
A flat discharge profile is characteristic. The discharge voltage, as expected, is dependent on
the discharge current and discharge temperature. Typically, the higher the current and the
lower the temperature, the lower the operating voltage. This is due to the higher IR drop
with increasing current and the increasing resistance at the lower temperatures. However,
because of the relatively low resistance of the nickel-metal hydride battery (as well as the
nickel-cadmium battery), this drop in voltage is less than experienced with other types of
portable primary and rechargeable batteries.
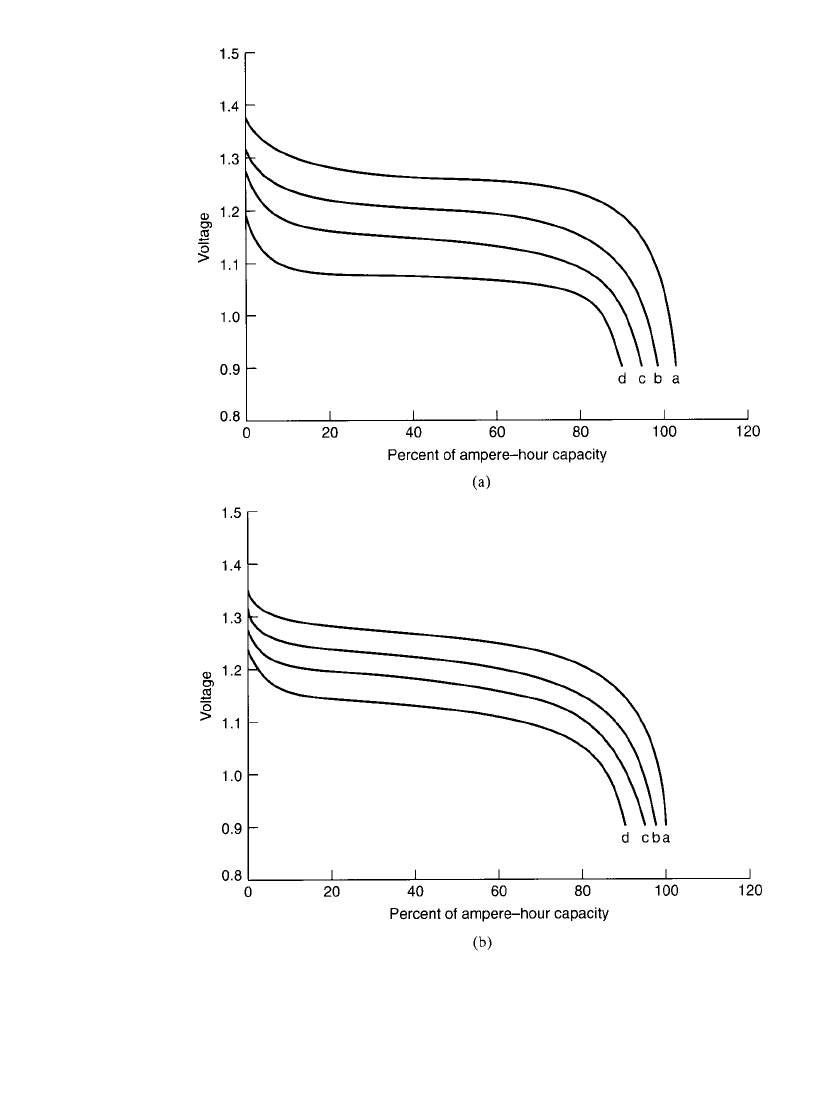
29.8 CHAPTER TWENTY-NINE
FIGURE 29.3 Discharge performance of sealed cylindrical nickel-metal hydride bat-
teries at (a)20⬚C; (b)45⬚C. Curves a—0.2C rate; curves b—1C rate; curves c—2C
rate; curves d—3C rate.
PORTABLE SEALED NICKEL-METAL HYDRIDE BATTERIES 29.9
FIGURE 29.3 (c)0⬚C; (d ) ⫺20⬚C. Curves a—0.2C rate; curves b—1C rate; curves
c—2C rate; curves d—3C rate (Continued ).
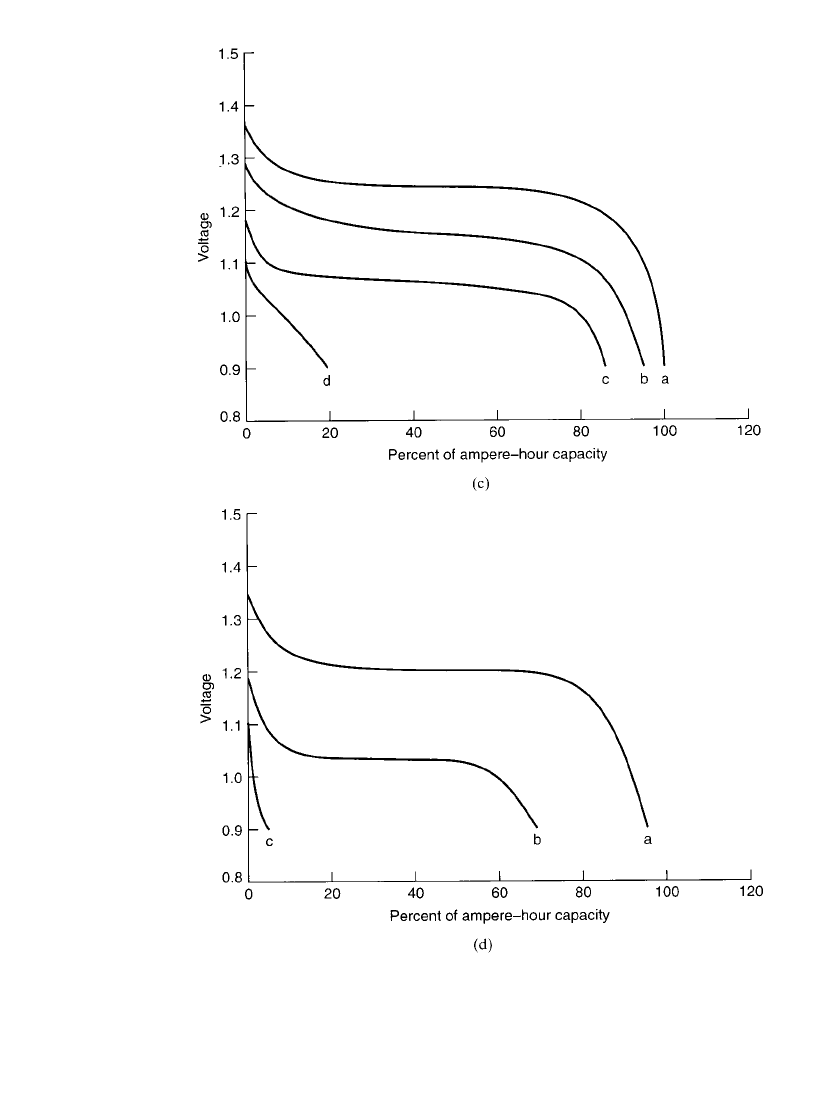
29.10 CHAPTER TWENTY-NINE
Button Batteries. Typical discharge curves for button-type sealed nickel-metal hydride bat-
teries at room and other temperatures are shown in Figs. 29.4a and 29.4b.
FIGURE 29.4 Discharge characteristics of nickel-metal hydride button batter-
ies (a) Discharge at 20⬚C. (b) Discharge at 0.2 C rate. (Courtesy of GP Batteries,
Inc.)
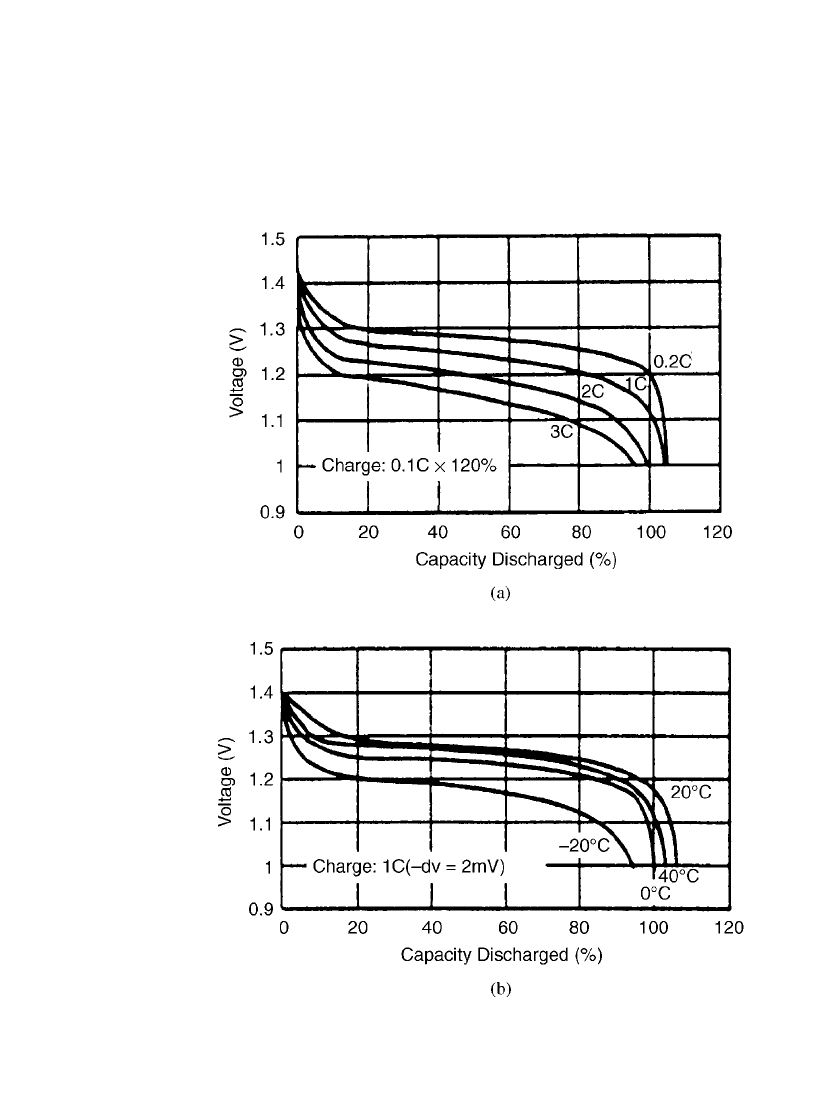
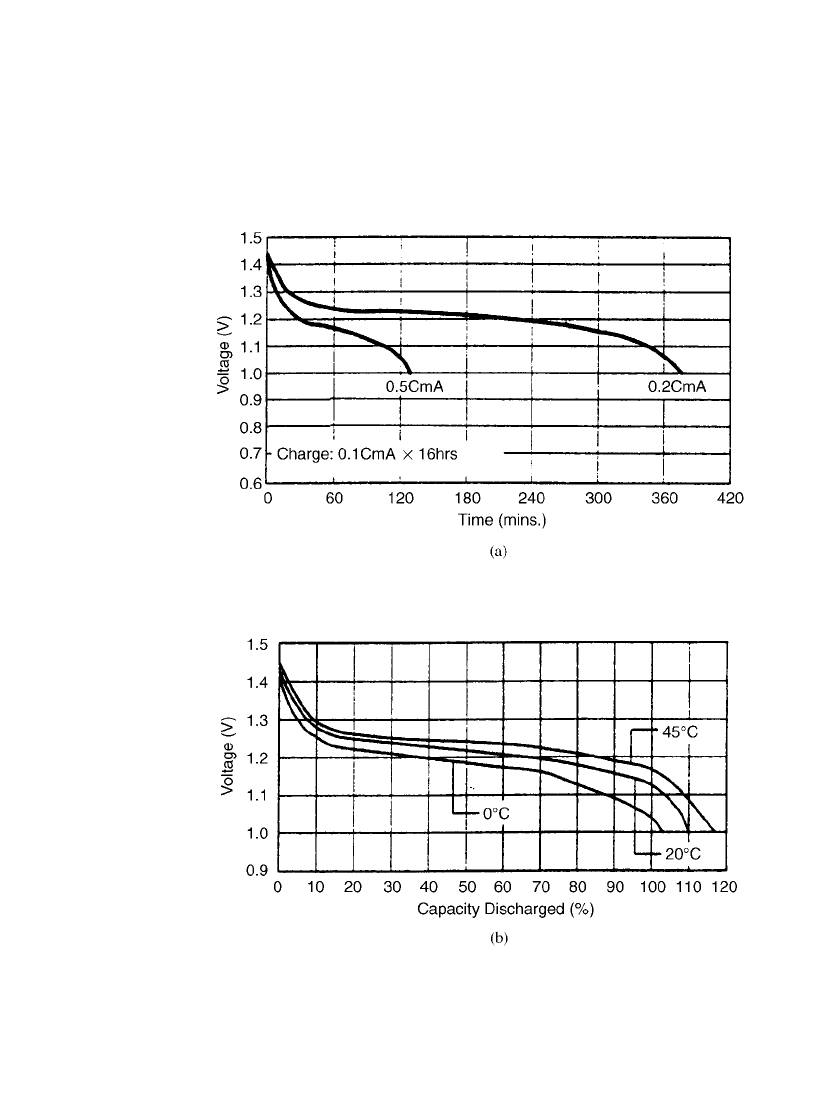
PORTABLE SEALED NICKEL-METAL HYDRIDE BATTERIES 29.11
Prismatic Batteries. Typical discharge curves for the prismatic sealed nickel-metal hydride
batteries at room and other temperatures are shown in Figs. 29.5a and 29.5b.
FIGURE 29.5 Discharge characteristics of nickel-metal hydride prismatic bat-
teries (a) Discharge at 20⬚C. (b) Discharge at 0.2 C rate. (Courtesy of GP Bat-
teries, Inc.)
