Lima J.J.Pedroso, de (ed.). Nuclear Medicine Physics
Подождите немного. Документ загружается.

438 Nuclear Medicine Physics
0°
Electron
Photon
Photon
E
θ
ϕ
E′< E
E′
T
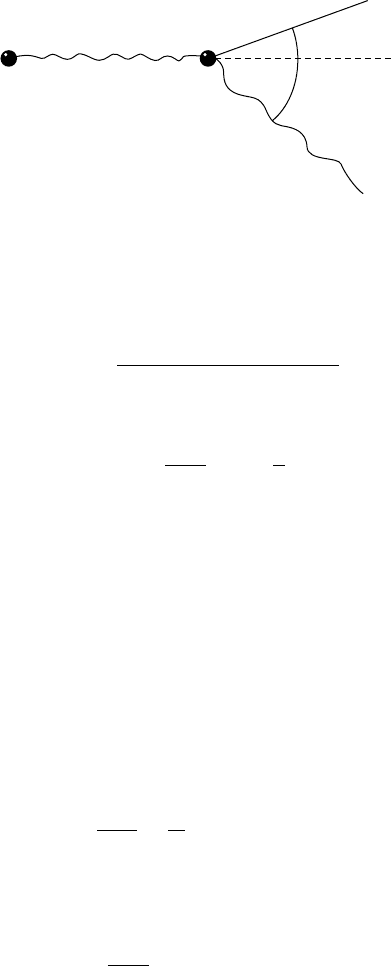
FIGURE 8.2
Kinematics of the Compton effect.
One can obtain the following kinematic equations for the Compton effect,
w
=
w
1 +(w/m
0
c
2
)(1 −cos φ)
, (8.11)
T = w −w
, (8.12)
cot θ =
1 +
w
m
0
c
2
tan
φ
2
, (8.13)
where m
0
c
2
(rest mass energy of the electron) is 0.511 MeV; and the energies
w, w
, and T are expressed in MeV.
8.2.4 Thompson Scattering
In the Thompson description, both the incident photon and the scattered
photon have the same energy. The electron does not acquire kinetic energy as
a result of the elastic collision.
Thompson pointed out that considering the differential cross-section per
electron for a photon scattered at an angle φ, per unit of solid angle, one can
write
d
e
σ
0
dΩ
φ
=
r
2
0
2
(1 +cos
2
φ), (8.14)
in units of (cm
2
sr
−1
per electron), where
r
0
=
e
2
m
0
c
2
= 2.818 ×10
−13
cm, (8.15)
which is named the classic radius of the electron; and the subscript e in
e
σ refers
to a cross-section per electron.

Dosimetry and Biological Effects of Radiation 439
We can conclude that there is a forward–reverse symmetry, meaning that
Equation 8.14 has the same value for φ = 0
◦
and φ = 180
◦
and has half of this
value for φ = 90
◦
.
The total cross-section per electron,
e
σ, is obtained by integration of Equa-
tion 8.14 over all possible scattering angles. A further simplification can be
introducedby assuming cylindrical symmetry and integrating over 0 ≤ φ ≤ π
and dΩ
φ
= 2π sin φ dφ.
e
σ =
π
φ=0
d
e
σ
0
= πr
2
0
π
φ=0
(1 +cos
2
φ) sin φ dφ, (8.16)
e
σ =
8πr
2
0
3
= 6.65 ×10
−25
cm
2
/electron. (8.17)
This leads to a value independent of the energy.
It is well known that this cross section can be viewed as an effective target
area and is numerically equal to the probability of occurrence of a Thomp-
son scattering when a photon crosses a layer that contains one electron
per cm
2
.
8.2.5 Klein–Nishina Cross Section for the Compton Effect
In 1928, Klein and Nishina applied the Dirac relativistic theory of the electron
to the Compton effect in order to obtain better values for the cross section.
The value obtained by Thompson (Equation 8.17), which is independent of
the energy, has an error that can be up to a factor of 2 for energies of the order
of 0.4 MeV. Klein and Nishina have had success in reproducing the values
experimentally obtained, even assuming free electrons, initially at rest.
The differential cross-section for the scattering of a photon over an angle φ,
per unit of solid angle and per electron, is given by
d
e
σ
0
dΩ
φ
=
r
2
0
2
w
w
2
w
w
+
w
w
−sin
2
φ
, (8.18)
where the energy w
is given by Equation 8.11 and the subscript e in
e
σ refers
to the cross-section per electron.
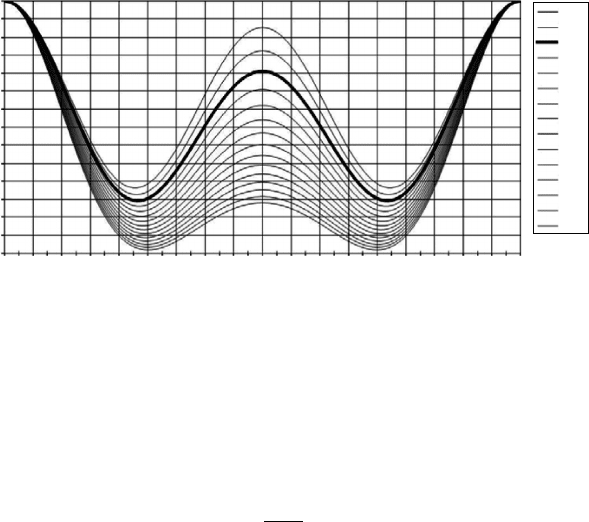
Equation 8.18 is graphically shown in Figure 8.3.
It can be seen that the highest probabilitiesoccur for angles near the incident
axis. For backscattering, that is, angles between 90
◦
and 270
◦
, the highest value
for the probability is at exactly 180
◦
.
For lower energies, w
= w, and Equation 8.18 is reduced to the Thomp-
son expression (Equation 8.14).
440 Nuclear Medicine Physics
20 40 60 80 100 120 140 160 180
Angle (degree)
200 220 240 260 280 300 320 310 360
150
140
130
120
110
100
90
80
70
60
50
40
30
20
10
0
0.3
0.35
0.4
0.45
0.5
0.55
K-N cross section/r
0
0.6
0.65
0.7
0.75
0.8
0.85
0.9
0.95
1
2
FIGURE 8.3
Klein–Nishina cross-section for several values of the energy in the range 10–150 keV.
The Klein–Nishina total differential cross section per electron is obtained
by integration of Equation 8.18 over all scattering angles,
e
σ = 2π
π
φ=0
d
e
σ
dΩ
φ
sin φ dφ
(8.19)
8.2.6 Corrections of the Bonding Energy
of the Electrons
In the theory of gamma radiation transport, the bond energy of the electrons
is, in general, approximately ignored or treated. The justification for this is
that for elements of low Z, the bonding energies in the K shell are small when
compared with the photon energies; whereas for materials with high Z (with
rather high bonding energy in shell K), these electrons are a small fraction of
the total.
In the Compton effect, a photon collides with one of the atomic electrons,
transferring some of the momentum. When the electron acquires enough
energy, it escapes from the atom or is excited to an unoccupied atomic level. If
the energyis very small for any of these possibilities, then the electron remains
bonded in its orbital, and the atom reacts as a single entity. In general, as the
bonding energies are small compared with the photon energies, the electron
is released, leaving the excited ion with an excitation energy equal to the
bonding energy of the shell from which the electron was removed. After the
previous process, the atom returns to the ground state through the emission
of fluorescent photons and Auger or Coster-Kroning electrons. The Comp-
ton effect is similar to the photoelectric effect, as in both interactions there

Dosimetry and Biological Effects of Radiation 441
is energy transfer, leading to the appearance of an energetic electron and an
ionized atom.
In general, as has already been mentioned, ionization or excitation of
the atom is not considered in the energy transfer process in the Compton
interaction. It is assumed that the bond energy of the electron is less than the
energy acquired in the collision; and, therefore, the ionization energy of the
atom is ignored. This approach was considered by Klein and Nishina when
they assumed that there is a free electron.
When the bond energy in the atom is considered, the treatment of the
collision becomes considerably more complex.
There are two conditions where the ionization energy of the atom is not
small compared with the kinetic energy of the electron. These are (a) for all
photon energies where a scratching collision occurs and (b) for photons with
lowenergywhere, for all collision angles,the bond energycannot be neglected
[2]. These conditions depend on the atomic number and the shell where the
collision occurs.
The bonding corrections are often treated in the Waller–Hartree or impulse
approximations [3], which take into account not only the K shell but also the
atomic electrons.
These calculations involve the application of a multiplicative factor, termed
the incoherent scatter function, S(q, Z), to the expression of the Klein–Nishina
differential cross section [4]
dσ(θ)
dΩ
= S(q, Z)
dσ
KN
(θ)
dΩ
(8.20)
where q is the momentum transferred. In the impulse approximation, S(q, Z)
represents the probability of an atom leading to any excited or ionized state
as a result of a sudden impulsive action that supplies a (recuo) momentum q
to an atomic electron.
The expression impulse approximation results from the assumption that the
interaction between both the radiation field and the atomic electron happens
in such a short time that the electron sees a constant potential.
The calculation of S(q, Z), which depends on knowledge of the atomic wave
function, can be made for hydrogen and for other atoms using several meth-
ods of approximations based on the models of Thomas–Fermi, Hartree, and
others.
Ribberfords and Carlsson [3] calculated σ
en
using the impulse approxima-
tion. They pointed out that the tables of Hubbel [5] and Storm and Israel [6]
have considerable errors for photon energies less than 200 keV, particularly
for materials of high atomic number.
Experimental results shows that both approximations, impulse and Waller–
Hartree, can give considerable errors for atomic numbers Z > 50. Though for
higher Z values, these approximations need more refinements, they can be
used for low Z materials.
442 Nuclear Medicine Physics
8.2.7 The Photoelectric Effect
Theoretical analysis of the photoelectric effect is difficult, because the wave
functions of the final states, which are solutions of the Dirac relativistic
equation of the scattered electron, can only be obtained in the exact form
as an infinite sum of partial waves. In addition, for high energies, a large
number of terms are needed.
This is more complex than the Compton effect due to the existence of the
bonding energy of the electron, and there is no simple equation, such as that
of Klein–Nishina discussed earlier.
The wave function of the initial state for the bond electron is, in general,
assumed to be an electron state in a central potential of the nucleus, and the
effect of all the remainder electrons in the atom are taken in account as if the
field of the nucleus has an electric charge Z −S
i
. The constant S
i
is named the
shielding constant and represents the decrease of the nuclear electric charge
arising from the presence of all the electrons of the atom. For further analysis,
see, for example, Davisson [7].
In the photoelectric effect, a photon disappears and an electron is ejected
from the atom. Instead of looking at this interaction as an effect between a
photon and an electron, it must be seen as a process between a photon and an
atom. In fact, complete absorption between only a photon and a free electron
cannot occur, because the linear momentum will not be conserved [8].
The nucleus absorbs the momentum but acquires relatively little kinetic
energy due to its large mass. It is clear that the photoelectric effect can only
occur if the incident photon has energy greater than the bonding energy of
the electron that will be removed. The hole created by the ejected electron is
filled by electrons from any outer orbital, which can simultaneously produce
fluorescence radiation, emission of Auger electrons, or both.
The competition between the emissions of a fluorescent K photon and the
emission of Auger electrons is described by Y
K
, termed the fluorescence yield
of the K shell, which is defined by the number of K photons emitted per hole
in the shell K. The probability that a photon K will be emitted is nearly 1 for
elements with high Z and almost zero for materials with low Z.
Inthe photoelectric effect, the photoelectronacquiresenergyw −E
s
,where
E
s
is the bonding energy of the shell from where the photon was ejected. In
the filling of the holes created, on average, part of the energy, E
s
, is emitted
as characteristic radiation, whereas part is deposited through Auger elec-
trons. For this reason, the mean energy transferred (to electrons),
¯
E
tr
,inthe
photoelectric process is given by
w −E
s
<
¯
E
tr
< w. (8.21)
For materials with high Z, this becomes more complicated [9]. For tissue
equivalent materials of interest in radiological applications, it is much sim-
pler, because in these materials the bonding energies of the K shell are very
small (approximately 500 eV); therefore, the photoelectron acquires almost
Dosimetry and Biological Effects of Radiation 443
all the photon energy, meaning that
¯
E
tr
= w and the coefficients of transfer
and attenuation can be considered to be equal. Since the photoelectric pro-
cess is important only for low energies, the “bremsstrahlung” produced by
the ejected electrons can be neglected, and then the absorption and transfer
coefficients are numerically identical.
The published tables are based on experimental results, complemented by
theoretical interpolations for other energies and absorption media, and give
satisfactory results for several regions of energy values.
8.2.8 Pair Production
Pair production is a process whereby a photon disappears, producing an elec-
tron and a positron. Often, this is mentioned as a process of materialization.
The pair production occurs only in a field of a Coulombic force, gener-
ally near an atomic nucleus; however, it can occur in a field of an atomic
electron. This latter process is named as triplet production, because the host
electron,which furnishes the Coulombic field, also acquires significant kinetic
energy due to the conservation of momentum. This results in emission of two
electrons and a positron from the site of interaction.
Obviously, a minimum of 2mc
2
= 1022 MeV will be needed for pair pro-
duction to occur in the nuclear field. Since we are more interested in energies
below 1 MeV, this process will not be further developed.
8.2.9 Rayleigh Scattering
Rayleigh scattering, also called resonant scattering by an electron, is an atomic
process where an incident photon is absorbed by a weakly bound electron.
The electron is excited to a state of higher energy, and a second photon of
the same energy is emitted; whereas the electron returns to its initial state,
such that there is no excitation. The recoil of the scattered photon is actually
taken by the whole atom, with a very small transference of energy, so that the
energy lost by the photon is negligible.
This process is elastic. The atom is neither excited nor ionized. Some authors
[9] have described Rayleigh scattering as a cooperative phenomenon that
involves all the electrons of the atom. The photons are scattered by the bound
electrons in a process where the atom is neither excited nor ionized. The
process occurs mainly at low energies, and for materials of high Z, in the
same region where the effects of the bonding electrons influence the Compton
cross section.
Since the scattering of the photon results from the reaction of the whole
atom, it is often termed coherent scattering.
Rayleigh scattering does not contribute to the kerma or the absorbed dose,
becausethereis no energyfurnished toany chargedparticle, and noionization
or excitation is produced.
444 Nuclear Medicine Physics
8.2.10 Photonuclear Interaction
In a photonuclear interaction, an energetic photon (>MeV) enters and excites
a nucleus that will emit a proton or a neutron. The protons contribute to
the kerma. However, the relative amount remains below 5% of that due to
the pair production; and, therefore, in general, these are not considered in
dosimetry. Neutrons do already have practical importance, because they pro-
duce problems in radiation protection. This happens in some clinical electron
accelerators, where energies involved can be greater than 10 MeV.
Since we are, in general, interested in energies below 1 MeV, we will not
make any further considerations about photonuclear interaction.
8.2.11 Interaction Coefficients
For x- and γ-rays, the mass attenuation linear coefficient is often used. This is
given by
μ
ρ
=
τ
ρ
+
σ
C
ρ
+
σ
coh
ρ
+
κ
ρ
(8.22)
whose units are (m
2
kg
−1
) and the components refer to photoelectric effect,
Compton effect, coherent scatter, and pair production, respectively.
The mass energy transfer coefficient is given by
μ
tr
ρ
= f
τ
τ
ρ
+f
C
σ
C
ρ
+f
κ
κ
ρ
(8.23)
also in units of (m
2
kg
−1
), and where the components refer to photoelec-
tric effect, Compton effect, and pair production, respectively. The weights
f
τ
, f
C
, and f
κ
are conversion factors which indicate for each interaction the
fraction of the photon energy that, eventually, is converted into kinetic energy
of electrons and is dissipated in the medium through losses in collisions, ion-
izations, and excitations. The coherent scattering is excluded, because in this
process there is no transference of energy.
The mass energy absorption coefficient for x- and γ-rays is given by
μ
en
ρ
=
μ
tr
ρ
(1 −g) (8.24)
in (m
2
kg
−1
), where g represents the fraction of the energy of secondary
charged particles (for photons, these charged particles are electrons) that is
emitted as bremsstrahlung.
Dosimetry and Biological Effects of Radiation 445
For compounds and mixtures, we have,
μ
ρ
mixture
=
i
f
i
μ
ρ
i
, (8.25)
μ
tr
ρ
mixture
=
i
f
i
μ
tr
ρ
i
, (8.26)
where f
i
is the relative fraction of the element i of the compound or mixture.
We can also write,
μ
en
ρ
mixture
=
i
f
i
μ
en
ρ
i
, (8.27)
or in the exact form
μ
en
ρ
mixture
=
μ
tr
ρ
mixture
(1 −g
mixture
). (8.28)
8.3 Interaction of Radiation with Matter: The Electrons
8.3.1 Introduction
As mentioned earlier, interactions of the photon with matter will eject
electrons from atoms.
At a first step, the photons transmit their energy to electrons, which will
then transfer their energy to matter; this second step is named deposition of
energy within matter. In the following part, we will describe the processes of
energy deposition.
The main difference between the interactions of photons and electrons is
that, in general, photons undergo a relatively small number of interactions
(something between 2 and 3 and 20 and 30, depending on their energy)
involving relatively large losses of energy; whereas the electron suffers a
large number of interactions (thousands), each involving only a very small
amount of energy. As a first approximation, we can consider that the electrons
continuously lose energy along their track.
8.3.2 Radiative Energy Loss
According to classic electromagnetic theory, it is well known that a charged
particle, such as the electron, submitted to acceleration (curvilinear tracks in
Coulombic fields, for example) emits radiation. Traditionally, the radiation
produced by electrons submitted to acceleration is named bremsstrahlung,or

446 Nuclear Medicine Physics
braking radiation. This type of radiation is called x-rays when it is produced
in an x-ray tube.
The energy lost by an electron is approximately proportional to its kinetic
energy. The fraction of energy lost by an electron through this process is only
1% of the total loss due to interactions within the medium, for electrons with
energy of the order of 1 MeV. Only for electrons with energy greater than
100 MeV does the radiative process dominate. In a material of high atomic
number, for example, lead, the situation is different and the radiative process
exceeds any other energy loss process even for 10 MeV.
8.3.3 Loss of Energy by Collision
Collisions with the atomic electrons are the most important mechanisms of
loss of energy in matter, resulting in excitation or ionization of the material
traversed.
The loss of energy, dE, along the track dl (dE/dl) is proportional to the
electron density of the material traversed.
Applying quantum mechanical theory, one obtains,
dE
dl
col
∝
q
2
ρ(Z/A)
v
2
ln(1/I), (8.29)
where q is the electric charge, ρ is the density of the material, Z/A is the
quotient between the atomic number and the atomic mass, v is the velocity,
and I is the mean ionization potential.
8.3.4 Stopping Power
The quotient (dE/dl) is known as the linear stopping power of a material for a
charged particle with energy E.
As already mentioned, the loss of the energy of the electrons has two main
components, one due to losses by collisions and the other due to radiative
losses (we will not consider here losses of energy by nuclear reactions).
We can write,
S =
dE
dl
col
+
dE
dl
rad
, (8.30)
that is, the total stopping power is equal to the sum of the collision stopping
power and the radiative stopping power.
Massexpressionsareoften used,dividing thestopping power bythe density
of the material, ρ.
Since the stopping power is proportional to the density, the mass stopping
power is independent of the density.
In dosimetric issues, the restricted mass stopping power, (S/ρ)
Δ
, is often
used, where losses of energy greater than Δ are not considered.

Dosimetry and Biological Effects of Radiation 447
8.3.5 Linear Energy Transfer, L
Δ
, LET
Considering the effects of electrons in matter and specifically with relation
to the biological effects of radiation, in general, we are more interested in
how the energy is deposited in the material irradiated rather than in how
the particle loses its energy. Some collisions of the electrons produce new
electrons that have sufficient energy to escape from the track of the initial
electron leading to small tracks. These electrons are called δ-rays, and their
energy is not deposited in the neighborhood of the initial electron track.
The energy locally deposited can be determined by ignoring all the losses
of the initial electron energy that produce δ-rays with energy above a given
value Δ. Therefore, L
Δ
, the restricted linear energy transfer is defined that
equals the stopping power if we restrict consideration to energy losses less
than Δ, that is,
L
Δ
=
dE
dl
Δ
. (8.31)
Generally speaking, Δ is expressed in keV. If Δ =∞, then there are no
restrictions to the loss of energy, and one can write L
Δ
= S
col
, which is termed
unrestricted linear energy transfer.
8.3.6 Range and CSDA
We can define the range R of a given charged particle in a given medium as
the mean value, p, of the length of the track traveled until rest (neglecting
thermal movement).
Additionally, we can also define the projected range,
t
, of the charged
particle of a given type and initial energy, in a given medium, as the expected
value of the greatest penetration depth, t
f
, of the particle along its initial
direction. In Figure 8.4, the concepts of p and t
f
are outlined.
Another important concept, available in tables published by several
authors, is the continuous slowing down approximation (CSDA) range.
For electrons, the CSDA range is calculated using the equation
R
CSDA
=
T
0
0
dT
dx
−1
dT. (8.32)
For materials with low atomic number, Z, the value of t
max
is comparable
to the CSDA range, which has useful consequences for the application of
tables of the range of electrons. It may be possible that these quantities will
not be useful to describe the electron penetration due to the large number of
interactions.
