Linden D., Reddy T.B. (eds.) Handbook of batteries
Подождите немного. Документ загружается.

PORTABLE SEALED NICKEL-CADMIUM BATTERIES 28.27
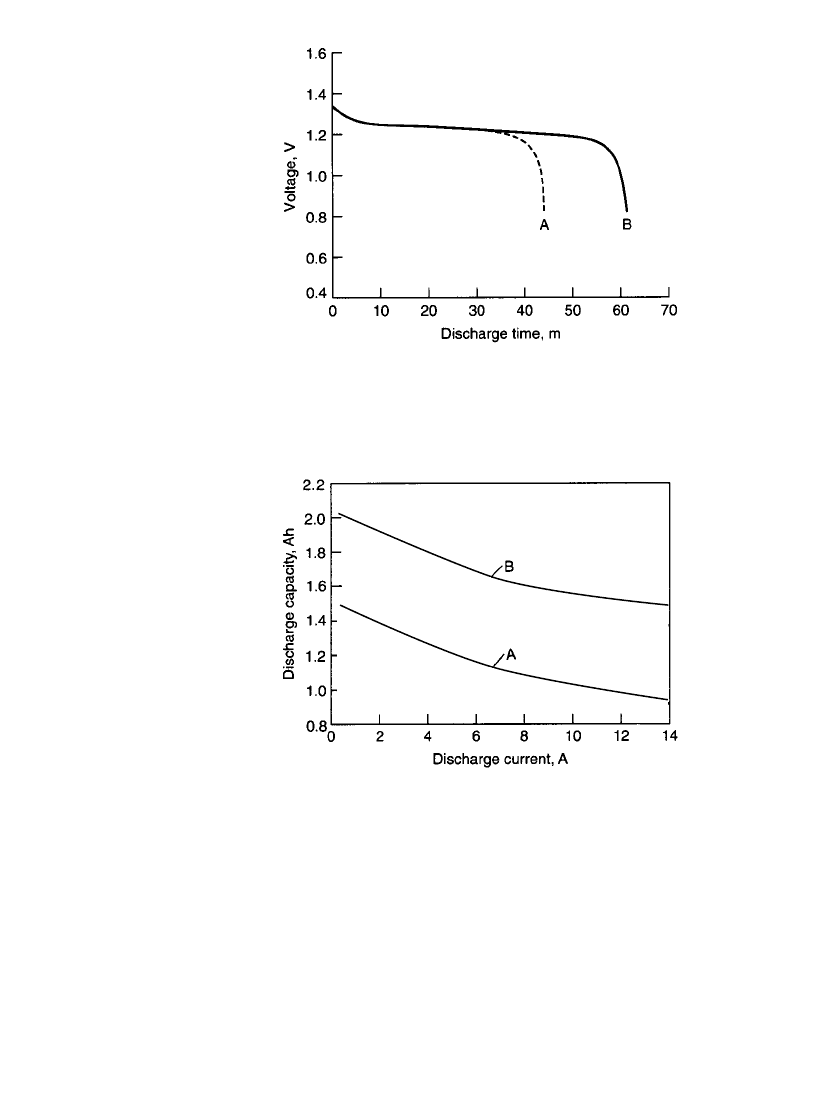
FIGURE 28.31 Comparison of discharge characteristics of
sub-C size standard battery (A) vs. high-capacity battery (B)
on discharge at 20⬚C. Discharge at C rate, charge at 0.1C
rate for 16 hours.
FIGURE 28.32 Comparison of performance of standard
battery (A) vs. high-capacity battery (B) (sub-C size), at 20⬚C.
28.6.2 Fast-Charge Batteries
These batteries have electrode structures and electrolyte distribution designs to enhance ox-
ygen recombination. They can be charged at the fast 1-h rate with charge control (such as
temperature-sensing and
⫺⌬V techniques) and at the C/ 3 rate without charge control because
of their ability to withstand this level of overcharge. They are also capable of performance
at high discharge rates, though this is achieved at the expense of a slightly reduced battery
capacity. The batteries used in these batteries have improved internal heat conductivity, which
results in a faster increase in surface temperature. This feature can be used advantageously
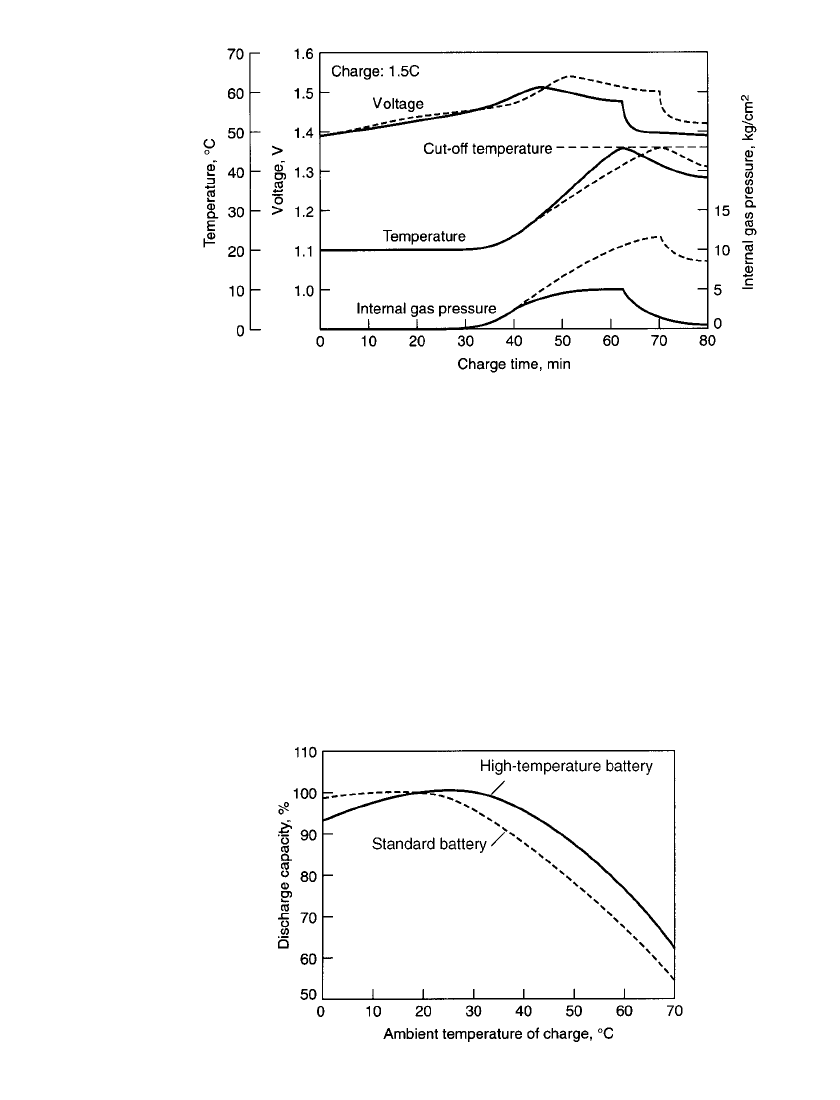
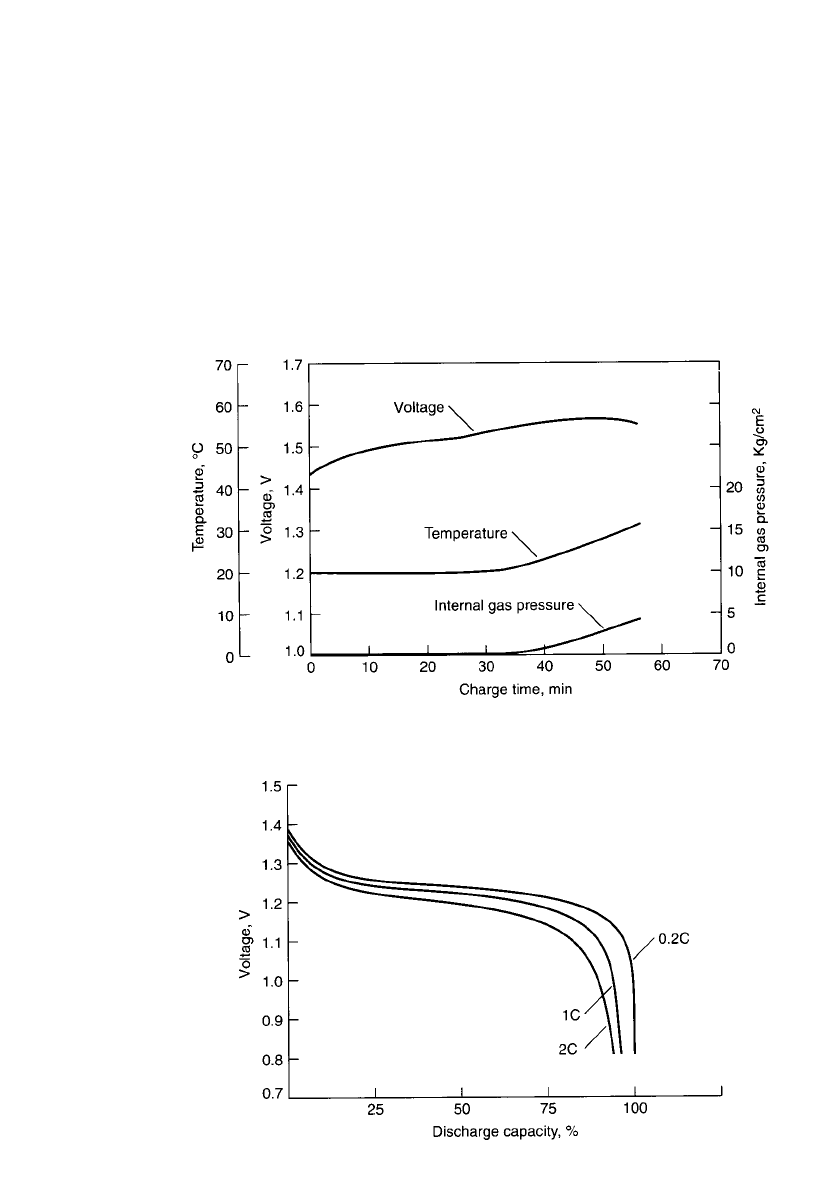
in a temperature-sensing fast-charge system. Figure 28.33 shows the charge characteristics
of a fast-charge battery compared to a standard one. The internal gas pressure of the standard
battery increases quickly during charging whereas that of a fast-charge battery stabilizes.
28.28 CHAPTER TWENTY-EIGHT
FIGURE 28.33 Comparison of charge characteristics of fast-charge (solid line) bat-
tery vs. standard battery (broken line).
28.6.3 High-Temperature Batteries
These batteries are designed to operate at high temperatures without the service life deteri-
oration and charging inefficiencies experienced with conventional designs. Figure 28.34 com-
pares the performance of the high-temperature battery with the standard battery as a function
of ambient temperature during charge. This type of battery is capable of charge-discharge
cycling at temperatures as high as 35 to 45
⬚C and is particularly designed for trickle charging
(C/20 to C /50 rate) at these high temperatures. The charge voltage of these batteries is
slightly higher than that of the standard battery due to the designed-in control of the oxygen-
generating potential.
FIGURE 28.34 Comparison of performance of high-
temperature battery vs. standard battery. charge—C/ 30 rate;
discharge—1C rate at 20⬚C.
PORTABLE SEALED NICKEL-CADMIUM BATTERIES 28.29
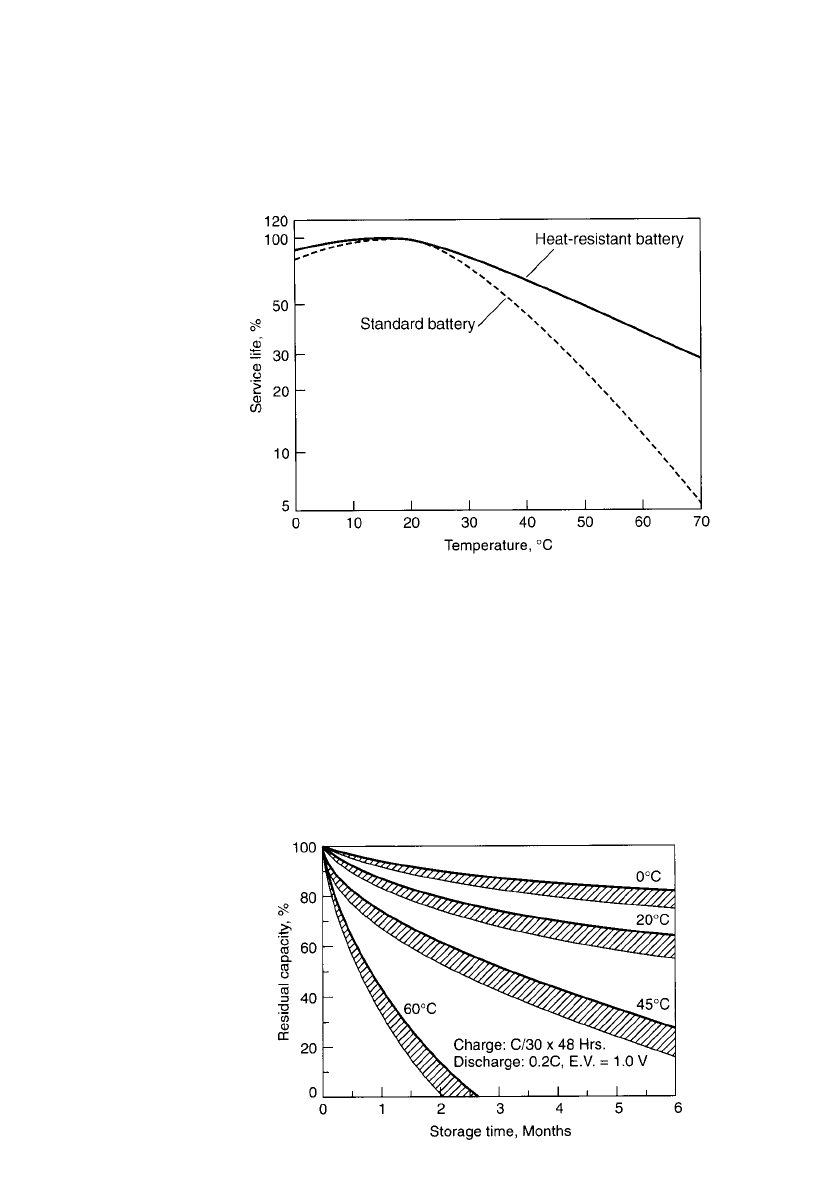
28.6.4 Heat-Resistant Batteries
These batteries are designed for fast charging at high temperatures. For example, charging
at the 0.3C rate is possible even at temperatures as high as 45 to 70
⬚C. Their performance
characteristics are similar to those of the standard battery. However, they have a superior
service life when used at high temperatures because of the use of specially selected materials
with minimum deterioration at high temperatures. Figure 28.35 compares the service life for
standard and heat-resistant batteries throughout the temperature range.
FIGURE 28.35 Comparison of performance of heat-resistant battery
vs. standard battery.
28.6.5 Memory-Backup Batteries
These batteries are used to provide battery backup for volatile semiconductor memory de-
vices. The key requirements for this type of battery are long life (up to 10 years in certain
applications), low self-discharge, and good performance at low discharge rates. Figure 28.36
shows the storage characteristics of the memory-backup battery. (This can be compared to
the characteristics of the standard battery shown in Fig. 28.19.) The low-rate discharge
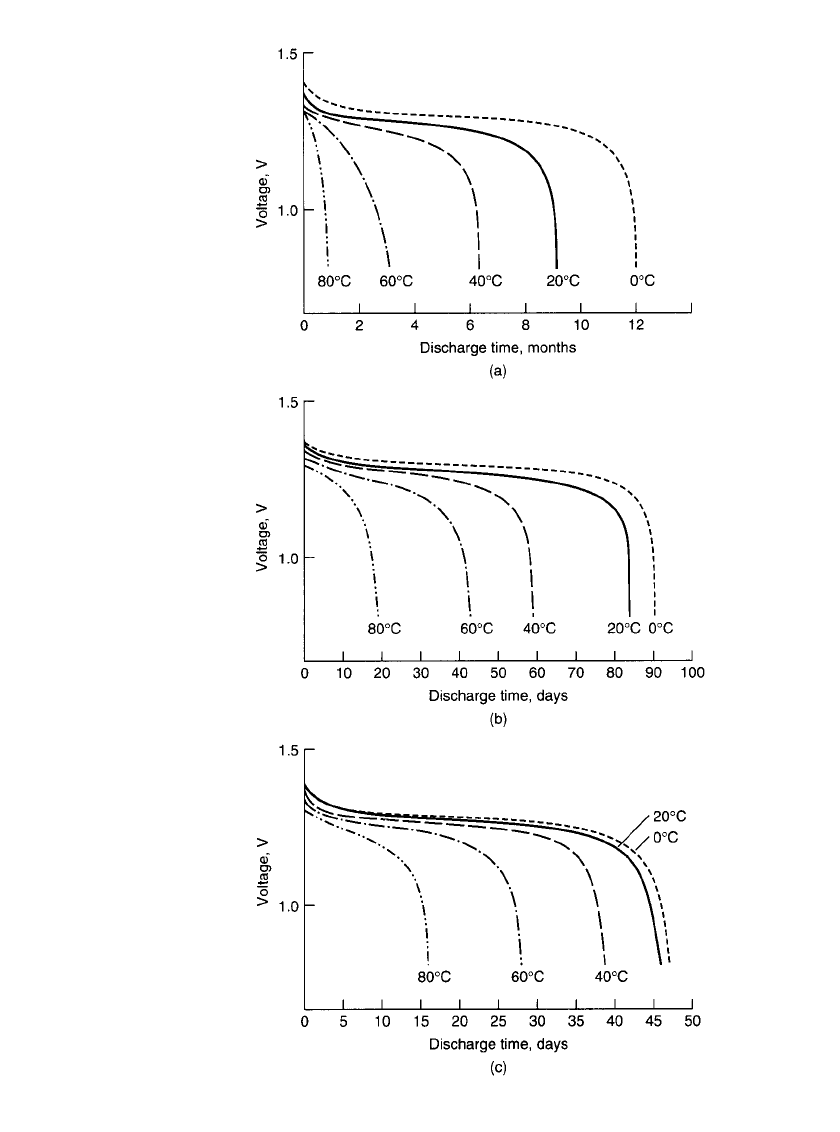
characteristics of the battery are plotted in Fig. 28.37. As the backup battery is designed for
low-rate use, its internal resistance is higher than that of the standard battery and its high-
rate discharge characteristics are not as good.
FIGURE 28.36 Storage characteristics of memory-backup
batteries.
28.30 CHAPTER TWENTY-EIGHT
FIGURE 28.37 Performance of memory-backup batteries.
Charge—C/ 30 for 48 h at 20⬚C. Discharge rate: (a) C / 10,000; (b)
C / 2000; (c) C/ 1000.
PORTABLE SEALED NICKEL-CADMIUM BATTERIES 28.31
28.6.6 Slim Rectangular Batteries
The constructional features of the slim rectangular battery are described in Sec. 28.3.3. The
advantage of the rectangular battery is that it permits more efficient battery design, elimi-
nating the voids that occur with the assembly of cylindrical batteries. The volumetric energy
density of these batteries can be about 20% higher than a battery using a cylindrical design.
Most of the performance characteristics are similar to those of the standard cylindrical
battery, except that it also incorporates some of the features of the high-capacity battery. Gas
recombination has been improved to permit charging at the 0.2C rate or less and 1-h charging
with charge control, preferably with
⫺⌬V sensing. This is illustrated in Fig. 28.38. The
voltage profile on discharge is flat, as with the cylindrical battery, as shown in Fig. 28.39.
However, because the resistance of the rectangular battery is higher, performance at rates
greater than 4C are not as good as with the cylindrical battery. Storage characteristics and
cycle life are similar to those of the cylindrical battery.
FIGURE 28.38 Charge characteristics of slim rectangular batteries at 20⬚C. Charge—
1.5C rate; ⫺⌬V ⫽ 10 mV.
FIGURE 28.39 Discharge characteristics of slim rectangular batteries at
20⬚C. Charge—0.1C rate for 16 h.
28.32 CHAPTER TWENTY-EIGHT
TABLE 28.3 Specifications of Typical Sealed Nickel-
Cadmiuim Single-cell Batteries
Battery
size
Capacity
at 0.2C
rate, mAh
Dimensions, max., mm
Diameter Height Weight, g
Cylindrical batteries
Standard batteries: Charging—standard, 0.1C rate, 14–16 h;
quick, 0.3C rate, 4–5 h
N
AAAA
1
⁄
3
AAA
AAA
1
⁄
2
AA
AA
A
SC
SC
D
D
F
M
170
120
55
270
300
650
550
1450
1550
4400
4800
7700
12000
12.0
8.0
10.5
10.5
14.5
14.3
17.0
22.9
22.9
33.0
33.0
33.2
43.1
29.3
42.5
15.8
44.4
30.3
50.2
28.5
43.0
43.0
59.5
61.1
91.0
91.0
9
6
4
11
14
23
19
45
47
160
145
230
400
High-capacity batteries:
Charging—standard, 0.1C rate, 14–16
h; quick, 0.3C rate, 4–5 h
AA
AA
A
A
A
SC
SC
D
M
880
1150
650
1200
1550
1900
2400
5400
25500
14.3
14.3
17.0
17.0
17.0
22.9
22.9
33.2
43.1
50.3
50.3
28.5
43.0
43.0
43.0
50.0
59.5
146.1
23
24
18
28
31
47
58
150
700
28.7 BATTERY TYPES AND SIZES
Table 28.3 lists some of the types of sealed nickel-cadmium single-cell batteries that are
manufactured and some of their physical and electrical specifications. Multicell batteries are
also manufactured, using these cells, in a variety of output voltages and configurations.
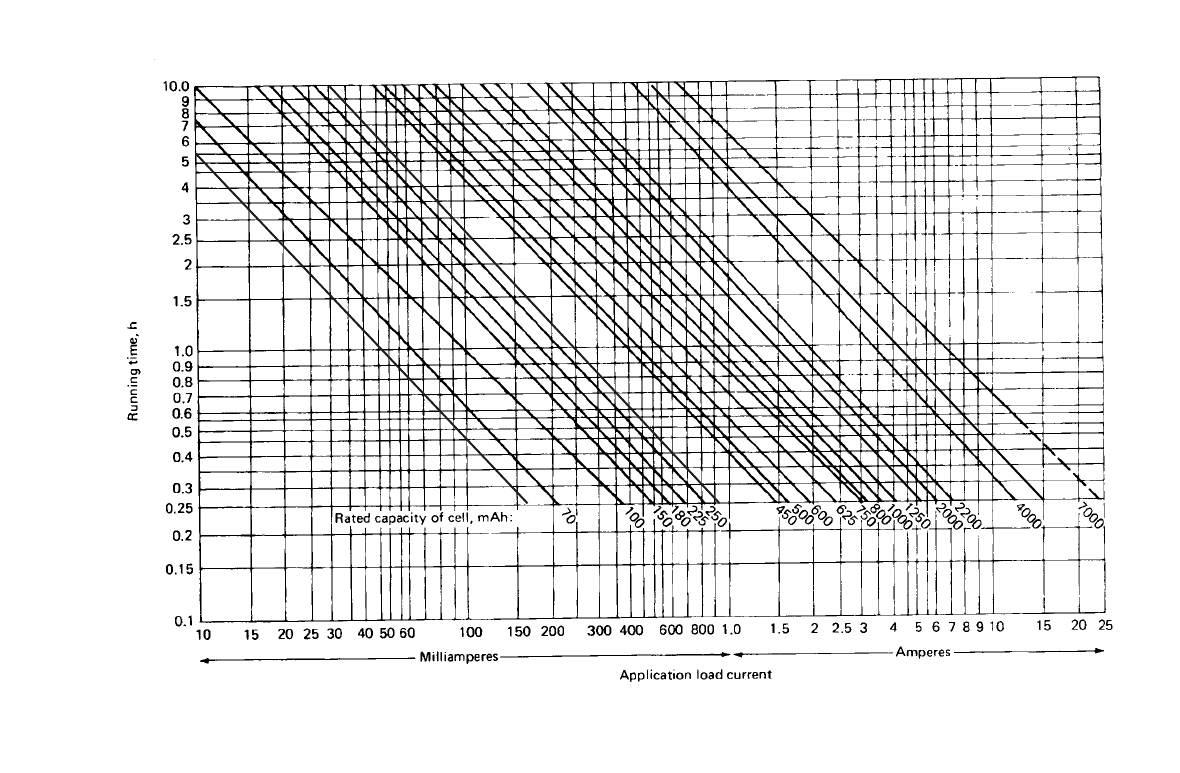
Figure 28.40 is a guide to determining the approximate battery size required for a given
performance requirement or application. These data are based on the performance of a stan-
dard battery at 20 to 25
⬚C. Allowance must be factored into the estimate to determine the
performance under other discharge conditions.
Manufacturers’ data should be consulted for specific details on dimensions, ratings, and
performance characteristics as they may be different from those shown.
PORTABLE SEALED NICKEL-CADMIUM BATTERIES 28.33
TABLE 28.3 Specifications of Typical Sealed Nickel-
Cadmiuim Single-cell Batteries (Continued)
Battery
size
Capacity
at 0.2C
rate, mAh
Dimensions, max., mm
Diameter Height Weight, g
Cylindrical batteries
Fast-charge batteries: Charging—standard, 0.1C rate, 14–16 h;
quick, 0.3C rate, 4–5 h; fast, 1.5C rate, 1 h
A
4
⁄
5
SC
SC
SC
SC
C
D
550
1250
1400
1850
2000
3200
4300
17.0
22.9
22.9
22.9
22.9
26.0
33.0
28.5
34.0
43.0
43.0
42.9
50.0
59.5
19
43
52
54
56
84
160
High-temperature batteries:
Charging—standard, 0.1C rate,
14–16 h
AA
SC
C
D
F
M
650
1650
3100
4500
7700
12000
14.3
22.9
26.0
33.2
33.2
43.1
48.9
43.0
50.5
59.5
91.0
91.0
23
49
78
145
230
400
Heat-resistant batteries:
Charging—standard, 0.1C rate, 14–16
h; quick, 0.3C rate, 4–5 h
2
⁄
3
AA
AA
4
⁄
5
SC
SC
C
300
650
1350
1800
2200
14.5
14.3
22.9
22.9
26.0
30.3
50.2
43.0
42.9
50.0
14
23
52
56
80
Slim rectangular batteries
Capacity at
0.2 C rate,
mAh
Dimensions, max., mm
Height Width Thickness Weight, g
Slim rectangular batteries: Charging—standard, 0.1C rate,
14–16 h; quick, 0.3C rate, 4–5 h; fast, 1.5C rate, 1 h
450
650
650
900
1200
48.0
48.0
67.0
67.0
67.0
17.2
17.2
17.2
17.2
17.2
6.3
8.5
6.3
8.5
10.7
17
22
24
30
38
28.34
55
300
1650
4600
FIGURE 28.40 Selector guide for sealed nickel-cadmium cylindrical batteries. Guide can be used to deter-
mine approximate required battery size, given the load and desired run (service) time. Data based on fully
charged battery and 20
⬚C operating temperature.

PORTABLE SEALED NICKEL-CADMIUM BATTERIES 28.35
REFERENCE
1. Y. Sato, K. Ito, T. Arakawa and K. Kobaya Kawa ‘‘Possible Causes of the Memory Effect Observed
in Nickel-Cadmium Secondary Batteries. J. Electrochemical Society, 143:L225 (October 1996).
BIBLIOGRAPHY
Cadnica Sealed Type Nickel-Cadmium Batteries, Sanyo Electric Co., Osaka, Japan.
Ford, Floyd E.: Handbook for Handling and Storage of Nickel-Cadmium Batteries: Lessons Learned,
NASA Ref. Publ. 1326, Feb. 1994.
Nickel-Cadmium Batteries, Charge System Guide, Panasonic Industrial Co., Secaucus, N.J.
Nickel-Cadmium Batteries, Technical Handbook, Panasonic Industrial Co., Secaucus, N.J.
Sealed NiCad Handbook, SAFT Corp. of America, Valdosta, Ga.
Sealed Nickel-Cadmium Accumulators, Sales Program and Technical Handbook, Varta Batterie AG,
Hanover, Germany.

29.1
CHAPTER 29
PORTABLE SEALED NICKEL-METAL
HYDRIDE BATTERIES
David Linden and Doug Magnusen
29.1 GENERAL CHARACTERISTICS
The rechargeable sealed nickel-metal hydride battery is a relatively new technology with
characteristics similar to those of the sealed nickel-cadmium battery. The principal difference
is that the nickel-metal hydride battery uses hydrogen, absorbed in a metal alloy, for the
active negative material in place of the cadmium used in the nickel-cadmium battery.
The metal hydride electrode has a higher energy density than the cadmium electrode.
Therefore the amount of the negative electrode used in the nickel-metal hydride cell can be
less than that used in the nickel-cadmium cell. This allows for a larger volume for the positive
electrode, which results in a higher capacity or longer service life for the metal hydride
battery. Furthermore, as the nickel-metal hydride battery is free of cadmium, it is considered
more environmentally friendly than the nickel-cadmium battery and may reduce the problems
associated with the disposal of rechargeable nickel batteries.
Most of the operating characteristics of the sealed nickel-metal hydride battery on dis-
charge are similar to those of the nickel-cadmium battery. The sealed nickel-metal hydride
battery, however, does not have the very high rate capability of the nickel-cadmium battery.
In addition, the behavior of the two systems on charge, particularly on fast charge, is dif-
ferent. The nickel-metal hydride battery is less tolerant of overcharge and requires control
of the cutoff of the charge, which may not always be required for nickel-cadmium batteries.
During the past five years, the specific energy and energy density of the nickel-metal
hydride battery has been increased by over 35% as a result of improvements in both the
positive and negative electrodes. Concurrently, improvements were made in its high-rate
performance and cycle life. Because of its higher energy density and other comparable per-
formance characteristics, the nickel-metal hydride battery is replacing the nickel-cadmium
battery in computers, cellular phones and other consumer electronic applications with the
possible exceptions of high-drain power tools and applications where low battery cost is the
major consideration. However, the nickel-metal hydride battery now is being replaced, in
turn, by the lithium-ion battery which has an even higher specific energy and energy density.
The metal hydride battery in larger sizes is also being considered for use in applications
such as electric vehicles, where its higher specific energy and good cycle life approach
critical performance requirements.
The advantages and limitations of the sealed nickel-metal hydride battery are summarized
in Table 29.1. The main advantage of the nickel-metal hydride battery compared to the
nickel-cadmium battery is its higher specific energy and energy density.
