Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

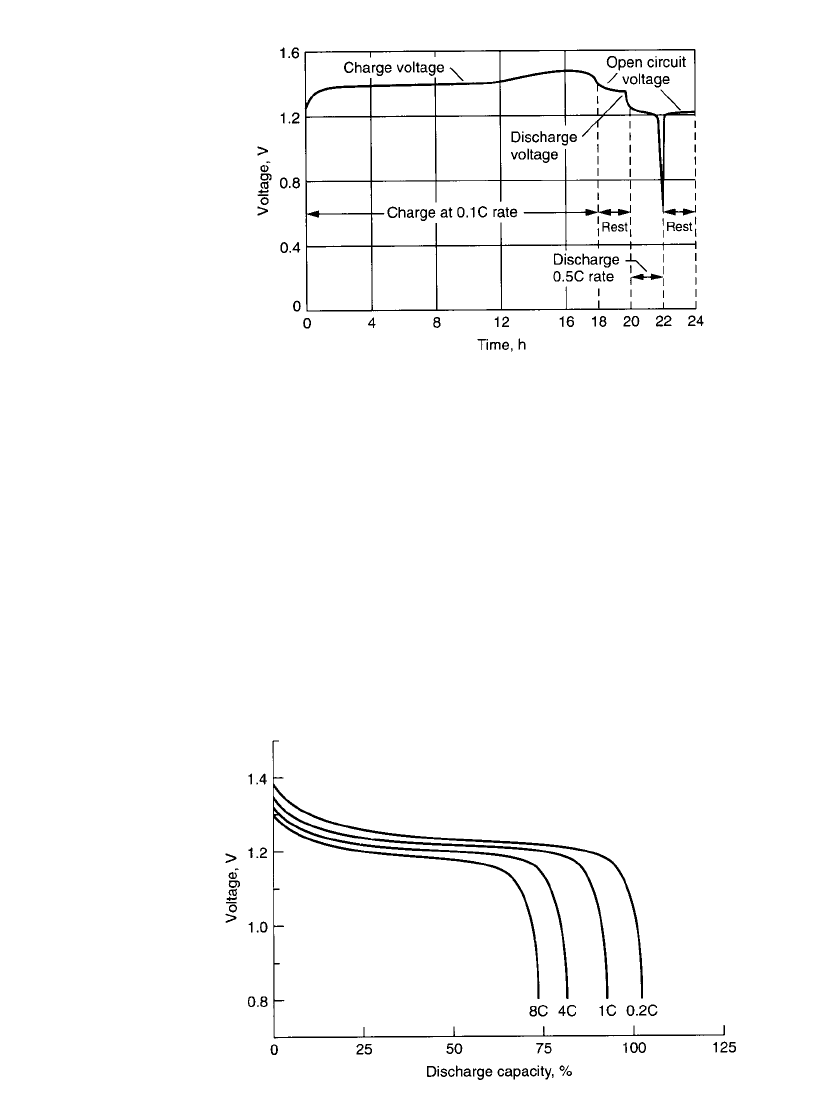
PORTABLE SEALED NICKEL-CADMIUM BATTERIES 28.7
FIGURE 28.6 Voltage profile of nickel-cadmium battery in a typ-
ical charge/ discharge cycle.
28.4.2 Discharge Characteristics
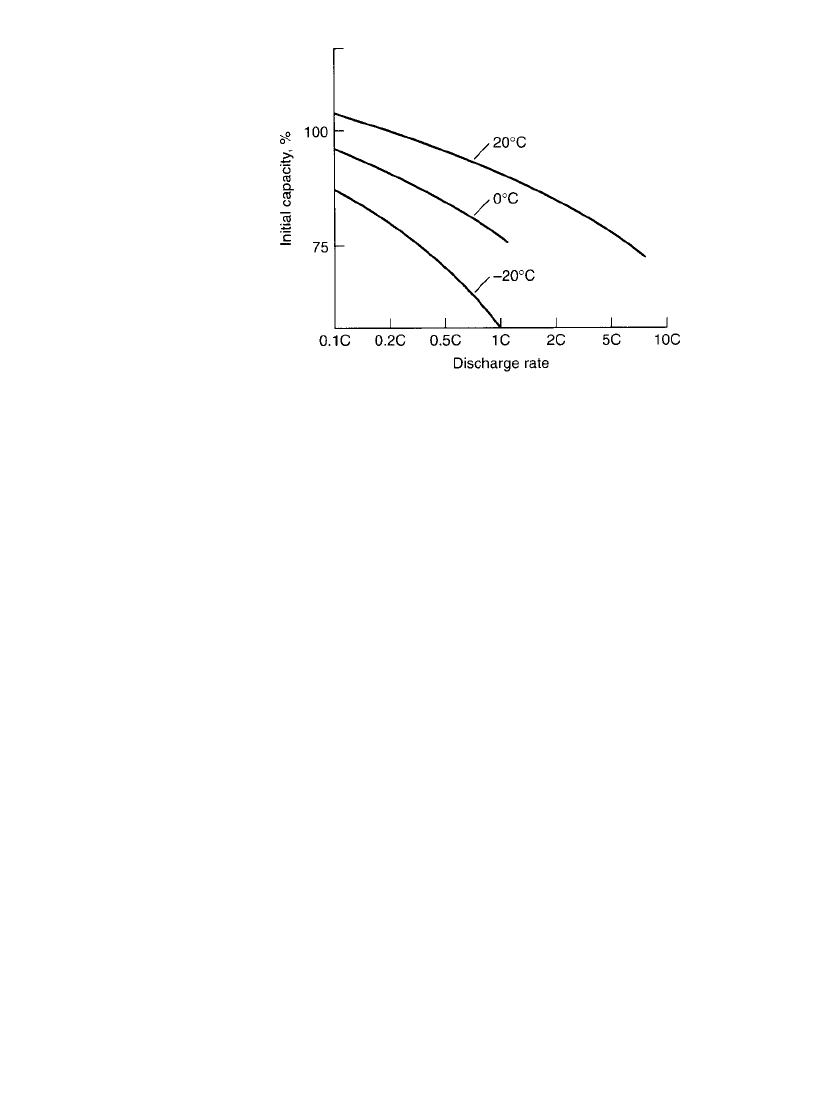
Typical discharge curves for the cylindrical battery at 20⬚C at various discharge loads are
shown in Fig. 28.7. The flat voltage profile, after the initial voltage drop, is characteristic.
The capacity that can be obtained from a battery is dependent on the rate of discharge,
the voltage at which discharge is terminated, the discharge temperature, and the previous
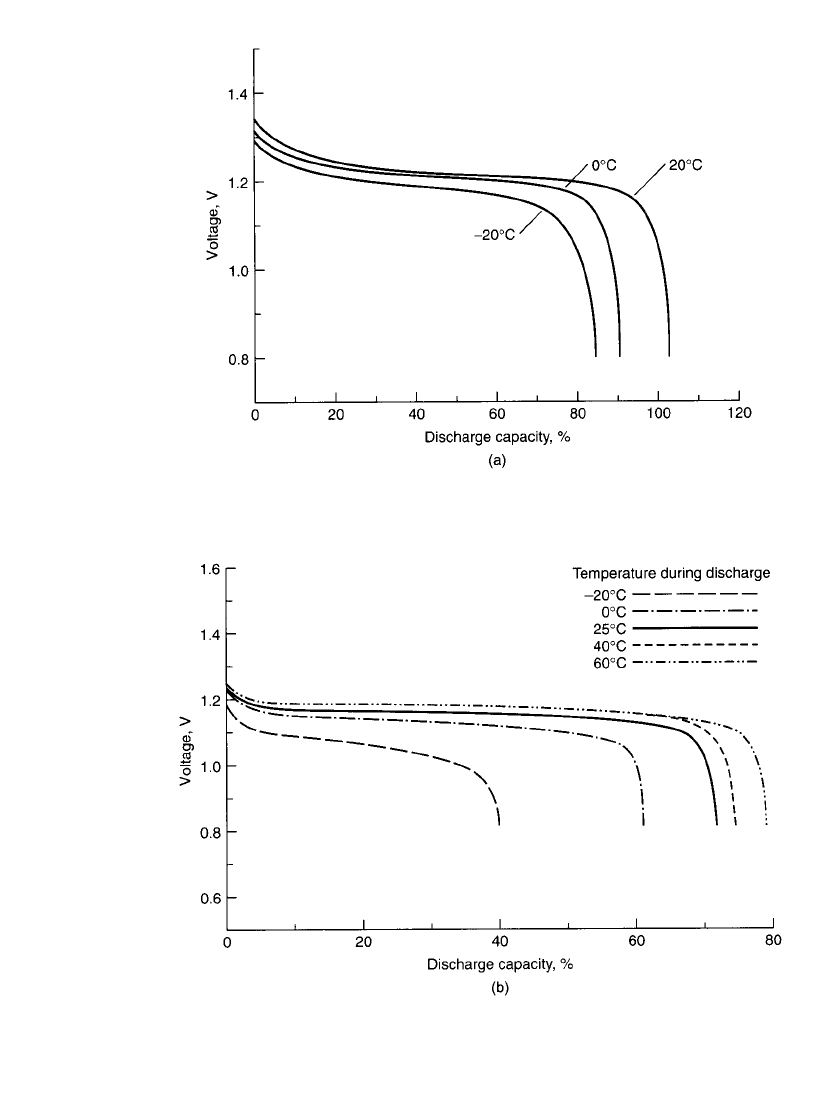
history of the battery. Figure 28.8 shows the percentage of rated capacity delivered during
discharges at various rates and temperatures. The midpoint voltage during discharge de-
creases as the discharge rate increases (see Fig. 28.11). If the battery were allowed to dis-
charge to a lower cutoff voltage, a greater percentage of the C/ 5 rate capacity will be
obtained. However, batteries or batteries should not be discharged to too low a cutoff voltage
as the battery may be damaged (see Sec. 28.4.6).
FIGURE 28.7 Constant-current discharge curves for sealed nickel-cadmium
batteries at 20⬚C, charge 0.1C,16h.(Courtesy of Sanyo Energy Corp.)
28.8 CHAPTER TWENTY-EIGHT
FIGURE 28.8 Percent of C /5-rate capacity vs. discharge
rate to 1.0-V cutoff for typical sealed nickel-cadmium battery.
28.4.3 Effect of Temperature
The sealed nickel-cadmium battery is capable of good performance over a wide temperature
range. Best operation is between
⫺20 and ⫹30⬚C, although usable performance can be
obtained beyond this range. The low-temperature performance, particularly at high rates, is
generally better than that of the lead-acid battery but usually inferior to that of the vented
sintered-plate battery. The reduction in performance at low temperatures is due to an increase
in the internal resistance. At high temperatures, the loss can be due to a depressed operating
voltage or to self-discharge.
Figure 28.9 shows some typical discharge curves of the sealed battery at various tem-
peratures at the 0.2C and 8C rates; Fig. 28.10 shows typical discharge curves at
⫺20⬚C. A
flat discharge profile is still characteristic, but at a lower operating voltage than at room
temperature. Figure 28.11 shows the effect of temperature on the midpoint voltage. Ambient
temperatures significantly above or below 20 to 25
⬚C have a depressing effect on the average
discharge voltage.
The effect of temperature and discharge rate on the capacity of a sealed nickel-cadmium
battery is shown in Fig. 28.12. These data are typical of standard batteries. Manufacturers
should be contacted to obtain performance characteristics of specific batteries.
PORTABLE SEALED NICKEL-CADMIUM BATTERIES 28.9
FIGURE 28.9 Constant-current discharge curves of sealed nickel-cadmium batteries at var-
ious temperatures. (a) 0.2C discharge rate. (b)8C discharge rate.
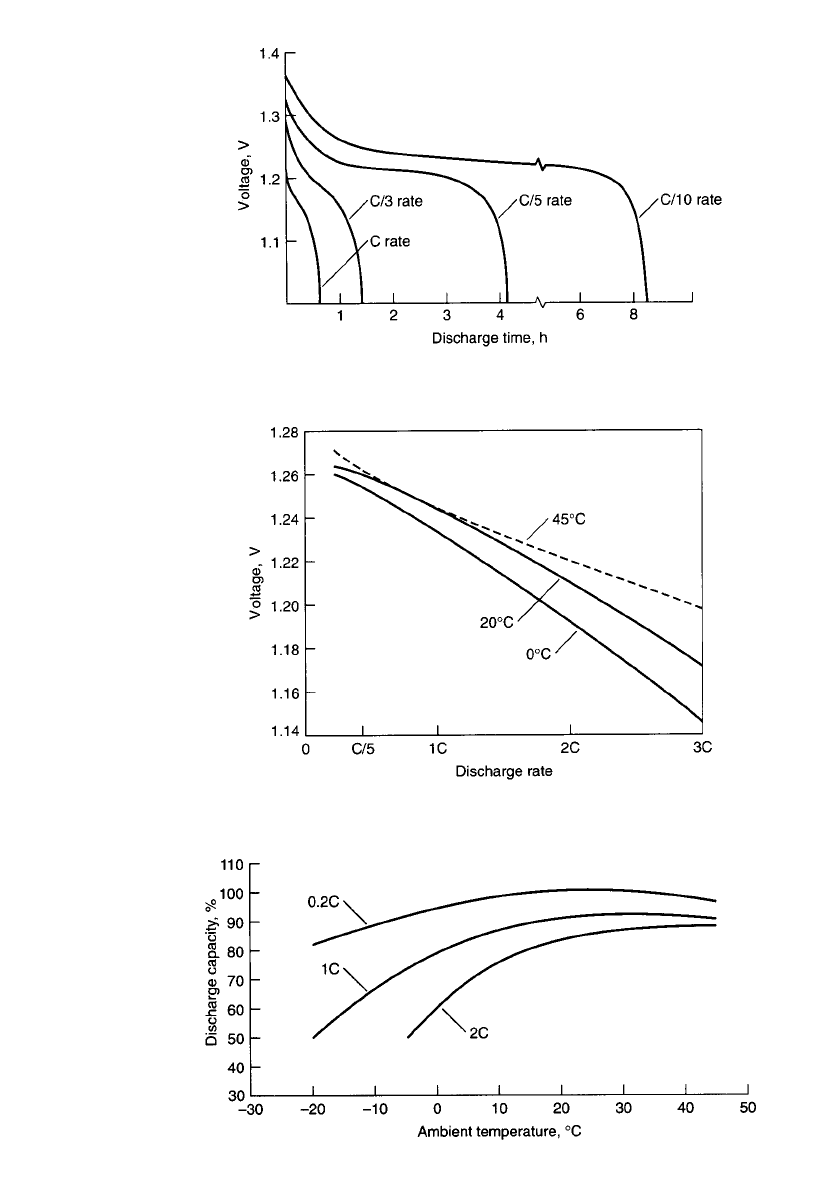
28.10 CHAPTER TWENTY-EIGHT
FIGURE 28.10 Constant-current discharge curves of sealed nickel-
cadmium batteries at ⫺20⬚C.
FIGURE 28.11 Midpoint discharge voltage vs. rate at various tem-
peratures for sealed nickel-cadmium batteries, 1-V cutoff.
FIGURE 28.12 Percentage of rated capacity vs. temperature at different discharge
rates for typical sealed nickel-cadmium batteries, 1.0-V cutoff.
PORTABLE SEALED NICKEL-CADMIUM BATTERIES 28.11
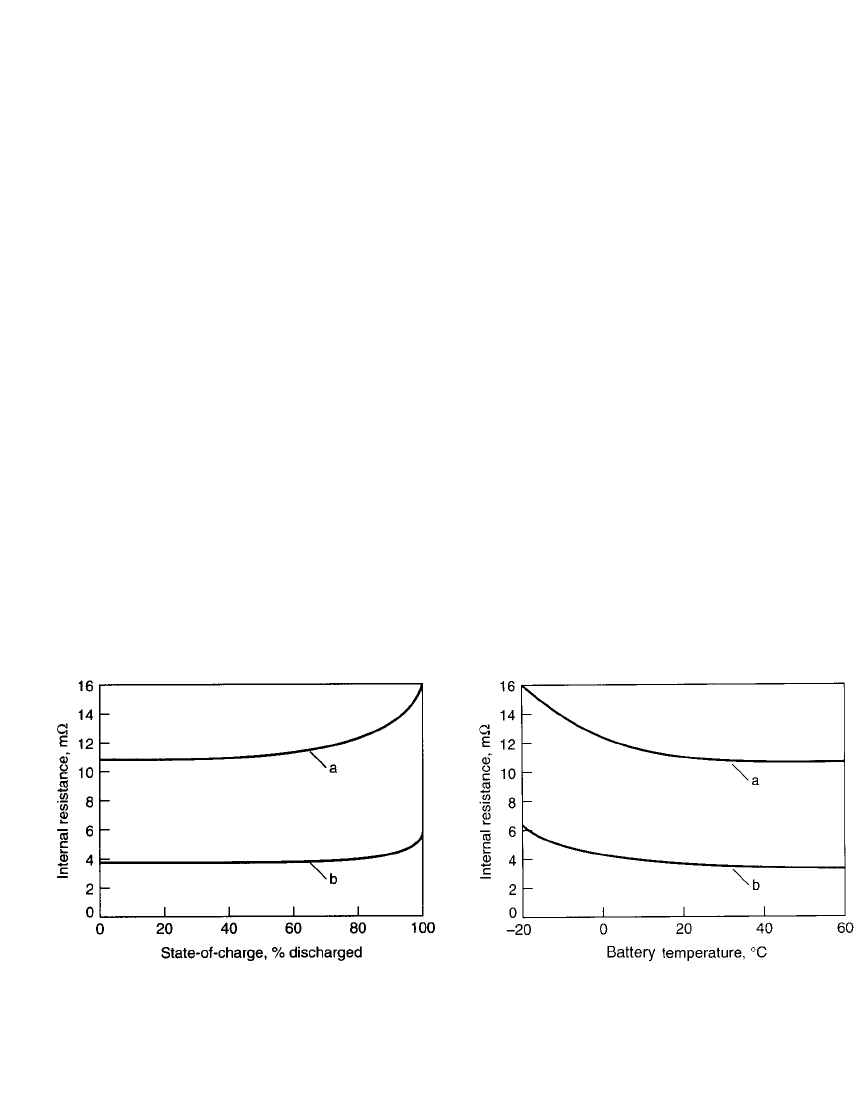
28.4.4 Internal Impedance
The internal impedance of a battery is dependent on several factors, including ohmic resis-
tance (due to conductivity, the structure of the current collector, the electrode plates, sepa-
rator, electrolyte, or other features of the battery design), resistance due to activation and
concentration polarization, and capacitive reactance. In most cases the effects of capacitive
reactance can be ignored. Polarization effects are dependent in a complicated way on current,
temperature, and time; they decrease with increasing temperature (see Chap. 2). The effect
may be negligible for pulses of short duration, that is, less than a few milliseconds.
The nickel-cadmium battery is noted for its low internal resistance due to the use of thin
and large-surface-area plates with good electrical conductivity, a thin separator with good
electrolyte retention, and an electrolyte having a high ionic conductivity. During discharge,
the activation and concentration polarization effects are negligible, at least at low and mod-
erate rates, and the internal resistance of the battery, and the discharge voltage, remains
relatively constant from the state of full charge to the point where almost 90% of the capacity
has been discharged. At that point the resistance increases due to the conversion of active
materials in the electrode plates, which tends to lower electrical conductivity. Figure 28.13
shows the change in internal resistance with the depth of discharge for two batteries of
different size and capacity. Figure 28.14 illustrates the effect of temperature. The internal
resistance increases as the temperature drops because the conductivity of the electrolyte and
other components is lower at the lower temperatures.
With use over time, a nickel-cadmium battery gradually loses capacity, resulting in a
gradual increase in internal resistance. This is caused by gradual deterioration of the separator
and electrodes and by loss of liquid through the seals, which changes the electrolyte con-
centration and level. The net effect is an increase in internal impedance.
FIGURE 28.13 Resistance vs. state of charge at
20⬚C, discharged at 0.2C rate, for sealed nickel-
cadmium batteries. a—AA-size battery, b—sub-C-
size battery. (Typical for sintered-plate electrode type
batteries.)
FIGURE 28.14 Resistance vs. temperature for fully
charged sealed nickel-cadmium batteries. a—AA-size
battery, b—sub-C-size battery. (Typical for sintered-
plate electrode type batteries.)
28.12 CHAPTER TWENTY-EIGHT
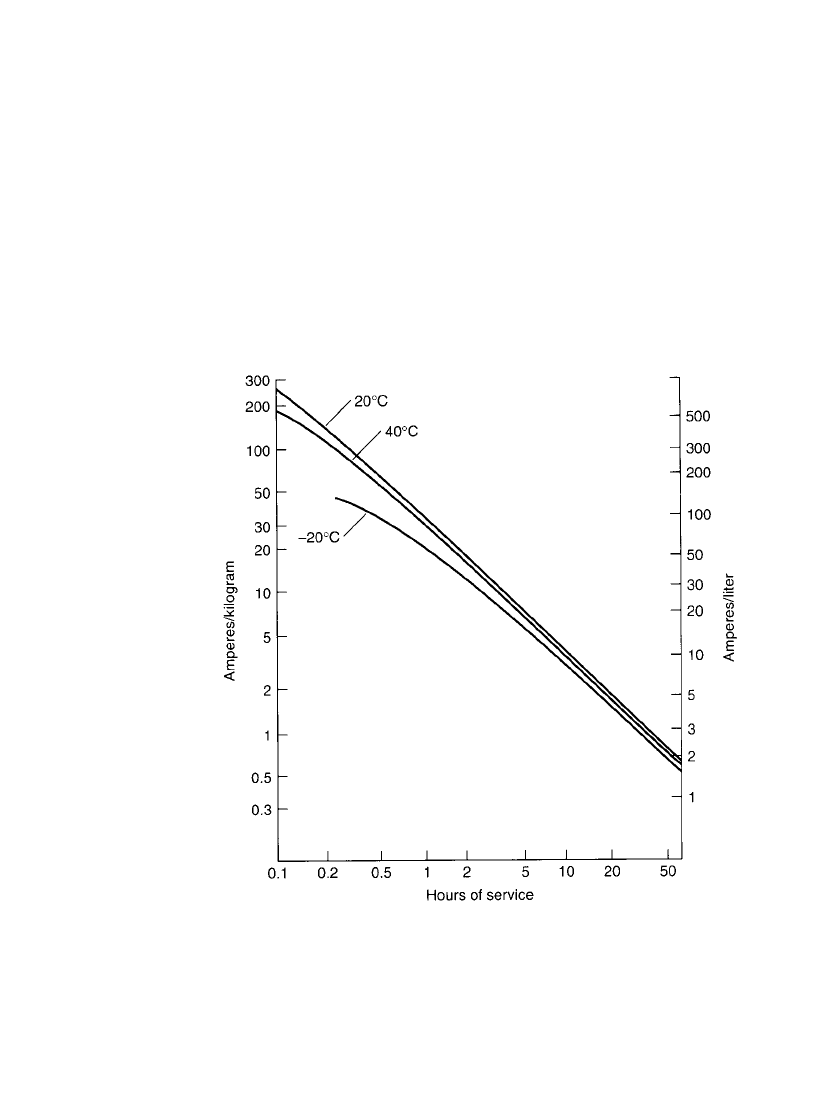
28.4.5 Service Life
The service life of a sealed nickel-cadmium battery, normalized to unit weight (kilograms)
and size (liters), at various discharge rates and temperatures is summarized in Fig. 28.15.
The curves are based on a capacity, at the C/ 5 discharge rate at 20
⬚C, of 30 Ah /kg and 85
Ah/ L, reflecting the performance of standard-type sealed cylindrical batteries. Manufacturers
should be contacted for performance characteristics of specific batteries.
FIGURE 28.15 Service life of sealed nickel-cadmium battery at various
constant-current discharge rates and temperatures; end voltage at 1.0 V.
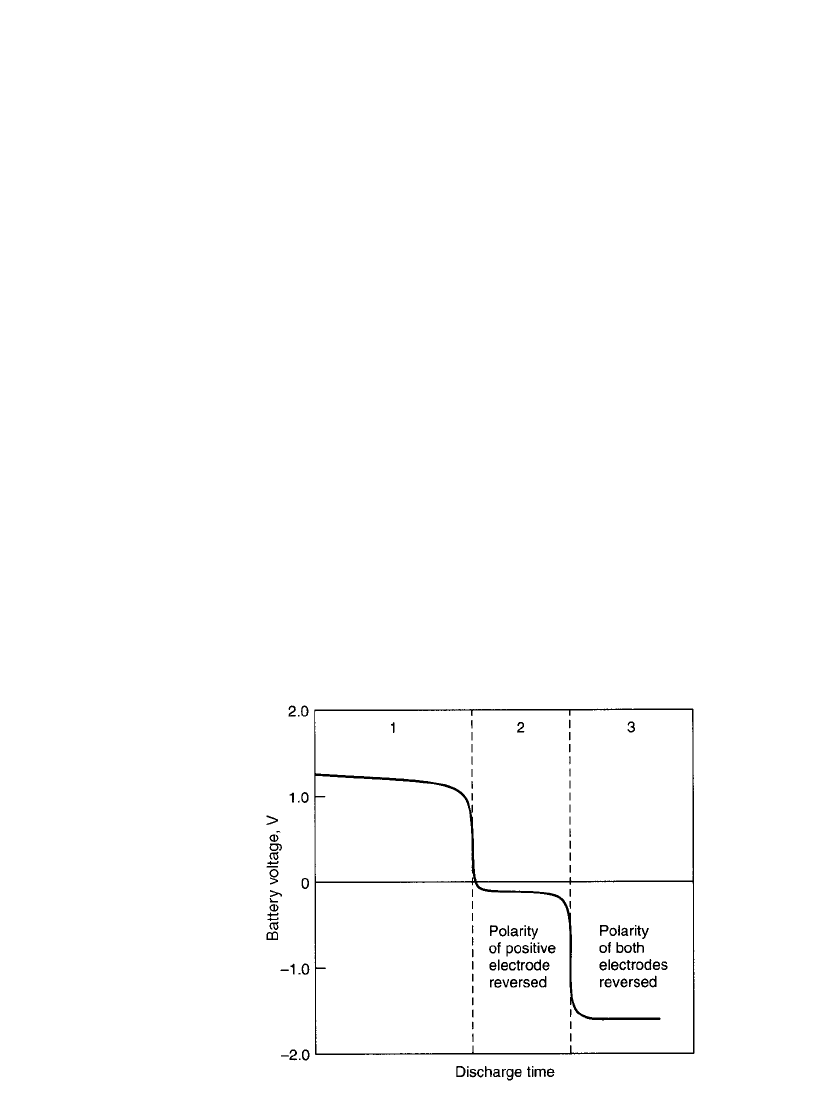
PORTABLE SEALED NICKEL-CADMIUM BATTERIES 28.13
FIGURE 28.16 Discharge of sealed nickel-cadmium battery
showing polarity reversal.
28.4.6 Reversal of Voltage Polarity
When three or more batteries are series-connected, the lowest-capacity battery can be driven
into voltage reversal by the others. The larger the number of batteries in series, the greater
the possibility of this occurring. During reversal, hydrogen may evolve from the positive
electrode and oxygen from the negative. Figure 28.16 shows the complete discharge curve
of a battery, including polarity reversal. Section 1 is the normal period of discharge with
active materials remaining on both electrodes. Section 2 represents the period when the
discharge has been extended and all of the active material on the positive electrode has been
discharged and hydrogen gas is generated at this electrode. Active material still remains on
the negative electrode and its normal discharge reaction continues. The battery voltage varies
with the discharge current, but stays at about
⫺0.2 to ⫺0.4 V. In Section 3 the negative
active material has been discharged and oxygen gas is generated at this electrode.
Continued discharging during polarity reversal will lead to high battery pressure and
opening of the safety vent. This then results in a loss of gas and electrolyte and breakdown
of the capacity balance of the positive and negative electrodes.
Some battery designs provide a limited amount of built-in protection against deep reversal
by adding a small amount of cadmium hydroxide to the positive electrode. The term used
for the material added to the positive electrode for reversal protection is ‘‘antipolar mass’’
(APM). When the positive electrode is completely discharged, the cadmium hydroxide in
that electrode is converted to cadmium, which, combining with the oxygen generated from
the negative electrode, depolarizes the positive, preventing hydrogen generation for a time.
This reaction occurs at about
⫺0.2 V. This reaction can sustain for only a limited time, after
which hydrogen is evolved from the positive electrode. Because hydrogen combines with
the battery materials to only a limited extent, repetitive battery reversal will gradually in-
crease a battery’s internal pressure, ultimately causing the battery to vent.
Discharging to the point of reversal should be avoided. In order to prevent voltage reversal
in any of the cells of a multicell battery, particularly with a series string of more than four
cells, the battery should not be discharged to a voltage below 0.8 V per cell. In applications
of multicell batteries where it is likely that the battery will be frequently discharged below
1.0 V per cell, a voltage-limiting device is recommended to avoid cell reversal.
28.14 CHAPTER TWENTY-EIGHT
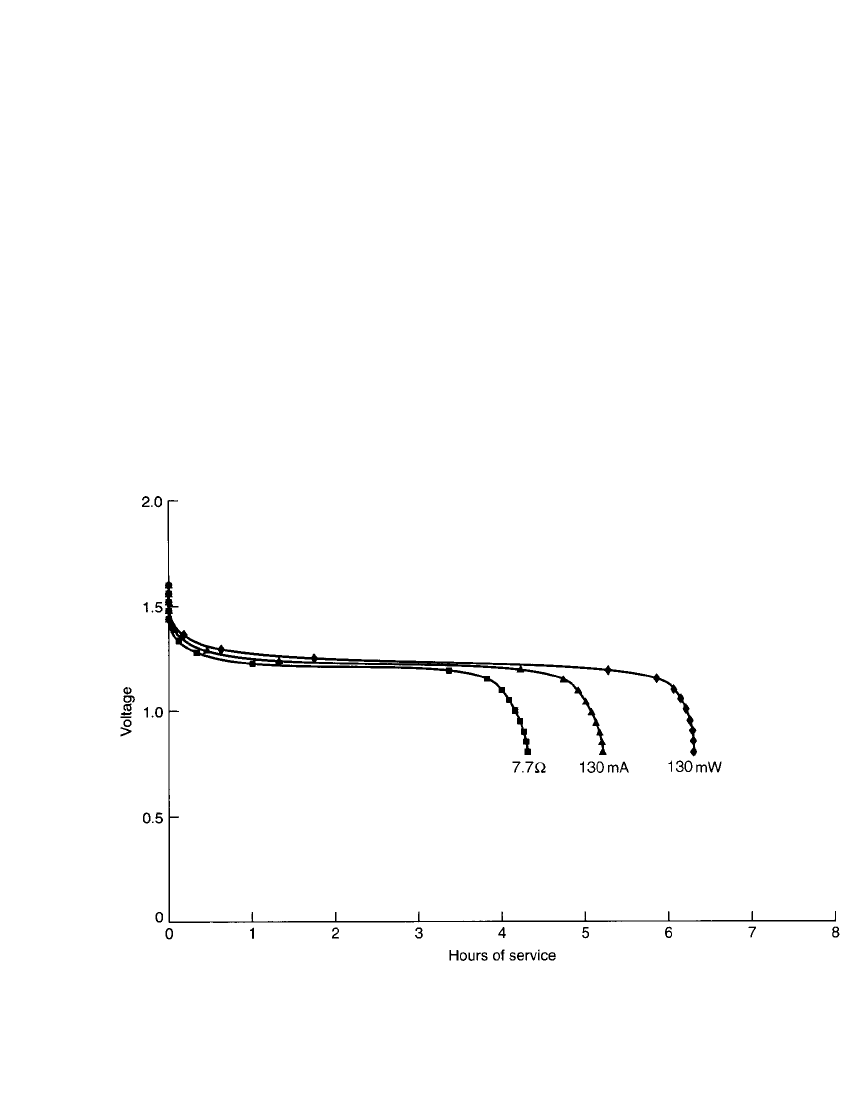
28.4.7 Types of Discharge
As discussed in Sec. 3.2.3, a battery may be discharged under different modes (such as
constant resistance, constant current, or constant power), depending on the characteristics of
the equipment load. The type of discharge mode selected has a significant impact on the
service life delivered by a battery in a specified application. The voltage profiles of a nickel-
cadmium battery discharged under the three different modes are plotted in Fig. 28.17. The
data are based on a discharge of a 650-mAh battery so that, at the end of the discharge (1.0
V per cell), the power output is the same for all modes of discharge. In this example the
power output is 130 mW. To discharge at 130 mW at 1.0 V, the constant-current discharge
is 130 mA (C/ 5 rate) and the constant-resistance discharge is 7.7
⍀. As shown, the longest
service life is obtained under the constant-power mode as the average current is the lowest
under this mode of discharge.
FIGURE 28.17 Discharge curves of AA-size (650-mAh) nickel-cadmium battery—constant power (⽧) vs.
constant current (䉱) vs. constant resistance (䡲).
PORTABLE SEALED NICKEL-CADMIUM BATTERIES 28.15
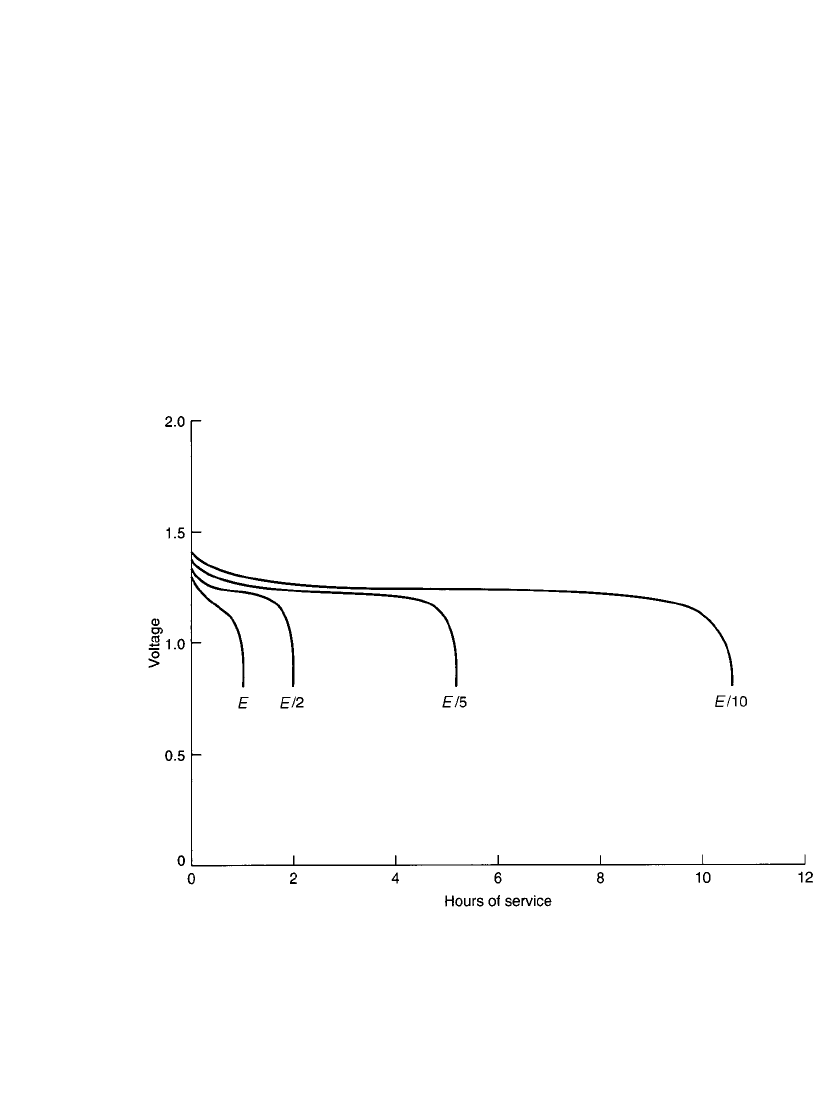
28.4.8 Constant-Power Discharge
The discharge characteristics of the nickel-cadmium battery under the constant-power mode,
at several different power levels, are shown in Fig. 28.18. These are similar to the data
presented in Fig. 28.7 for constant-current discharges, except that the performance is pre-
sented in hours of service instead of percent discharge capacity. The power levels are shown
based on the E-rate. The E-rate is calculated in a manner similar to calculating the C-rate,
but based on the rated watthour capacity. For example, for the E /5 power level, the power
for a battery rated at 780 mWh is 156 mW.
FIGURE 28.18 Constant-power discharge curves at various E rates for sealed nickel-cadmium bat-
teries at 20⬚C.
28.16 CHAPTER TWENTY-EIGHT
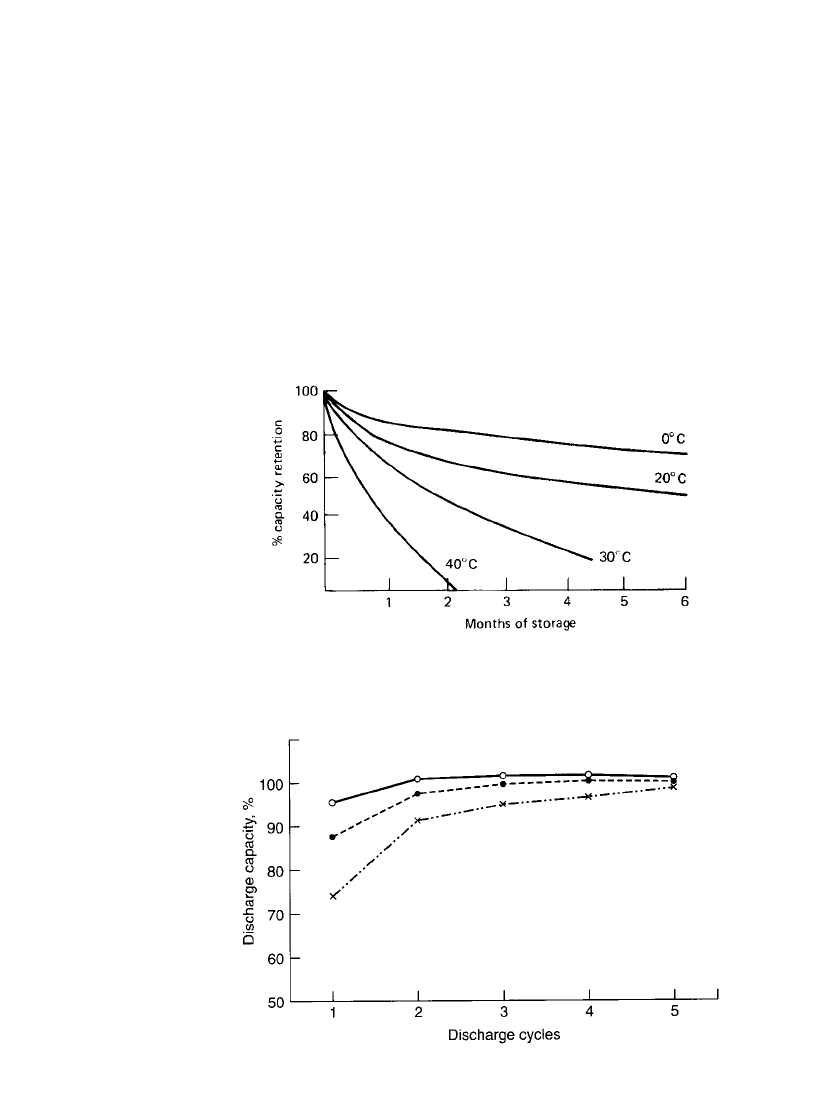
28.4.9 Shelf Life (Capacity or Charge Retention)
Nickel-cadmium batteries lose capacity during storage. The rate of this self-discharge is a
function of storage temperature and battery design. Figure 28.19 can serve as a guide for
the shelf life (capacity or charge retention) at several temperatures for typical standard type
nickel-cadmium sealed batteries. Specifically designed batteries, as discussed in Sec. 28.6,
may have considerably different charge retention characteristics. For example, the button
batteries designed for memory-backup application have significantly better charge retention
characteristics than the standard lower-resistance higher-discharge-rate cylindrical batteries.
Sealed nickel-cadmium batteries can be stored in a charged or a discharged condition.
Except for extended storage at high temperatures, they can be restored to full capacity after
storage by recharging (two or three charge-discharge cycles). Figure 28.20 illustrates the
capacity recovery after prolonged storage at several temperatures. The recovery time may
be longer after high-temperature storage.
FIGURE 28.19 Capacity retention (shelf life) of standard type
sealed nickel-cadmium batteries.
FIGURE 28.20 Capacity recovery of standard type sealed nickel-
cadmium batteries, discharge after 2-year storage at 0.2C rate. Storage
temperatures: at 20⬚C(䡩); 35⬚C(●); and 45⬚C(⫻).
