Leroy C., Rancoita P.-G. Principles Of Radiation Interaction In Matter And Detection
Подождите немного. Документ загружается.

January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
220 Principles of Radiation Interaction in Matter and Detection
and, finally, Eq. (3.9) can be re-written as
M
at
≈ M
A
u. (3.11)
In summary, to a first approximation the relative atomic mass, A (also referred to
as atomic weight, see page 15), of a nuclide is expressed by its mass number. Fur-
thermore (see Appendix A.2), if we assume m
nucl
≈ 938.92 MeV/c
2
, i.e., the mean
value between the proton and neutron masses, from an inspection of Eqs. (3.8, 3.9)
we can note that the variation
k
of the binding energy per nucleon divided by m
nucl
slightly affects the resulting value of M
at
and the expression (3.11) is approximated
to about or better than a percent also for light nuclei. From Eq. (3.10) one obtains
B
e
≈ 7.42 MeV/nucleon. Since numerically A ∼ M
A
, in this chapter the symbol A
may be found to replace M
A
, particularly in theoretical expressions or calculations
(e.g., see Sects. 3.2.2-3.2.3) to indicate the number of nucleons in a nucleus with a
compact notation.
3.1.1 Radius of Nuclei and the Liquid Droplet Model
The size or the radius of a nucleus is not completely defined because the nucleus
cannot be considered as a rigid sphere. However, under the approximation of a
spherical shape, the mean nuclear radius can be determined by scattering with
particles such as electrons, neutrons, alpha’s, etc. From these measurements, it was
concluded that the nuclear radii are proportional to the cubic root of their mass
numb er. From the earliest scattering investigations by Rutherford and Chadwick,
it was derived that, except for the lightest nuclei, the nuclear radius, r
n
, is given by
the relation [Bethe and Ashkin (1953); Povh, Rith, Scholz and Zetsche (1995)]:
r
n
' r
0
M
1/3
A
[fm], (3.12)
where r
0
' 1.2 fm = 1.2 × 10
−13
cm. For these nuclei, the mean nuclear density,
ρ
0
, is approximately constant and given by:
ρ
0
'
M
A
m
p
4
3
πr
3
n
=
M
A
m
p
4
3
π
³
r
0
M
1/3
A
´
3
=
3 m
p
4 πr
3
0
(3.13)
≈ 2 × 10
14
[g/cm
3
].
The mean density of the nuclear matter is extremely large relatively to ordinary
matter.
k
For all isotopes from
3
H up to
238
U, the binding energy per nucleon ranges from ≈
2.6 MeV/nucleon up to ≈ 8.8 MeV/nucleon; for
2
H (deuterium) it is ≈ 1.1 MeV/nucleon.
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
Nuclear Interactions in Matter 221
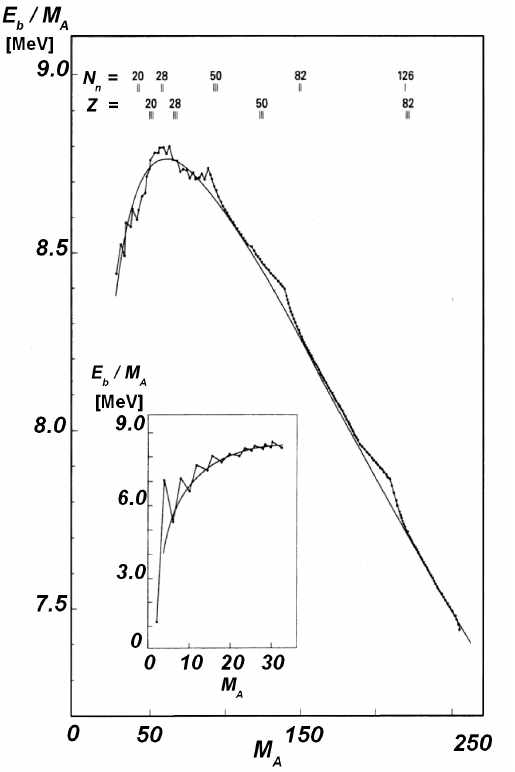
Fig. 3.1 Binding energy in units of MeV per nucleon in case of stable nuclei with even values
of mass number M
A
as a function of M
A
(adapted and reprinted with permission from Povh,
B., Rith, K., Scholz, C. and Zetsche, F. (1995), Particles and Nuclei: an Introduction to the
Physical Concepts, Figure 2.4, page 18, Springer-Verlag Publ., Berlin Heidelberg New-York;
c
° by
Springer-Verlag 1995). The continuous line is determined by the Weizs¨acker–Bethe mass formula
given in Eq. (3.14) (at page 223).
3.1.1.1 Droplet Model and Semi-empirical Mass Formula
In nuclear physics, the analogy with the physics of an incompressible fluid was
suggested by the fact that the nuclear density (Sect. 3.1.1) and binding energy per
nucleon (Sect. 3.1) are almost constant. Thus, the nucleus can be regarded as a
liquid drop, in which an almost constant binding energy per nucleon corresponds to
a constant heat of vaporization independent of the droplet size.

January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
222 Principles of Radiation Interaction in Matter and Detection
The liquid drop model was used by von Weizs¨acker and Bethe to evaluate nuclear
masses ([von Weizs¨acker (1936); Blatt and Weisskopf (1952); Bethe and Ashkin
(1953); Finkelnburg (1964)]; see also Chapter 2, Section 3 in [Povh, Rith, Scholz
and Zetsche (1995)]). In addition, a semi-empirical mass formula was proposed in
which the overall nuclear binding energy can be computed on the base of several
contributions, whose relative magnitudes are given empirically by adapting a few
parameters to measured nuclear masses. In this framework with the parameters a
v
,
a
s
, a
c
, a
a
and δ
p
defined at page 223 (from [Povh, Rith, Scholz and Zetsche (1995)]),
five contributions to the binding energy, E
b
, are considered:
• Volume Energy
The major contribution to E
b
is given by nucleon interactions mediated by
the nuclear forces. As already mentioned, the binding energy per nucleon
E
b
/M
A
slightly varies, except for light (and very heavy) nuclei. Further-
more, the quasi-constancy of the mean nuclear-matter density indicates
that the nuclear forces are short ranged and involve the nearest nucleon
neighbors. The binding volume-energy can be expressed by:
E
v
b
' a
v
M
A
.
• Surface Energy
Nucleons located at the nuclear surface are necessarily less bound than
those inside the nucleus, because less nucleons are surrounding them. The
number of surface nucleons is proportional to the nucleus surface, which, in
turn, is proportional to M
2/3
A
[Eq. (3.12)]. The effect of the binding surface
energy is to decrease the overall binding-energy, thus:
E
s
b
' −a
s
M
2/3
A
.
• Coulomb Energy
The electrostatic repulsion among protons has a long range characteri-
stic. The resultant Coulomb-energy will decrease the total amount of the
nuclear binding-energy. In the electrostatic theory, the energy due to a
net charge Ze uniformly distributed over a sphere of radius R is given by
−
3
5
(Ze)
2
R
. To a first approximation, the Coulomb-energy term can be eva-
luated as:
E
C
b
' −a
c
Z
2
M
1/3
A
.
• Asymmetry Energy (neutron excess)
Light stable nuclei are those for which N
n
≈ Z. For heavier nuclei, the
number of neutrons usually exceed the number of protons. The neutron
excess increases as the mass number increases. Therefore, a neutron excess
term depending on N
n
−Z must be present in the equation. In addition, it
has to vanish for N
n
' Z. The asymmetry term is given by:
E
a
b
' −a
a
(N
n
− Z)
2
4M
A
.

January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
Nuclear Interactions in Matter 223
• Pairing Energy
Nuclei with an even number of both protons and neutrons show a trend
of high stability. Nuclei having an odd number of one type of nucleon and
an even number of the other type are less stable. Nuclei with doubly odd
numbers of protons and neutrons are even more unstable. This pairing effect
is taken into account by the additional term:
E
p
b
' −
δ
p
√
M
A
.
As mentioned above, the semi-empirical mass formula was derived by von
Weizs¨acker and Bethe for the atomic mass M
at
(Z, M
A
) of an atom constituted
by M
A
nucleons, Z of them being protons; it can be summarized in the following
way:
M
at
(Z, M
A
) = N
n
m
n
+ Zm
p
+ Zm − E
b
= N
n
m
n
+ Zm
p
+ Zm − a
v
M
A
+a
s
M
2/3
A
+ a
c
Z
2
M
1/3
A
+ a
a
(N
n
− Z)
2
4M
A
+
δ
p
√
M
A
, (3.14)
where the parameters are (from [Povh, Rith, Scholz and Zetsche (1995)])
a
v
= 15.67 MeV/c
2
,
a
s
= 17.23 MeV/c
2
,
a
c
= 0.714 MeV/c
2
,
a
a
= 93.15 MeV/c
2
,
δ
p
= −11.2 MeV/c
2
for even-even nuclei (even value of M
A
),
δ
p
= 0 MeV/c
2
for even-odd and odd-even nuclei (odd value of M
A
),
δ
p
= +11.2 MeV/c
2
for odd-odd nuclei (even value of M
A
).
3.1.2 Form Factor and Charge Density of Nuclei
The classical interaction of a charged particle, like an electron, with a massive
object of charge Ze, like a nucleus, is described by the classical Rutherford diffe-
rential cross section (Sect. 1.5), in which spin dependent effects and target recoil
are neglected. The same equation is derived following a non-relativistic quantum-
mechanical approach, using the Born approximation, for the case of a point-like
target of charge Ze. For a non-point like target, it is possible to demonstrate that
the scattering cross section on nucleus can be rewritten as (see for instance Sec-
tion 5.2 of [Povh, Rith, Scholz and Zetsche (1995)], and also Section 6.3 of [Segre
(1977)]):
µ
dσ
dΩ
¶
Rutherford, non point like
=
µ
dσ
dΩ
¶
Rutherford
|F (~q)|
2
, (3.15)
where ~q is the tri-momentum transfer in the scattering process and F (~q) is the
so-called nuclear form-factor given by
F (~q) =
Z
n
c
(~r) exp
µ
i~q ·~r
~
¶
d
3
r,

January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
224 Principles of Radiation Interaction in Matter and Detection
where ~r is the radial position with respect to the scattering center, d
3
r is the
infinitesimal volume located at ~r, and n
c
(~r) is the normalized charge-density distri-
bution at ~r. The latter is related to the charge-density distribution by:
ρ
c
(~r, Z) = Zen
c
(~r)
with
Z
ρ
c
(~r) d
3
r = Ze
Z
n
c
(~r, Z) d
3
r = Ze.
As a consequence, we have:
F (0) =
Z
n
c
(~r) d
3
r = 1.
At relativistic energies, spin effects cannot be neglected anymore and, for an
electron interaction, they are taken into account by the Mott differential cross sec-
tion (as mentioned at pages 85 and 123). Neglecting recoil effects, the Mott cross
section can be written as:
µ
dσ
dΩ
¶
Mott
=
µ
dσ
dΩ
¶
Rutherford
µ
1 − β
2
sin
2
θ
2
¶
, (3.16)
where θ is the scattering angle. The meaning of the right-hand multiplicative term
in Eq. (3.16) can be understood, for instance, considering that for β → 1 it becomes
∼ cos
2
(θ/2). Thus, for θ ' 0
◦
, the cross section becomes ' 0, which accounts for
helicity conservation in the scattering process.
As seen above, the extended charge-distribution of the nucleus can be described
by introducing the nuclear form-factor. Often, for charge distributions which have a
spherical symmetry, form factors depend on the value of the tri-momentum transfer
(|~q|) and is indicated as F (q), or F (q
2
). The form factor can be determined by
measuring the experimental differential cross section and taking the ratio to the
Mott differential cross section (see for instance [Povh, Rith, Scholz and Zetsche
(1995)]), namely:
µ
dσ
dΩ
¶
exp
=
µ
dσ
dΩ
¶
Mott
|F (q)|
2
.
The first measurements of nuclear form-factors were carried out during the years
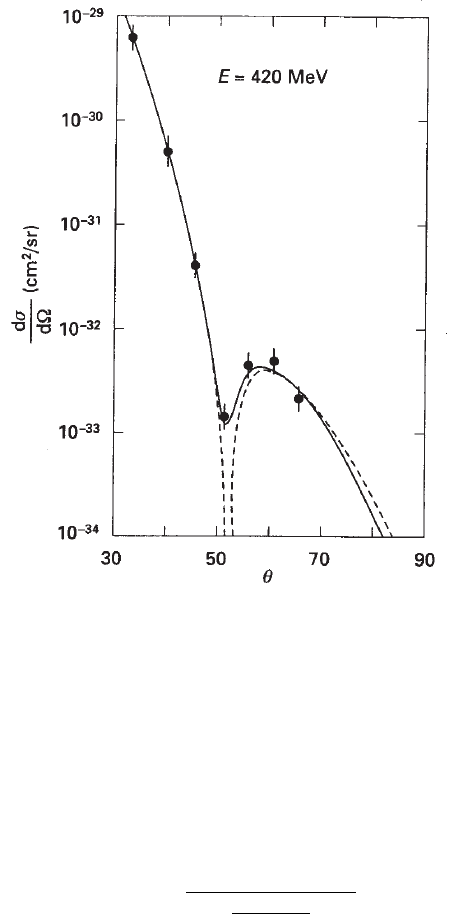
1950s. In Fig. 3.2, the differential cross section of electrons on
12
C nuclei is shown
as a function of the electron diffusion angle. It was obtained by electron scattering
at 420 MeV [Hofstadter (1957)]: the shape of the differential cross section depends
on the nuclear form-factor. The dashed line corresponds to the expected differential
cross section calculated using the Born approximation for the electron scattering
on the diffused surface of a homogeneous sphere. The differential cross section exhi-
bits the typical diffraction-pattern, in which a minimum is present at θ ' 51
◦
,
i.e., |~q|/~ ' 1.8 fm
−1
.
Systematic experimental measurements have shown that the nucleus has a charge
distribution decreasing gradually towards the surface. Therefore, one could conclude
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
Nuclear Interactions in Matter 225
Fig. 3.2 Measurement of the form factor of
12
C nucleus obtained by the scattering of 420 MeV
electrons (adapted and reprinted, with permission, from the Annual Review of Nuclear Science,
Volume 7
c
° 1957 by Annual Reviews www.annualreviews.org; [Hofstadter (1957)]). The differential
cross section is shown as a function of the electron scattering angle. The dashed line corresponds
to the expected electron scattering on the diffused surface of a homogeneous sphere, following the
Born approximation. The continuous line corresponds to a fit to the experimental data.
that Eq. (3.13) is valid only as a simplified expression. Under the approximation of
nuclear spherical charge-symmetry and in agreement with the experimental data,
the radial charge-density ρ
c
(r, Z) is given by
ρ
c
(r, Z) =
ρ
c
(0, Z)
exp
h
r−C
c
(M
A
)
Z
0
i
+ 1
, (3.17)
where r is the radial distance from the center of the nucleus in fm, C
c
(M
A
) '
1.07 M
1/3
A
fm for M
A
≥ 30, Z
0
' 0.545 fm and ρ
c
(0, Z) is determined from the
condition:
Ze = 4π
Z
∞
0
ρ
c
(r, Z) r
2
dr.

January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
226 Principles of Radiation Interaction in Matter and Detection
3.1.3 Angular and Magnetic Moment, Shape of Nuclei
Many nuclei have an intrinsic angular momentum
~
I
s
, which represents the total
angular momentum of the nucleus and is given by the sum of the orbital momentum
and the spin of all protons and neutrons inside the nucleus.
~
I
s
is always an integer
or a half integer in units of ~ :
|
~
I
s
| = I
s
~. (3.18)
Often, I
s
is referred to as the nuclear spin
∗
and varies between 0 and
9
2
for all
known nuclei in their ground state. Nuclei with an even number of nucleons (even
mass number) have an integral value of I
s
. In most cases, they do not have angular
momentum at all, i.e., I
s
= 0. In particular, even-even nuclei have a ground state
with I
s
= 0. Nuclei with odd mass number always have a half-integral spin.
Nuclei can exist in excited states of higher energy, as will be discussed in the next
part of this section. The angular momenta of excited states may differ from those of
the corresponding nuclear ground states. It has been shown that there are selection
rules for transitions between energy states of the nucleus or from neighboring nuclei.
Since transitions between nuclear states with very different angular momenta
are strongly forbidden, there exist long-lived excited nuclear states. Nuclei in these
long-lived excited states are called Nuclear isomers. Isomeric nuclei, have the same
charge and mass, but are in different energy states, i.e., have different arrangements
of their nucleons. An important difference between a normal excited nuclear state
and a nuclear isomer is that, for the latter one, the transition probability to a
more stable state, particularly to the ground state, is very small. So, like in atomic
physics, the isomeric nucleus can be called a metastable nuclear state.
As for the case of electron shell, a magnetic moment is related to the angular mo-
mentum of the electrically charged constituents of the nucleus. These constituents,
by their orbital motion, generate electric current densities which contribute to the
overall magnetic moment in addition to other effects, like the intrinsic magnetic
moment of nucleons. In Appendix A.2, the measured proton and neutron magnetic
moments are given in units of the so-called nuclear magneton, µ
N
, defined as:
µ
N
=
e~
2m
p
.
The positive (negative) sign of the proton (neutron) magnetic moment indicates
that it is directed along (opp osite) to its angular momentum.
Heavy nuclei show a deviation from a spherical nuclear shape of the order
of 1%. An ellipsoidal shape agrees better with the experimental results. Usually,
there is a contraction in the direction of the spin axis. This asymmetry is repre-
sented by associating an electric quadrupole moment with the nucleus. The p ositive
∗
At page 232, Finkelnburg (1964) noted that this notation is misleading: this designation is
correct only for elementary particles, like protons and neutrons. For nuclei consisting of protons
and neutrons,
~
I
s
represents the total angular momentum of the nucleus.
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
Nuclear Interactions in Matter 227
quadrupole sign is assigned to an extension in the direction of the spin axis, while
a negative value corresponds to a shortening along this direction.
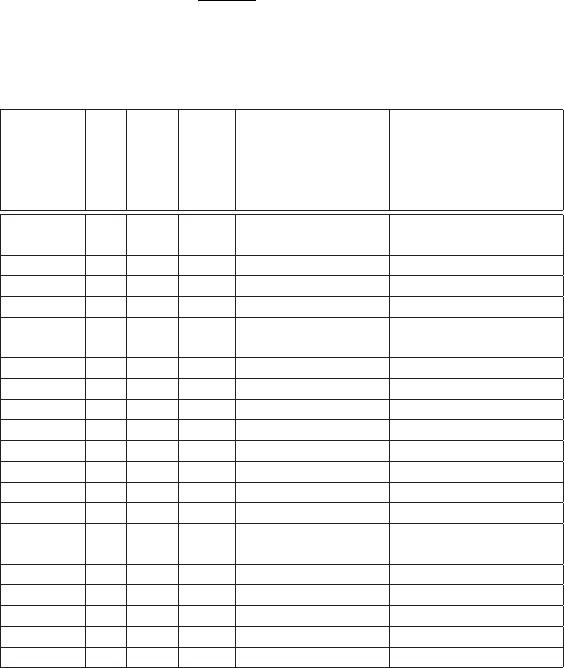
In Table 3.1, values of the nuclear spin, nuclear magnetic and electrical
quadrupole are shown for several nuclides ([Stone (2001)] and references therein).
3.1.4 Stable and Unstable Nuclei
An aggregate of nucleons may become a stable and bound nucleus only if the num-
ber of one nucleon-type does not largely exceed the other nucleon-type. There is
no experimental evidence of a stable nucleus consisting exclusively of neutrons or
exclusively of protons.
Unless replenished artificially, unstable nuclides in any given sample decay at a
rate −dN
s
(t)/dt, where the minus sign is introduced because N
s
(t) diminishes as
the time, t, increases. The decay rate is proportional to the number of nuclides in
the sample at the time t and is expected to become independent of t, i.e.,
dN
s
(t)
dt
= −λ
s
N
s
(t), (3.19)
Table 3.1 Spin, nuclear magnetic moment and electric quadrupole moment
for some stable nuclides in their ground state determined by recent measure-
ments (see [Stone (2001)] and references therein).
Element Z M
A
Spin Nuclear Magnetic Electric Quadrupole
Moment Moment
µ
N
10
−24
cm
−2
H 1 1 1/2 2.79284734(3)
2 1 0.857438228(9) 0.00286(2)
He 2 3 1/2 -2.12749772(3)
Be 4 9 3/2 -1.1778(9) 0.0529(4)
C 6 13 1/2 0.7024118(14)
N 7 14 1 0.40376100(6) 0.02001(10)
15 1/2 -0.28318884(5)
O 8 17 5/2 -1.89379(9) -0.26(3)
Al 13 27 5/2 3.6415068(7) 0.1402(10)
Si 14 29 1/2 -0.55529(3)
K 19 41 3/2 0.21489274(12) 0.060(5)
Ca 20 43 7/2 -1.317643(7) -0.049(5)
Mn 25 51 5/2 3.5683(13) 0.42(7)
Ga 31 69 3/2 2.01659(5) 0.17(3)
Zr 40 91 5/2 -1.30362(2) -0.206(10)
Ru 44 99 5/2 -0.641(5) 0.079(4)
101 5/2 -0.719(6) 0.46(2)
Nd 60 145 7/2 -0.656(4) -0.314(12)
Ta 73 181 7/2 2.3705(7) 3.17(2)
W 74 183 1/2 0.11778476(9)
Ir 77 191 3/2 0.1507(6) 0.816(9)
Pb 82 207 1/2 0.58219(2)

January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
228 Principles of Radiation Interaction in Matter and Detection
where λ
s
is called decay constant: it is the inverse of the mean lifetime, τ
s
:
λ
s
≡
1
τ
s
.
Finally, by integrating Eq. (3.19) between the limits N
s
(t = 0) = N
0
at t = 0 and
N
s
(t) = N
s
at the time t, we have:
N
s
= N
0
exp (−tλ
s
) = N
0
exp
µ
−
t
τ
s
¶
.
The treatment, extended to the case of more than one nuclide in a substance and
to fluctuations in radioactive decays, can be found in [Bethe and Ashkin (1953)].
Among the combinations of nucleons which form bound nuclear aggregates, only
a relatively small fraction of them generates stable nuclides. For instance, β-stable
nuclides for are the ones whose number of neutrons and protons belong to the
narrow band shown
∗
in Fig. 3.3 [Bohr and Mottelson (1969)]. For stable nuclei, an
approximated empirical relationship between the mass number M
A
and the atomic
numb er Z is given by [Marmier and Sheldon (1969)]:
Z =
M
A
1.98 + 0.0155 M
2/3
A
. (3.20)
This formula shows, for M
A
< 40, that stable nuclides are those for which Z '
N
n
. For the heaviest nuclei, the number of neutrons can exceed the number of
protons up to ≈ 50%.
Dynamical instabilities lead to spontaneous nuclear break-up into two or more
parts, i.e., α-decay, fission and other related phenomena. The β-instability proceeds
via a change in the atomic number Z by the emission or absorption of an electron,
namely via β-decay or electron capture.
A large amount of nuclei emits positrons or electrons under reactions, result-
ing in a net charge change of one unit. The β-radioactive decay originates from
fundamental weak processes involving nucleons:
n → p + e
−
+ ¯ν
e
(3.21)
p → n + e
+
+ ν
e
, (3.22)
where ¯ν
e
and ν
e
are the electronic antineutrino and neutrino, associated with the
electron and the positron, respectively. Also, the process, in which an external
orbital electron is absorbed, can occur:
p + e
−
→ n + ν
e
. (3.23)
The process
n + e
+
→ p + ¯ν
e
is theoretically possible, but there is no positron available around the nucleus. Pro-
cesses which proceed following Eqs. (3.21, 3.22) are called electron (β
−
) and positron
(β
+
) β-decay, respectively. Equation (3.23) describes the electron capture pro-
cess. The other nucleons in the nucleus can supply to or remove the energy needed
for these processes from the nucleon, undergoing the transformation.
∗
The Fig. 3.3 is known as a Segr`e chart.
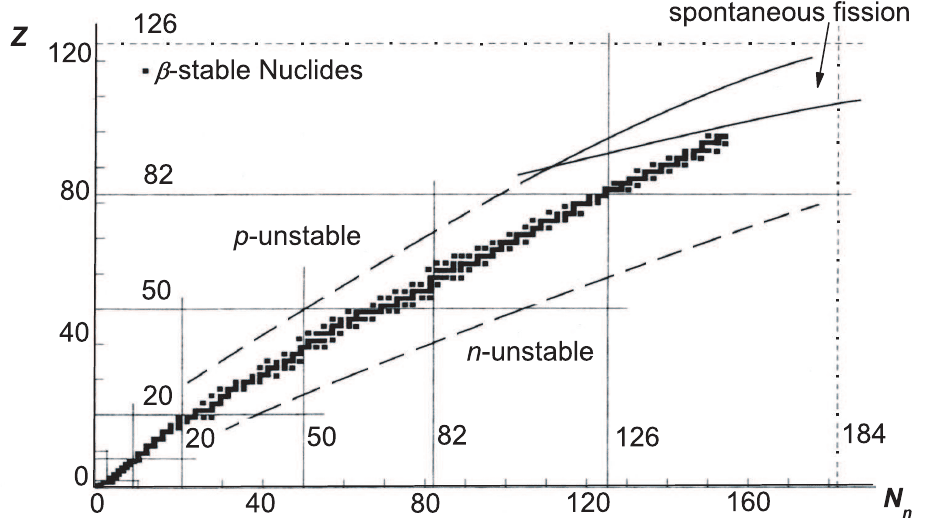
January 9, 2009 10:21 World Scientific Book - 9.75in x 6.5in ws-bo ok975x65˙n˙2nd˙Ed
Nuclear Interactions in Matter 229
Fig. 3.3 Z versus N
n
distribution of β-stable nuclides (adapted with permission from [Bohr and Mottelson (1969)]).
