Lima J.J.Pedroso, de (ed.). Nuclear Medicine Physics
Подождите немного. Документ загружается.

298 Nuclear Medicine Physics
receptors. Regarding tyrosine kinase receptors, we can refer to the platelet-
derived growth factor-B receptor (PDGF-R), the vasoendothelial growth
factor receptor (VEGF-R), the insulin receptor, the insulin-like growth fac-
tor type-1 receptor, the EGF-R, and the fibroblast growth factor recep-
tors 1 and 4. With regard to the nontyrosine kinase receptors, we have
the leukemia inhibitory factor receptor and the β-subunit of interleukin-2
receptor [192].
Similar to trastuzumab for the treatment of breast cancer, other antibodies
and peptide antagonists have been developed to explore receptors as thera-
peutic targets. As a consequence, several receptors are considered as targets,
particularly the gastrin-releasing peptide receptor, the EGF-R, the PDGF, and
VEGF receptors.
As in the trastuzumab example, too, the use of ligands labeled with radio-
active isotopes can give information about the correct choice of therapy as
well as allow the monitoring of anticancer therapy [193].
Severalligands have been tested in this context. Examples are theligands for
the endothelin receptors such as PD156707 labeled with
11
C(
11
C-PD156707)
and FBQ3020 labeled with
18
F(
18
FBQ3020), the oxytocin analog ligands such
as DOTA-lys8-vasotocin labeled with
111
In (
111
In-DOTA-lis8-vasotocin), and
a specific ligand for melanoma, N-(2-diethylaminoethyl)-2-iodobenzamide
labeled with
123
I(
123
I-N-(2-diethylaminoethyl)-2-iodobenzamide).
6.4.13 Molecular Image as Target
After reviewing the several types of approaches to the use of NM, we can say
that molecular imaging provides information about many metabolic steps of
angiogenesis, hypoxia, and the different transformations and genetic changes
of cells.
In oncology, the added value of molecular imaging is the possibility of
obtaining functional information related to cell differentiation and thera-
peutic response. This means that discriminating between an inflammatory
reaction and a tumor recurrence, or knowing in advance whether the tumor
will respond to a specific therapy, gives the clinician the ability to wisely
choose which patients to include in a particular therapeutic protocol.
After analyzing the available possibilities for evaluating the various
metabolic pathways through functional imaging, when a malignant trans-
formation occurs, we can say that the agents that can differentiate between
inflammation and tumor recurrence are apoptosis and DNAmarkers, because
they are able to accumulate in cell nuclei. Agents that are able to provide
information in terms of predicting therapeutic response are usually enzy-
matic markers or ligands for membrane receptors. The most commonly
used enzymatic markers are those of glycolysis, hypoxia, and tyrosinase;
whereas the most important receptors are the estrogenic and the androgenic
receptors.

Imaging Methodologies 299
6.5 CNS: Physiological Models and Clinical Applications
6.5.1 Introduction
Now that you have reached this section, we may say that your CNS has
performed important and relatively essential tasks for the reading, appre-
hension, and understanding of the book’s subjects, matters, and goals. You
have probably not noticed the significantly high amount of substrates you
had to use nor even the cellular interaction and coordination mechanisms
you triggered. We are convinced that other readings have already given you
the opportunity to understand that the use of these substrates and fairly
complex mechanisms of the functioning of many millions (approximately
100 ×10
9
) of SNC cells can nowadays be investigated by means of medical
imaging. However, you may have also noticed that new medical imaging
techniques enable us to build functional maps [194] of the CNS, which, to
some extent, refute some of the functional anatomy knowledge so rigorously
and artistically developed by basic research [195] and clinical [196–198] sci-
entists. Very recently, we were stunned by some remarks about the need to
change concepts that we thought were well established: Contrary to the num-
ber of neuronal cells decreasing from birth on, as was previously thought, it
is now considered as certain that these cells can be re-created during our life
[199] to offset functional failure due to disease or trauma—neuronal plas-
ticity. Further, the concept of an anatomical and functional map known as
“homunculus” seems to be under reconsideration [200] in view of the results
of medical imaging research, especially functional magnetic resonance and
optical imaging [201].
The objective of this section is to make you think a little more deeply about
the anatomical and functional bases of CNS medical images, especially those
using radioactive molecules as radiopharmaceuticals. More than just inform-
ing you, our intention is rather to stimulate your curiosity and provide you
with some clues to develop your potential interest in functionally investigat-
ing the CNS by means of medical images obtained with radionuclide-labeled
molecules, that is, radiopharmaceuticals.
So, come along with us through a short summary of the anatomy, physi-
ology, pharmacology, and neuronal interaction mechanisms relevant to the
most important clinical applications in this field of knowledge.
6.5.2 Anatomical Basis
The anatomy of the CNS describes the morphology and macroscopic and
microscopic components of the brain and spinal cord. Here, we will only
remind you of some important aspects to help understand medical images.
The morphological study of the spinal cord and cranial pairs is almost
exclusivelycoveredby magnetic resonanceradiology, which playsthe leading
300 Nuclear Medicine Physics
role in diagnosing disease and evolutive changes. Radiopharmaceuticals are
much more often associated with the study and investigation of brain physi-
ology and pathophysiology, either supra- or infra-tentorial. Therefore,we will
focus on the brain, including the cerebellum and midbrain, with the medulla
oblongata and the pons, where we can find a reticular substance that is func-
tionally implicated in some neurological diseases currently under extensive
research.
6.5.2.1 Brain
Some authors argue that several anatomical features of the brain can be
used as an index of mental capacity [202], including brain weight, predom-
inance of left hemisphere, and the complexity of surface brain gyri of the
frontal and parietal lobes. All these have been reported in brain autopsies by
famous scientists. Even you if find it hard to fully believe in such morpho-
logical assumptions of human intelligence, you will have to consider their
importance in the assessment of qualitative and quantitative studies of brain
functions and diseases [203].
The average weight of an adult brain is approximately 1300 to 1400 g, and
the average volume is about 1400 mL. These figures from postmortem studies
are not error free, especially due to water loss. Structural studies with very
precise spatial and volumetric resolution are possible with MRI. They enable
us to establish such in vivo values by means of automated and semi-automated
algorithms for the extraction of the skull and extracranial structures[204]. This
methodologygives us an in vivo brain volume of 1286.4 ±133mLand 1137.8 ±
109 mLin normal male and female volunteers, respectively. The weight is then
calculated from these volumes using the equation
p
c
= V
T
×1.0365 g mL
−1
+V
L
×1.00 g mL
−1
(6.72)
in which V
T
is the total volume in milliliters and V
L
is the cerebrospinal fluid
volume in milliliters [205,206].
Brain volume is age-related that is, it increases exponentially during child-
hood and adolescence to achieve a peak between 12 and 25 years of age.
From then until the age of 80, the brain volume slowly decreases, with a
reduction of about 26% of the peak value between 71 and 80 years of age. At
that point, brain volume is lower than that of healthy 2- or 3-year-old children.
These findings are very similar to those reported in research using data from
postmortem studies [207].
The brain surface is composed of gray matter (cell bodies of neuronal cells)
gyri with gaps between them—the sulci—filled in by meninges. This surface
is divided into four lobes, as depicted in Figure 6.36: frontal (8), parietal (1),
temporal (7), and occipital (2) in each of the hemispheres. There are well-
defined geographical boundaries between the frontal and the parietal lobe—
central sulcus or the fissure of Rolando—between the temporal and frontal
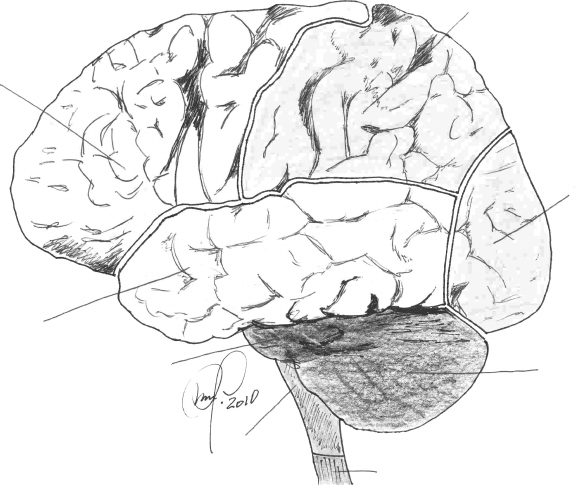
Imaging Methodologies 301
1
2
3
4
5
6
7
8
FIGURE 6.36
Brain surface anatomy as an intracranial component of the central nervous system. For reference,
the cerebellum (3), the medulla oblongata (5), the pons or pons Varolii (6), and the upper end of
the spinal cord (4) are shown.
lobe, including the most anterior and inferior regions of the parietal lobe—
Sylvianor lateralfissure.However,thedivision betweenthe restofthe parietal
lobe and the temporal lobe, between the parietal and the occipital lobes and
between the temporal and the occipital lobes, is simply functional and has no
well-defined geographical representation.
The parietal-temporal-occipital association cortex is located at the most
posterior and inferior region of the cortex (brain surface composed of the out-
ermost brain tissue made of cell bodies—gray matter). As its name implies,
it extends from the parietal cortex to the most posterior area of the temporal
cortex and invades the occipital cortex. Small sulci separate cortical areas with
different cytoarchitecture and functions (52 Brodmann areas; 15 were origi-
nally described) in each lobe. However, there are no geographical boundaries
in the above mentioned association cortex that make an accurate distinction
between parietal, temporal, and occipital lobes possible. Some other functions
arereferenced to cortical areas in two other brain lobes called subsidiary lobes;
one is the insular cortex, which forms the medial wall of the Sylvian fissure or
sulcus, and the other is the limbic cortex or lobe, located above the most ante-
rior (cephalic) or rostral region of the midbrain next to the corpus callosum.
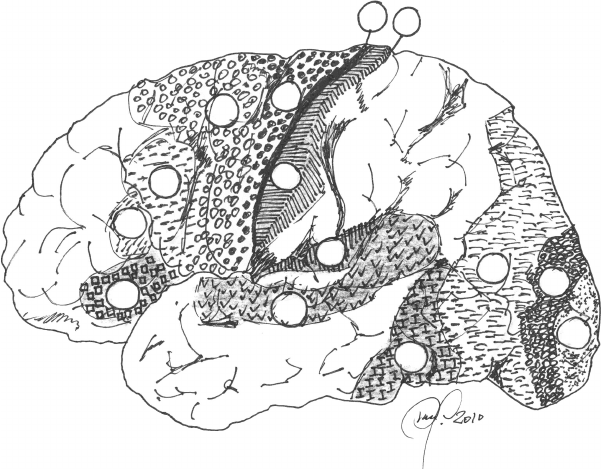
The Brodmann functional areas (Figure 6.37) are a very important working
302 Nuclear Medicine Physics
medium for imaging experts and neurosurgeons conducting brain functional
provocation studies during presurgery preparation in neuro-oncology and
epilepsy.
Within the brain, besides lateral ventricles and most of the white substance
made of axons (some of which are very long) of neuronal cells with soma
(cell body) in the cortex, there are also gray matter nuclei with very impor-
tant regulation and transition functions. These nuclei accommodate synapses
(transitions from presynaptic neurons to postsynaptic neurons) in which neu-
ronalinformation is transmitted either electrically or chemically. Several types
of neurotransmission belonging to different physiological–pharmacological
systems, with such different actions as stimulation or inhibition, may coexist
in the same nuclei. These nuclei can be identified or visualized only by means
of tomography techniques. This chapter does not set out to describe tomo-
graphic anatomy in detail, but in the next section we give an overall idea of
it. We briefly describe some of the problems we face when comparing images
from the same brain section, in the same subject, showing different functions
in the same structures.
1
19
18
17
37
2
3
4
41
44
22
45
47
6
FIGURE 6.37
Brodmann areas on the outer surface of the left hemisphere. Some are worth mentioning as an
example: 1, 2, and 3 (sensory); 4 and 6 (motor and extrapyramidal); 17, 18, and 19 (visual); 22, 37,
41, and 47 (auditory); 44 and 45 (Broca’s speech production).
Imaging Methodologies 303
6.5.2.1.1 In Vivo Comparative Anatomy
The knowledge about CNS morphology and structure, especially the brain,
was fundamentally based on postmortem dissection studies. Even the micro-
scopic structure, described so clearly and accurately by Ramón y Cajal, was
characterized at the expense of painstaking cadaver studies. Today, com-
puterized tomography and MRI studies detect important changes in brain
morphology and architectural structure with a spatial resolution of less than
one millimeter in some cases. Compared with autopsy-derived data, the CNS
structural design above and below the brain tentorium is obtained in an
elegant manner and can be shown in concordance and with the same orienta-
tions. The advent of new sequences used by the most recent MRI instruments
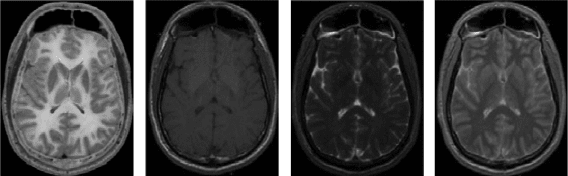
and software broughtfunctionality to brain images. Each image obtained with
a different sequence also has a different meaning (Figure 6.38).
Even this ability of MRI to offer us high resolution, fine images of the brain
structures with functional characteristics does not depict “life” that is, cog-
nitive function, blood flow, perfusion, or substrate use. To do that, we need
in vivo functional markers, such as radiopharmaceuticals. Figure 6.39 shows
slices taken at the same level as those in Figure 6.38, but this time each slice
has its functional and chemical meaning in vivo. On the left, the distribution
of a radioligand (
99m
Tc-exametazime) whose brain uptake is proportional to
the regional cerebral blood flow (perfusion) is shown. On the right, the dis-
tribution in the same subject (normal volunteer), at the same level, of another
radiopharmaceutical (
123
I-ioflupane), whose image shows the uptake distri-
bution in the basal ganglia, which is directly proportional to the distribution
of dopamine transport systems located on the membrane of the presynaptic
neuronal end, is shown.
All these images need suitable coordinates for intermodality and interfunc-
tion referencing. By defining planar representation grids (such as those in
Figure 6.39) and, more importantly, volumetric representation grids, such as
those defined by Talairach [209], it is possible to superimpose images using
FIGURE 6.38
Images of the same slice (at the level of basal ganglia, with caudate nucleus, lentiform nucleus,
and thalamus in both hemispheres) of one cadaver. The leftmost image is a picture (gray shades)
of the cadaver slice, followed by sequence MR images to demonstrate, from left to right, T1, T2,
and proton density.
304 Nuclear Medicine Physics
100
80
60
40
20
0
20
40
60
60 40 20 0 20 40 60
mm
60 40 20
0
20 40 60
mm
mm
100
80
60
40
20
0
20
40
60
mm
FIGURE 6.39
Comparative functional anatomy obtained from the same normal volunteer, at the level of basal
ganglia, in a transversal slice parallel to the anterior commissure-posterior commissure reference
line, as is usual in neuroimaging. The two slices obtained on different days are referenced to
a grid (1 cm
2
units) similar to the one used by Talairach in his Co-Planar Stereotaxic Atlas of the
Human Brain. (Adapted fromJ. Talairach,P. Tournoux.Translated by Mark Rayport. Thieme Medical
Publishers, Inc., Stuttgart, New York, NY, 1988.)
software and relatively complex algorithms. These images are the coregistra-
tion of anatomical and functional information. Further, these images allow
us to obtain composite maps of the correlation between different functions.
Coregistration techniques are already part of routine clinical application,
for instance, in PET studies with
18
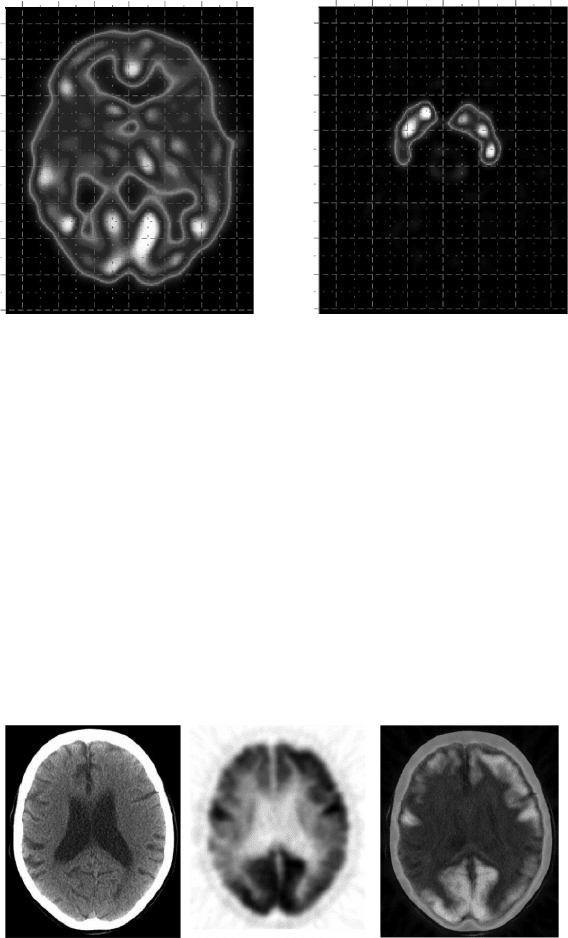
F-DG and CT. Figure 6.40 illustrates an
example of this. The image on the right is the coregistration of the voxel-
by-voxel information from the two images on the left. The leftmost of these
FIGURE 6.40
The images are of transversal slices at the same level (lateral ventricles). From left to right: x-
ray attenuation map (computerized axial tomography, CAT, or CT), distribution map of brain
glucose metabolism (
18
F-DG), and voxel-by-voxel coregistration of both images, that is, glucose
metabolism distribution superimposed on the attenuation map (CT).
Imaging Methodologies 305
represents the attenuation map, and the middle image represents a distribu-
tion map of glucose metabolism in the brain. In this specific case, a marked
reduction of brain metabolism in the parietal cortices (more to the right than
to the left) can be seen, and in the frontal cortices, too (lower than in the pari-
etal cortices), with the metabolism conserved in the motor cortices of both
hemispheres and in the occipital visual cortex. The leftmost CT image only
shows slight ventricular dilatation without further significant abnormalities.
This pattern of cortical hypometabolism is typical of a CNS degenerative dis-
ease evolving into memory loss and other cognitive disturbances typical of
dementia. This is a case of asymmetric Alzheimer’s dementia.
This example shows the simplest method of coregistering images, as the
object (brain) is encased in a static shell (as long as the subject does not move
their head), and the positioning of each voxel containing different informa-
tion is the same in the two acquisitions. In this case, there is no need for
external fiducial markers to act as a reference while the superimposition
program is run. These fiducial markers are contained in the images them-
selves. The two instruments that acquire the images are contiguous, and the
object just moves automatically and with (computer determined) precision
between the CT (left image) and PET (middle image). In other cases, the phys-
ical proximity of data acquisition instruments is not compatible with object
immobilization (the subject), so coregistration has to be digitally performed,
based on more elaborate techniques (see Section 6.3.3). Most of these tech-
niques require the use of mathematical algorithms to manipulate the data in
several ways and directions; consequently, the final product is not error free.
To complicate the coregistration system even more, images have to be often
rescaled, because, during acquisition, voxels of different sizes are used and
this immediately introduces a scale-correction problem. This can be solved,
but artifacts may arise. This can be seen in a recent work comparing the
coordinates from several methods used for brain imaging functional data
analysis [210].
6.5.2.2 Cerebellum
The cerebellum, formed by two lateral lobes and a median lobe, the vermis, is
located under the occipital lobes and is separated from them by a membrane
called the tentorium cerebelli. That is why it is considered infratentorial, simi-
lar to the midbrain, the medulla oblongata, and the pons. The main cerebellar
function is motor coordination, which is very important for maintaining body
posture and balance. For this reason, it receives axons from the brain cor-
tex after they have crossed the middle line. The right cerebellar hemisphere
receives axons originating in the left brain hemisphere and vice versa; the
left cerebellar hemisphere receives axons from the right brain hemisphere.
The cerebellum and periphery (muscular structures) are connected through
the superior, middle, and inferior cerebellar peduncles on the right and left
sides.
306 Nuclear Medicine Physics
6.5.2.3 Midbrain (Mesencephalon)
Referencesto thebrain stemarecommon in theliteraturepublished inEnglish.
This includes the cerebellum, the uppermost part of the spinal cord, the
medulla oblongata, and pons. The latter two together form what is called
the midbrain pons in the Anglo-Saxon literature. In Portugal, we consider
the mesencephalon as the pons or pons Varolii, located between the medulla
oblongata below and the cerebral base above, to which it is connected by the
cerebral peduncles. All the axons communicating between the brain and the
spinal cord and passing through the cerebellum have to pass through the mid-
brain. For the purpose of this book, this section will refer both to the actual
midbrain itself and to the Anglo-Saxon brain stem, because we want to make
it clear that the 12 pairs of cranial nerves that mediate the senses, that is, sight,
hearing, taste, and smell, arise from the lateral sides of the brain stem. Also, all
of them have specific representationin the brain through connections between
their source nuclei and the cortical areas directly related to their functions.
However, it is even more important to mention the formatio reticularis or retic-
ular formation or matter; although this is chiefly a mesencephalic structure,
it also extends to the brain stem from the upper end of the spinal cord to the
base of the cerebral peduncles. The reticular formation or matter is a mixture
of interneuronal arrays forming an intricate network of functional regulation
and modulation between sensitive and sensory neuronal input and peripheral
motor region output of what we call the reflexes.As far as we know, it is in this
mesencephalic area that some inputs are enhanced and maximized, whereas
others are minimized or even suppressed. The main functions of the reticu-
lar formation or matter are based on four types of connections: (1) upward
to the thalamus and cortex, which act as an activation or warning system.
This is especially important in pain perception and its unpleasant connota-
tion; (2) downward to the spinal cord and periphery, which modulate skeletal
muscle tension; (3) input, using cranial pairs, allowing it to modulate heart
rate and blood pressure originating from the carotid sinus (neurovegetative
reflex); and (4) downward through the reticulospinal bundle, ending at the
dorsal horn of the spinal cord and attenuating pain by reducing the effects of
pain-mediating sensitive nerves. In brief, it serves as a high-level controller
between the spinal cord and the brain, which helps responses to undesirable
events,always on thealert andreadytochoose thebestresponseunder normal
functional conditions. Due to its micro-structural complexity, with many axon
branches and neuronal interconnections, reticular formation or matter dys-
function causes poorly identified syndromes, which are sometimes medically
disparaged [211,212].
6.5.3 Physiological and Pharmacological Basis
Substrate input to the CNS obeys pharmacokinetic laws. If we exclude absorp-
tion, that is, the route from the administration site to blood circulation, this
Imaging Methodologies 307
includes a distribution that is directly proportional to local blood flow and,
consequently, to the percentage of blood volume per tissue mass unit (for
the purpose of our case the CNS, chiefly the brain). The substrate may be
present in blood, more specifically in plasma or serum, in its free form or
bound to plasma proteins or blood corpuscles, that is, red blood cells, white
blood cells, and platelets. The substrate concentration available to appear in
brain tissue cells is mainly the substrate concentration in its free form. How-
ever, according to pharmacokinetic principles, a substrate or a drug may be
metabolized (hepatically or by other means) or excreted (hepatically and/or
renally), which tends to reduce plasma-free concentration. This means that
substrates (for the purpose of this book on NM, radiopharmaceuticals) are,
afterintravenous administration,available tobrain tissuedepending on blood
flow distribution. They will have to cross the blood–brain barrier and find
their more-or-less specific binding sites; they may be metabolized in either
the CNS or other organs, and finally a part of the molecules will be excreted,
either via the liver or the kidneys.
The brain and the rest of the intracranial CNS are irrigated by the carotid
(carotid arteries run along the neck’s anterolateral side) and vertebral (ver-
tebral arteries run beside the cervical spine) arterial systems. These arterial
systems communicate with each other both extra- and intracranially. Intra-
cranial communication is better organized. The Circle of Willis is well known:
it is formed by the anterior and posterior communicating vessels connect-
ing anterior and posterior cerebral arteries, respectively. If not permanently
patent, these communications become so in pathological states, so that the
right system may easily supply blood to the left system and vice versa, and
the posterior system may supply blood to the anterior system and vice versa.
Besides these communications, there is also a capillary distribution network
that allows communication among all neighboring vascular territories in the
brain, especially at their boundaries, also called watershed boundaries. It is
essentially here that the development of reactive hyperemia after vascular
insult can be observed, along with the formation of new collateral capillary
vessels to supply ischemic territories in areas of ischemic penumbra, that is,
the halo surrounding the territory without blood supply.
The vascular and capillary network of the brain is not a rigid system of
branching tubes. Quite on the contrary, as noted earlier, it is an intricate,
flexible, and dynamic system of multidirectional vascular routes connecting
terminal arterioles to venules. The main driving force for regional cerebral
blood flow is the so-called perfusion pressure. This is the difference between
the arterial influx pressure and the outflow pressure in the veins. Cerebral
blood flow has self-regulation mechanisms that maintain a relatively con-
stant blood flow even under the influence of a number of factors. Under
physiological conditions, any change in brain metabolism evokes a blood
flow change of similar amplitude and direction. The brain vascular network
reacts to changes in arterial CO
2
concentration, in ionic (Ca
2+
,K
+
) concen-
tration, and in the interstitial concentration of drugs such as adenosine and
