Lima J.J.Pedroso, de (ed.). Nuclear Medicine Physics
Подождите немного. Документ загружается.

308 Nuclear Medicine Physics
neuropeptides. All these can be used to trigger vascular stress and thus, neu-
ronal metabolism. Under pathological conditions, in particular during the
development of brain ischemia, flow-metabolism dissociation occurs. Rather
than showing the normal behavior described above, blood flow changes may
not produce recognizable metabolism changes or trigger opposite metabolic
responses.
Once they arrive at the capillaries, the substrates or drugs then face cell
barriers separating blood from brain tissue. These cell barriers comprise
the capillary endothelium, a basement membrane, and astrocyte endfeet
(podocytes) amid a variable amount of interstitial liquid. These barriers are
explained next.
6.5.3.1 Barriers
The components of the CNS aredemarcated by three types of barriers that sep-
arate blood from the brain parenchyma with extracellular fluid (blood–brain
barrier), blood from cerebrospinal fluid (blood–cerebrospinal fluid barrier),
and cerebrospinal fluid from the brain parenchyma (brain–cerebrospinal fluid
barrier). These barriers play an important role in the formation of images of
the CNS. Intrathecal administration (in the cerebrospinal fluid of the spinal
cord subarachnoid space) of a myelography solute shows cerebrospinal fluid
distribution and, hence, the state of the brain–cerebrospinal fluid barrier.
This imaging method has been superseded by the tissue definition charac-
teristics of MRI, which enable an evaluation of the brain–cerebrospinal fluid
interface with very good spatial resolution. However, MRI does not provide
information on cerebrospinal fluid kinetics, especially on its production and
absorption. These can be better defined by intravenous administration of
sodium pertechnetate (
99m
Tc), which diffuses from blood serum into intra-
ventricular space through choroid plexi (blood–cerebrospinal fluid barrier,
i.e., production) and by intrathecal administration of, for instance, indium-
111- or technetium-99m-labeled DTPA, which, similar to cerebrospinal fluid,
will be absorbed into blood through the arachnoid villi or arachnoid gran-
ulations and through the CNS and pia mater capillary walls. The other
barrier—the blood–brain barrier—is highly selective and regulates substrate
uptake. It therefore regulates the uptake of potentially useful radiopharma-
ceutical molecules for the investigation of physiopharmacological functions
and processes of the brain.
6.5.3.1.1 Blood–Brain Barrier
In the early twentieth century, Ehrlich [213,214] found out during his research
studies on chemotherapeutical agents that intravenous dye injection did not
cause any coloring in the brain, unlike all the other organs. Later, when study-
ing how various substances moved from blood into the brain and what effects
these substances had on the CNS, Stern and Gautier [215] realized that (1)
there was a barrier between the blood and the brain that excluded some
Imaging Methodologies 309
substances from the CNS and they called it the blood–brain barrier—barrière
hématoencéphalique; (2) substances unable to cross this barrier could not be
found in the cerebrospinal fluid; and (3) some of the substances unable to
cross the barrier moved from the cerebrospinal fluid to the CNS, for exam-
ple, iodine. Although not completely right, Stern and Gautier’s hypothesis
established for the first time the selectivity feature of the blood–brain bar-
rier [217]. It still holds good today and is in many ways better understood.
The other very important feature of the blood–brain barrier, liposolubility,
was suspected by Becker and Quadbeck [218] and determined with the use
of quantitative analysis algorithms with defined parameters for membrane
crossing (rate constants) [219,220].
The concept of blood–brain barrier was thus based on two physical-
chemical characteristics: selectivity and liposolubility. Although liposolubil-
ity is easy to understand and locate in cell membranes of capillary wall cells
(endothelial cells) and their adjacent cells (astrocytes), the barrier selectivity
is far more difficult to anatomically ascribe. Some authors [221,222] con-
cluded that the endothelium was responsible for selectivity, whereas others
[223] noted that the endothelium was covered by a protoplasmic layer—the
podocytes—of astrocytes closely knitted by a basement membrane and that
there were no apparent gaps in these membranes [224]. The contact points
between two endothelial cells without gaps are called tight junctions and
are thereafter regarded as the structural location of the blood–brain barrier
according to Brightman and Reese [225]. These researchers showed that after
intraventricular injection, horseradish peroxidise diffuses through the inter-
stitial cell space around brain capillaries but cannot reach the bloodstream
and stops next to the tight junctions.
The blood–brain barrier [226] is permeable to liposoluble substances and is
apparently built of brain capillary endothelial cells, with their tight junctions.
Another of its properties is selectivity. Together, these two features explain
the results of many studies showing the absence of pharmacological effects
of nonlipophilic drugs on the CNS after intravenous administration. How-
ever, the same nonlipophilic, hydrosoluble drugs can cross the cerebrospinal
fluid–brain barrier when given by intrathecal route and can cause measurable
pharmacological effects.
6.5.3.1.2 Structural Components of the Blood–Brain Barrier
The blood–brain barrier concept has been evolving a long time, and it is still
fundamentally based on the CNS capillary endothelial cells. Their functional
and morphological features are modulated by the astrocytes with which they
are in very close contact [227,228]. The endothelial cell layer has no gaps
as such. On the contrary, due to the intervention of astrocytes, there are
tight junctions, which build up a barrier that can only be crossed by means
of transport mechanisms in the cell barriers, without any type of passage
through pores. The modulation of the endothelial layer by glial cells was
shown by cross-experiments in which a nonvascular cell tissue transplanted
310 Nuclear Medicine Physics
into bird embryonic coeloms originated a blood–brain-type barrier [229]. On
the other hand, when coelomic tissue grafts were transplanted into embry-
onic brain tissue, capillaries were seen to have the same characteristics as
peripheral capillaries, that is, they had pores in the endothelial membranes
and there were no tight junctions. This was later confirmed by Janzer and
Raff [230] when they showed that astrocytes are responsible for forming tight
junctions in nonneuronal tissue endothelial cells. To supplement these mod-
ulating and inducing capacities of astrocytes on endothelial cells, it was also
shown that these glial cells increase the frequency, size, and complexity of
tight junctions that are formed in cell growth media. Cultured endothelial
cells develop fragmented tight junctions of about 13 μm, whereas the size
of endothelial cells grown together with astroglia increases to approximately
165 μm.
Endothelial cells and astrocytes share yet another structure—the peri-
vascular basement membrane (BM)—which is composed of a fibrous base-
ment sheet with high electron density inserted between two much less dense
electronic layers (one in contact with the endothelial cell membrane and the
other in contact with the astrocyte cell membrane) The basement sheet con-
tained glycoproteins, laminin, fibronectin, variable amounts of different types
of collagen, and glycosaminoglycans [232].
This means that the blood–brain barrier should be thought of as a phys-
iological barrier composed of three structures: endothelial cells with tight
junctions, a perivascular basement membrane, and astroglia (astrocytes).
These three components of the blood–brain barrier make it selective and,
according to Crone [233], show well-defined physiological features, which
are as follows:
1. It is a cell layer ensheathed by a continuous basement membrane
2. Endothelial cells are connected to one another by tight junctions
3. Permeability to hydrophilic substances (nonelectrolytes) is very low
4. Ionic permeability is very low
5. Water conduction is very low
6. Passive permeability to solutes is mainly through intercellular junc-
tions
7. It has facilitated transport mechanisms for some organic solutions
8. It shows stereospecificity is saturable and allows competitive inter-
actions
9. It has induction mechanisms
10. It has high electric resistance (∼2000 cm
2
) [234]
11. Its permeability is increased by high osmolarity levels
12. Na
+
–K
+
pumps are located on the abluminal membrane of endo-
thelial cells

Imaging Methodologies 311
All these characteristics help one way or the other to define the most suitable
transport mechanism for each substance or substrate present at the blood–
brain barrier during regional blood flow distribution [235]. For instance, it is
increasingly accepted that endothelial cells are active in both the regulation
of brain extracellular fluid components and the brain’s amino-acid content
[236]. Active amino-acid transport occurs on the abluminal membrane, the
facilitated transport of other amino acids is regulated on the luminal side, and
the neutral and long amino acids cross the barrier via mechanisms located on
the two sides of the endothelium [236].
6.5.3.1.3 Transport Mechanisms
Intracerebral radiopharmaceutical uptake is governed by the same properties
as solute uptake, which is primarily determined by the nonionized and, there-
fore, lipophilic fraction of each of the components in question. The product
uptakeand its relationto thecirculatingportion not taken up can be quantified
by the indicator diffusion technique. According to Crone [237], permeability
across the blood–brain barrier is given by the following formula:
P =−
F
S
ln(1 −E), (6.73)
in which P is the permeability or partition coefficient, F is the blood or plasma
flow, S is the capillary surface area, and E is the initial extraction fraction of
the substance in question.
6.5.3.1.3.1 Influence of Liposolubility or Lipophilicity Although the first con-
tact and limitation to the passage of solutes from the blood to the CNS is the
endothelialcellmembrane, dueto itspropertiesoflipophilicity and selectivity,
the blood–brain barrier is not fully impervious to water and polar solutions.
Apparently, water and some low molecular weight polar solutions can pass
through hydrocarbon chains in cell membrane lipid bilayers. Further, the
tight junction dynamics is such that in some cases a small percentage of them
may open and allow the nonselective crossing of small ions [238]. According
to Equation 6.73, during the first passage through the blood–brain barrier,
the higher the permeability (partition coefficient), the higher the amount of
substance taken up by the CNS. As the (endothelial) capillary permeability
increases, the amount of substance crossing the blood–brain barrier depends
more and more on regional blood flow, to the extent that in a theoretical
situation of maximum possible lipid solubility, the CNS uptake is linearly cor-
related with brain flow, as shown by Equation 6.74, which defines diffusion
ability [237,239]:
P
S
= Q ln(1 −E), (6.74)
in which P
S
is ability to diffuse through the barrier, that is, the permeabil-
ity surface product, Q is cerebral blood flow, and E is substance extraction.
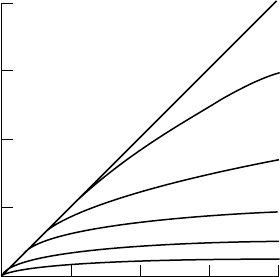
312 Nuclear Medicine Physics
50
5
2
1
0.5
0.2
432
Q – blood flow (mL
· g
–1
· min
–1
)
PS (mL
· g
–1
· min
–1
)
Q.E (mL · g
–1
· min
–1
)
1
1
2
3
4
FIGURE 6.41
Theoretical curves based on Equation 6.74, also called the Crone–Renkin equation, in which
product flow by extraction (Q.E) is compared with blood flow (Q). Q.E represents blood volume
at any time (minute) at the barrier, and it is directly proportional to substance flow or the amount
entering the brain per minute. Given that the equation assumes that back-diffusion is zero, the
Q.E product is also proportional to unidirectional substance flow from blood to brain through
the blood–brain barrier.
The graph in Figure 6.41, modified from Bradbury [240], shows the relation
between cerebral blood flow and blood extraction into cerebral tissue for
different P
S
values.
Considering that the endothelial capillary surface is linear and that there
is no back-diffusion from the brain back to the blood, the brain uptake of
a given compound is not significantly affected by blood flow when this is
higher than 0.5 mL g
−1
min
−1
and the P
S
is lower than 0.2 mL g
−1
min
−1
.
However, for P
S
of5mLg
−1
min
−1
or higher (meaning high extraction and
lipid solubility), brain uptake is chiefly determined by blood flow, and so it
can be said that brain uptake is limited by regional cerebral blood flow. Con-
sequently, this high liposolubility property has been used as the key factor
in the development of radiopharmaceuticals for brain function studies, espe-
cially of regional blood flow, for example,
123
I-labeled iodoantipyrine and
85
Krypton. Another example of high liposolubility is
133
Xenon. In this case,
there is overt back-diffusion through the barrier, because is neither metabo-
lized in tissues nor does it bind to any cell structure unless its concentration
in blood is the same as or higher than in the brain. After peak concentra-
tion (for the dose administered) is achieved in the brain, if there is no arterial
input of
133
Xenon all molecules entering the bloodstream will be taken down-
stream (from arteries to veins) in proportion to regional capillary blood flow
(washout rate).
Imaging Methodologies 313
6.5.3.1.3.2 Influence of Osmolarity or Osmotic Pressure For the examples of
interest in this chapter, osmolarity explains the movement of water molecules
through the blood–brain barrier. The P
S
of the blood–brain barrier is hard to
determine, even for radionuclide-labeled water.Water diffusioninto the brain
and from there to the bloodstream is partially dependent on blood flow and
is totally dependent on it for relatively low flow levels [241]. Paulson et al.
[242] calculated filtration coefficient or rate values (i.e., membrane osmotic
permeability) higher than the diffusion permeability rate, with a 4:3 ratio. This
led them to conclude that any osmotic pressure gradients across the blood–
brain barrier are higher than potential hydrostatic pressure gradients. Any
initial water filtration due to hydrostatic pressure will be immediately limited
byan osmoticgradient ofhigher value and in theopposite direction.However,
if for any reason there is a rupture in the blood–brain barrier, filtration of
substances from the plasma to cerebral tissues (interstitial space) is possible,
with or without proteins. This is most likely the mechanism underlying so-
called vasogenic cerebral edema.
6.5.3.1.3.3 Influence of Binding to Plasma Proteins Binding to plasma proteins
significantly influences pharmacokinetics and interaction between pharma-
ceuticals and substances in the bloodstream. Free and plasma protein-bound
states are consideredto have a significant influence on brain uptake. However,
some authors still disregard this, even in relatively recent studies. In general,
the drug’s lipophilic portion readily crosses the blood–brain barrier, whereas
nonlipophilic molecules stay in the bloodstream to be excreted or deposited
in tissues or organs other than the brain. Besides this, the amount of the
plasma protein-bound fraction also directly influences the passage through
the blood–brain barrier. This is further complicated by the type of bond, that
is, reversible or irreversible. A reversible bond introduces an important vari-
able in the amount of drug available to cross the barrier (free fraction), which
changes according to the free and protein-bound fraction concentration. In an
irreversible bond, the concentration of the free fraction available to cross the
barriers is constant.
However, there are some exceptions to this rule that are not fully under-
stood. Despite having significant serum protein-bound fractions, the steroid
hormones
3
H-progesterone and
3
H-testosterone show brain uptake percent-
ages of between 80% and 100%, regardless of their serum protein-bound
fraction concentration [243]. Another example is tryptophan, 90% of which
is bound to plasma albumin. However, brain uptake after intracarotid bolus
injection of
14
C-tryptophan is not influenced by the presence of albumin [244].
Seemingly, tryptophanwas separated, as though sucked, fromits bindingpro-
tein (albumin) during the first passage through the cerebral capillary. Three
mechanisms can be suggested to explain this:
1. Fast albumin dissociation due to strong brain uptake, with a conse-
quent very fast reduction in the free fraction.
314 Nuclear Medicine Physics
2. Differential changes in local physiological pH.
3. Metabolite displacement possibly due to antagonism, which can
change the association constant of the tryptophan–albumin complex.
The exact mechanism is still unknown. Nevertheless, it seems that the disso-
ciation constant for the tryptophan–albumin complex is significantly higher
than the dissociation constant measured in vitro [245].
In fact, the explanation for both this event and what happens with thy-
roid hormones, which is similar, is still unclear. Thyroid hormones are polar
compounds, but they enter the brain by a specific transport mechanism
[246]. However, hormones are still introduced into cerebral capillaries with
their serum protein-bound fraction. The hormones are disconnected from the
proteins within capillaries by mechanisms that are also still unknown.
6.5.3.1.3.4 Other Influences Binding to erythrocytes (red blood cells) and
endothelial sequestration can similarly reduce the brain uptake rate of
lipophilic compounds.
Washout of molecules from the brain into the bloodstream and its rate of
change are different from what occurs in extracerebral tissues. Here, high
molecular weight molecules or suspended particles that cannot cross the cap-
illary endothelial barrier from the interstitial fluid to blood are removed by
lymphatic drainage. In the brain, washout of even low molecular weight polar
solutes is difficult. These cannot cross the blood–brain barrier from the inter-
stitial tissue to blood unless there is a specific transport mechanism through
the endothelium. Nonetheless, there are nonspecific removal mechanisms for
several polar solutes (nonlipophilic) that slowly penetrate the interstice, such
as
35
S-sulphate and
14
C-inulin. There are three, nonmutually exclusive mech-
anisms [247] to explain the existence of convection in washout of polar solutes
from the brain interstice to blood:
1. Secretion of interstitial fluid through the capillaries to the blood
2. Circulation through perivascular spaces
3. Wave movements, particularly in arterial spaces
6.5.3.1.3.5 Specific Transport within the Blood–Brain Barrier If lipophilicity
were the only determining feature of the blood–brain barrier crossing rate to
enter cerebral tissues, then the brain would be deprived of adequate amounts
of several substrates it needs to survive, such as glucose and many amino
acids. Due to their relatively low partition ratio, these substrates cannot cross
the blood–brain barrier by simple (i.e., nonfacilitated) diffusion mechanisms.
Monosaccharides, carboxylic acids, neutral amino acids, dicarboxylic acids,
and some amines can cross the barrier with the help of active or facilitated,
specific transport mechanisms. These are stereospecific, saturable transport
mechanisms that can be inhibited by specific antagonists. They, therefore,
Imaging Methodologies 315
depend on energy expenditure. So, for example, monosaccharides have a
highly stereospecific transporter with high affinity for
D-glucose. Peptides
are another example, presenting as large, polar molecules [248,249]. With-
out an appropriate, specific transport mechanism across the blood–brain
barrier, it would be impossible for peptides to play their important role in
neurotransmitter synthesis or to be neurotransmitters themselves (Table 6.2).
6.5.3.2 Factors Influencing Cerebral Capillary Permeability
Rather than being defined by the permeability ratio, cerebral capillary per-
meability is usually defined by the above mentioned P
S
product, because
the exact endothelial area by unit of cerebral tissue weight is unknown. Per-
meability to liposoluble (lipophilic) substances is primarily influenced by
regional cerebral blood flow distribution and, of course, by the P
S
product.
Consequently, permeability increases with flow, for instance, in hypercap-
nia and, thus, capillary vasodilatation and diminishes with regional cerebral
blood flow reduction during hypocapnia, causing vasoconstriction [253]. Spe-
cific transport mechanisms, both facilitated and active, can be modulated by
a very variable number of factors [254–257], which change according to brain
tissue energy needs. Fasting [255] and porto-caval anastomosis [258] dramati-
cally change selective transporters in the blood–brain barrier, most likely due
to the marked metabolic changes they cause.
6.5.3.2.1 Importance to NM
The special selective permeability characteristic of the blood–brain barrier, in
particular its lipophilicity, prompted the research for development of radio-
ligands able to cross the barrier and show brain functions through uptake by
CNS cells. However, some radiopharmaceuticals that do not cross an intact
TABLE 6.2
Examples of Transport Systems through the Blood–Brain Barrier
Transport Michaelis
(Maximum Constant
Capacity) (V
max
) (Apparent)
Substance Type (Example) (μmols/g per min) (K
m
) (mM)
Monosaccharides [250] (D-glucose) 2–4 7–11
Monocarboxylic acids [251] (
L-lactate) 90 2
Neutral amino acids [251,252] (
L-leucine) 30–60 0.025–0.1
Basic amino acids [251] (
L-arginine) 8 0.09
Amines [251] (choline) 11 0.34
Nucleosides [251] (adenosine) 0.75 0.025
Purines [251] (adenine) 0.05 0.01
316 Nuclear Medicine Physics
blood–brain barrier are interesting for specific clinical applications, especially
in oncology and neurological infectology.
After intravenous injection, sodium pertechnetate (
99m
TcO
−
4
) presents at
the blood–brain barrier in its ionic form, hence it does not cross an intact
blood–brain barrier. However, it is secreted into cerebrospinal fluid through
the choroid plexi (blood–cerebrospinal fluid barrier), giving an idea of how
this barrier works in the blood–cerebrospinal fluid direction. Sodium pertech-
netate was the first radiopharmaceutical to mark an intact blood–brain barrier,
but negatively that is, by showing the normal brain as an empty image and
revealing uptake in areas of barrier disruption, as in cerebral infarction and
tumors, in which sodium pertechnetate accumulates in extracellular fluid. Its
main disadvantage is choroid plexi visualization, which in less-experienced
hands can lead to false positive results. Therefore, whenever sodium pertech-
netate is needed, its use should be preceded by the secretion blockade of
choroid plexi, for instance, with sodium perchlorate. More recently, other
radiopharmaceuticals have been used (
99m
Tc-labeled glucoheptonate and
diethylene triamine pentaacetic acid) that arenot secreted by the choroid plexi
and, thus, do not need blocking drugs. Thallium chloride (
201
Tl) is another
example of a radiopharmaceutical that does not cross an intact blood–brain
barrier. In recent times, the clinical importance of these radiopharmaceuticals,
especially thallium chloride, has gained a new life, because they can be used
in the differential diagnosis of cerebral tumor recurrence and fibrosis after
surgery and/or irradiation [259] and in the differential diagnosis between
infection (cerebral toxoplasmosis) and intracerebral lymphoma in patients
with viral immunodeficiency syndrome [260].
Thelipophilicity of the blood–brain barrierthat givesit selectivityprompted
the development of neutral and lipophilic compounds able to cross an intact
barrier. These compounds (radioligands) behave like chemical microspheres,
that is, they cross the endothelial-basement membrane-astrocyte barrier from
blood to brain to turn into polar compounds in the cerebral tissue, thus
becoming unable to cross the barrier in the opposite direction.
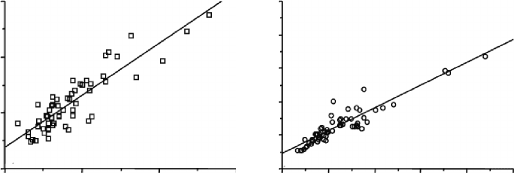
This causes intracerebral retention directly proportional to tin (
113
Sn)-
radiolabeled microsphere distribution in the capillary network (Figure 6.42)
and, thus, proportional to the regional cerebral blood flow distribution.
This proportionality happens with highly varied levels of regional cere-
bral blood flow. In the white matter (lower blood flow levels), there is
apparently an inflation of flow values by perfusion markers compared with
microspheres. The correlation is better in the usual flow level range of gray
matter (the regression line shows a significantly high correlation coefficient,
r = 0.924).
These radiopharmaceuticals, generally called cerebral or regional cerebral
blood flow perfusion markers (rCBF), have a clinical application with SPECT
to examine patients with cerebral ischemia and in the differential diagno-
sis of dementia, especially frontotemporal dementia and Alzheimer’s dis-
ease.
123
I-IMP (iodo-amphetamine),
99m
Tc-ECD (l, l-ethylcysteinate dimer),
Imaging Methodologies 317
200 400 600 800 1000 Bq/MB
q
Bq/MBqBq/MBq
113
Sn-Microspheres
113
Sn-Microspheres
99m
Tc-HMPAO
Gray matterWhite matter
00 100 200 300
0
200
y = 90.954 + 0.68211x
r = 0.924
y = 39.322 + 0.93719x
r = 0.885
400
600
800
1000
0
100
200
300
FIGURE 6.42
Comparison between distribution of a lipophilic cerebral blood flow marker (
99m
Tc-hexa-
metazime) and distribution of
113
Sn-microspheres in white and gray matter samples from dog
brain (Adapted form D. C. Costa. A Study of the First
99m
Tc-labelled Radiopharmaceutical for the
Investigation of Cerebral Blood Flow in Man. PhD Thesis, University of London, 1989.)
99m
Tc–hexametazime, and
99m
Tc–HMPAO (hexa-methyl-propylene-amine-
oxime) are among the substances available on the market.
The physiological dynamics of cerebrospinal fluid circulation is especially
well demonstrated by isotopic cisternography studies using
99m
Tc- o r
111
In-
labeled DTPA. After intrathecal injection (usually in the epidural space at the
lumbar spine level), the radiopharmaceutical is distributed by the cisternae
and eventually reabsorbed in the blood through the arachnoid villi or granu-
lations. At the moment, there is no other imaging technique that can show the
physiology of cerebrospinal fluid distribution and reabsorption as innocu-
ously as isotopic cisternography. This technique serves not only to diagnose
hydrocephalus in the differential diagnosis of intracranial hypertension [262]
but also to predict the outcomes of potentially useful surgery.
The other clinical application of this isotopic cisternography technique,
screening for cerebrospinal fluid loss [263], pertains to very fine anatomical
resolution techniques, such as magnetic resonance. It is, however, still use-
ful in cases where magnetic resonance gives rise to false negative results.
However, more important is that the loss can be quantified with radio-
pharmaceuticals but not with magnetic resonance.
6.5.3.3 Neurotransmitters
In the CNS, brain, and beyond the blood–brain barrier, cell-to-cell, that is,
interneuronal, interaction and communication are central to all information
processing theories. The molecular neurobiology era, which has been grow-
ing for some years within neurosciences, is based on knowledge of neuronal
transmission and the role of neurotransmitter molecules in the many brain
functions. Neurotransmission requires the relevant neurotransmitters to be
first synthesized at the presynaptic neuron and then transported to the neuron
