Lennarz W.J., Lane M.D. (eds.) Encyclopedia of Biological Chemistry. Four-Volume Set . V. 4
Подождите немного. Документ загружается.

SUMO Activating Enzyme (E1 Enzyme)
SUMO E1 activating enzyme is composed of two
subunits, Aos1 and Uba2 (for alternative names see
Table I), both of which are essential for the function.
Aos1 and Uba2 share striking homology to the N- and
C- terminal halves of ubiquitin E1 enzyme, respectively.
SUMO E1 activating enzyme is present throughout the
cell, with a strong enrichment in the nucleoplasm. A
single SUMO E1 activating enzyme is expressed in most
organisms, even those containing several distinct SUMO
proteins. Current data suggest that mammalian SUMO
E1 works with equal efficiency on each of the three
mammalian SUMO proteins, although it has no affinity
for other ubiquitin-related proteins.
SUMO Conjugating Enyzme (E2 Enzyme)
A single SUMO E2 conjugating enzyme, Ubc9, is
expressed in cells (see Table I for alternative names).
While it is strikingly similar to ubiquitin conjugating
enzymes, it works exclusively on SUMO proteins. As for
the E1 enzyme, Ubc9 does not appear to discriminate
between different SUMO proteins expressed within a
particular organism. Ubc9 localizes predominantly in
the nucleus, but is also present in the cytoplasm and at
nuclear pore complexes. A striking difference between
the ubiquitin-conjugating and the SUMO-conjugating
system is the ability of Ubc9 to directly bind to and
modify SUMO targets. However, with a few exceptions,
the efficiency of modification in the absence of E3 ligases
is extremely poor.
SUMO Ligases (E3 Ligase)
Three types of SUMO E3 ligases are currently
known (Table I). The first type is encoded by the
TABLE I
Nomenclature for SUMO and its Conjugating Enzymes
Human Baker’s yeast
SUMOs SUMO1/PIC 1/Ubl1/sentrin1/
GMP1/hSMT3
Smt3
SUMO2/sentrin3/Smt3a
SUMO3/sentrin2/Smt3b
E1 activating enzyme
Subunit 1 Aos1/SAE1/Sua Aos1
Subunit 2 Uba2/SAE2 Uba2
E2 conjugating enzyme Ubc9/UBE2I Ubc9
E3 ligating enzymes Pias1/GBP/DEAD/H box-
binding protein 1/ARIP
Siz1/UII 1
Pias3 Siz2/Nfi1
Piasx
a
/ARIP3
Piasx
b
/Miz1/Siz2
Piasy
RanBP2/Nup358
Pc2
Known proteins involved in the SUMO conjugation pathway. Since
most of them were independently identified by several groups, different
names exist in literature.
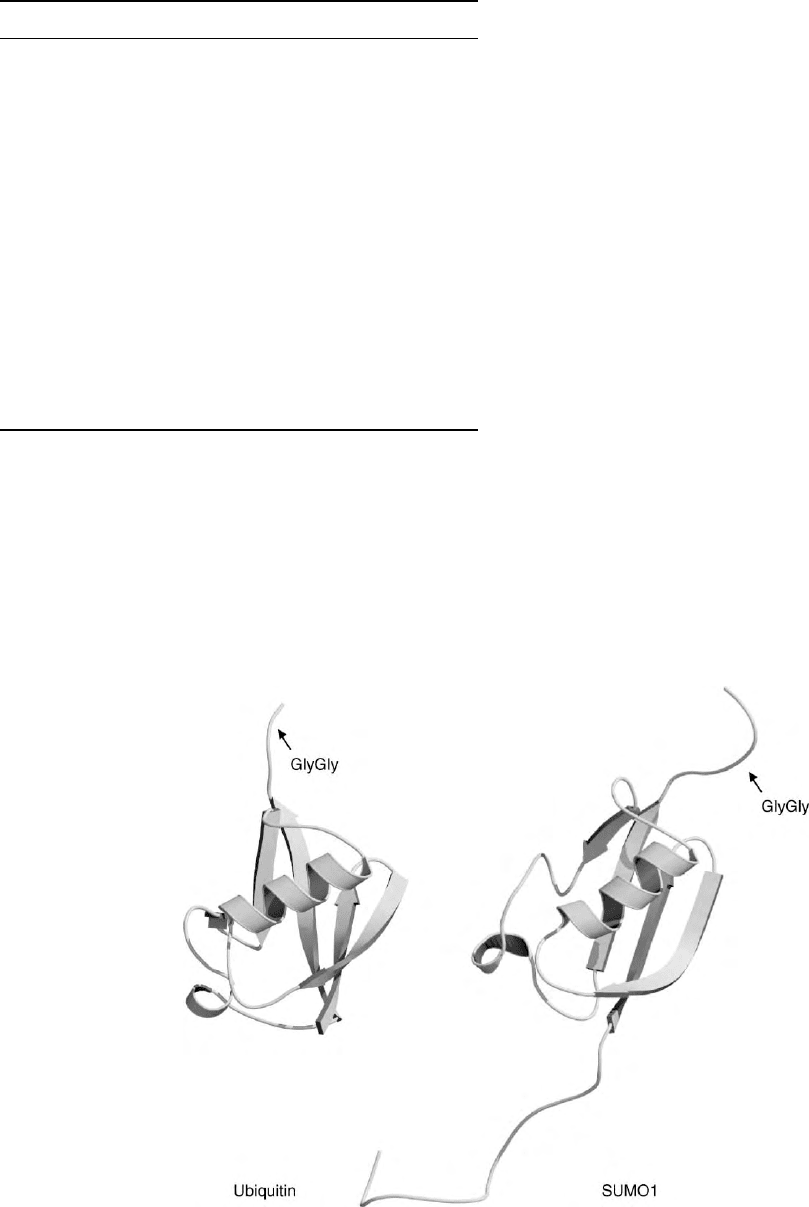
FIGURE 1 Ubiquitin and SUMO comparison. Although ubiquitin and SUMO1 share only 18% amino acid identity, the structural fold is very
similar. The indicated C-terminal double glycine motif is the attachment site to substrates. The images (generated using Moscript 2.1.2 and
Raster3D) were kindly provided by Dr. Peter Bayer.
SUMO MODIFICATION 131
“Protein inhibitor of activated STAT” (PIAS) family.
These proteins contain a predicted RING finger-like
structure that is essential for their function as E3 ligases.
They bind directly to Ubc9 and to selected SUMO
targets, and stimulate their modification both in vivo
and in vitro. Baker’s yeast contains two PIAS type E3
ligases, Siz1 and Siz2. These proteins seem to be
responsible for most, but probably not for all, SUMO
targets, and have partially overlapping target specificity.
The second type is currently represented by a single
(vertebrate specific) 358 kDa protein, RanBP2/Nup358
(Ran binding protein/nucleoporin). RanBP2 is a com-
ponent of nuclear pore complexes, and thus may
couple sumoylation and nucleocytoplasmic transport.
Interestingly, the catalytic domain of RanBP2 does not
contain a RING finger motif, and also shows no other
homology to ubiquitin E3 ligases. The Polycomb group
protein Pc2 appears to represent a third type of an E3
ligase, as it bears no similarity to other E3 ligases.
DECONJUGATION
Overview
Isopeptide bonds between ubiquitin-related proteins and
their cellular targets are reversible in nature due to the
presence of specific enzymes that recognize and cleave
the bond. Currently, a single family of SUMO-specific
isopeptidases is known (Table II). All members contain a
200 amino acid catalytic core that is distantly related to
viral cysteine proteases but shows no similarity to
ubiquitin-specific enzymes.
Interestingly, SUMO isopeptidases serve two physio-
logical functions in vivo, cleavage of isopeptide bonds,
but also maturation of nascent SUMO (C-terminal
hydrolase activity). This distinguishes the SUMO system
from the ubiquitin system, where two distinct enzyme
families serve to fulfill these functions.
SUMO Isopeptidases
The founding member of this family is S. cerevisiae
Ulp1 (ubiquitin like protease). Bakers yeast contains
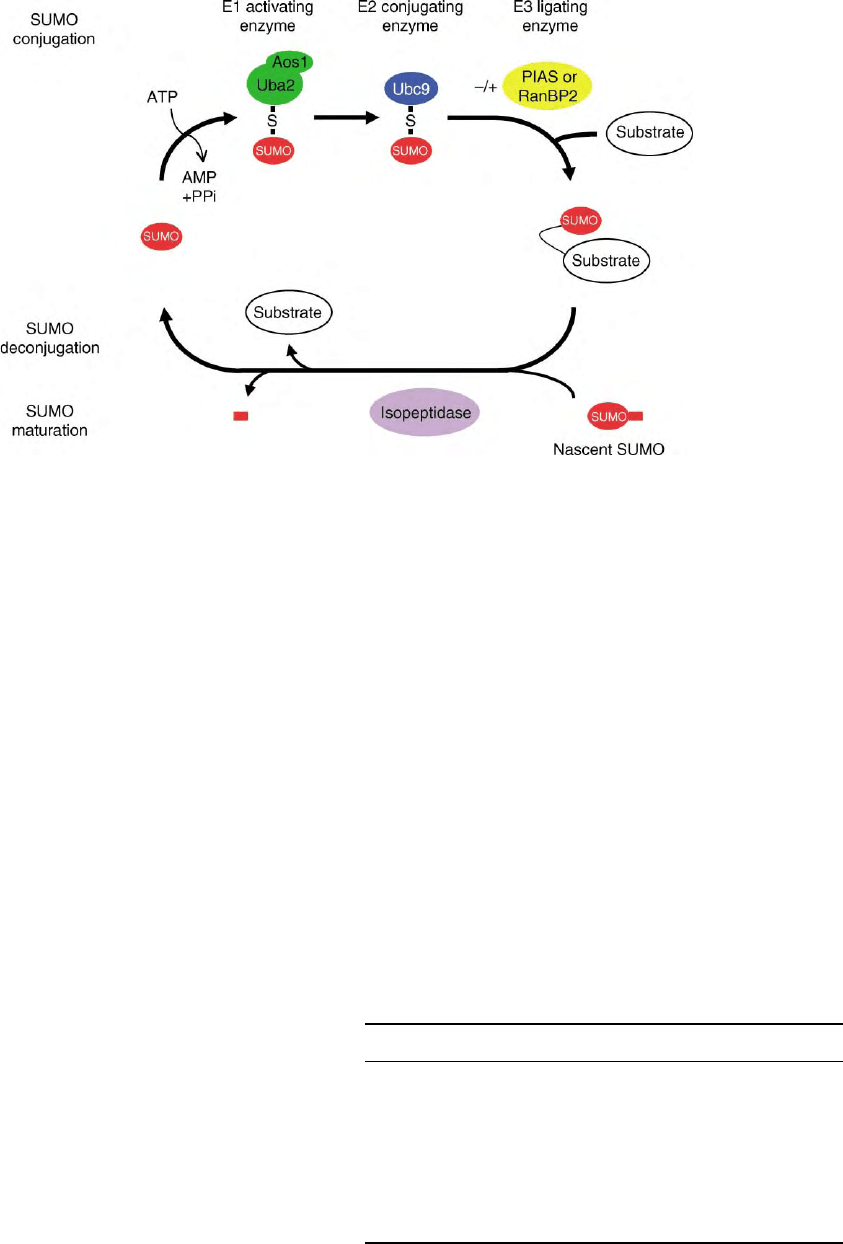
FIGURE 2 The SUMO modification pathway. SUMO is synthesized as a precursor that requires proteolytic processing. A C-terminal
hydrolase/isopeptidase removes several C-terminal amino acids to expose the GlyGly motif essential for conjugation. SUMO conjugation involves
an enzymatic cascade: In an ATP-dependent step, mature SUMO first forms a thioester with the E1 activating enzyme, the heterodimer Aos1/Uba2.
Subsequently, it is transferred to the E2 conjugating enzyme, Ubc9. SUMO is then either directly or in an E3 ligase-dependent step conjugated to the
substrate. SUMO de-modification is performed by isopeptidases.
TABLE II
Nomenclature for SUMO Deconjugating Enzymes
Human Mouse Baker’s yeast
Senp1 SuPr-2 Ulp1
Senp2/KIAA1331 Senp2/Smt3IP2
p
/Axam2
and Supr-1
p
Ulp2/Smt4
Senp3/Susp3/SSP3 Senp3/Smt3IP1/SuPr-3
Senp5
Senp6/Susp1/SSP1/KIAA0797
Senp7/Susp2/SSP2/KIAA1707
SUMO isopeptidases share a 200 amino acid core domain. Known
proteins containing this core domain are listed in this table. The
proteins characterized as SUMO isopeptidases are indicated in bold.
p
Indicates different isoforms or alternative splice products.
132 SUMO MODIFICATION
two enzymes of this family, Ulp1 and Ulp2. Ulp1 is
required for viability in yeast, whereas Ulp2 is not
essential. They localize to different intracellular
compartments, with Ulp1 being enriched at nuclear
pore complexes, and Ulp2 being diffusely distributed
throughout the nucleus. Mammals on the other hand
have at least six distinct genes for SUMO isopeptidases
(see Table II). Due to alternative splicing, the number of
enzymes expressed is probably much larger. Only a few
of these proteins have been characterized in some detail.
They vary significantly in size and intracellular localiz-
ation. It is plausible to assume that different isopepti-
dases exert different target specificity in large part
through their physical localization. Whether they also
differ in enzymatic properties, such as substrate speci-
ficity or kinetics of cleavage, remains to be seen.
SUMO Target Proteins
Sumoylation as a means to regulate protein function
appears to be quite a common mechanism. Modification
has been documented for more than 70 different target
proteins, and this number is expected to increase
significantly. Based on current knowledge, some gener-
alizations can be made about the nature of the targets,
motifs required for modification, consequences of
modification, and regulation.
KNOWN TARGET PROTEINS
Based on immuno-fluorescence analysis, most targets for
sumoylation are constitutively or transiently associated
with the nuclear compartment. Consistent with this,
most known targets can be associated with nuclear
processes such as chromatin remodeling, DNA repair,
transcription, or nucleocytoplasmic transport (amongst
them are histone deacetylases, topoisomerases, thymine-
DNA glycosylase, PCNA, p53, PML, heat shock
factors, steroid hormone receptors, I
k
B
a
, RanGAP1,
and many others). Other pathways to which SUMO has
been linked are, e.g., progression through mitosis
(mitotic arrest of yeast mutants defective in SUMO
conjugation or deconjugation; yeast septins, topoisome-
rase II and Pds5 are modified specifically in mitosis),
or infection with viruses (examples for viral SUMO
targets are: cytomegalovirus immediate early proteins
IE1 and IE2, adenovirus type 5 E1B-55 kDa, bovine
papillomavirus E1).
CONSENSUS SITES FOR MODIFICATION
In contrast to ubiquitinylation, for which a unifying
motif has not been identified, sumoylation of most
targets seems to be specified by a short consensus
sequence in target proteins. This motif consists of
just four amino acids, CK £ E/D, and includes the
lysine residue that forms an isopeptide bond with
SUMO. C stands for a bulky aliphatic amino acid
residue. Additional structural features are probably
required to ensure accessibility to the conjugation
machinery. Some motifs are present at the very N- or
C-terminal end of a protein, others are flanked by
proline residues that may induce a loop structure.
Consistent with this, RanGAP1, which is an extremely
efficient SUMO target, presents its sumoylation motif
in an accessible loop.
FUNCTIONAL CONSEQUENCES
FOR
MODIFICATION
Similar to phosphorylation, sumoylation seems to have
many different functional consequences that depend on
the specific target protein. Considering SUMO’s size, it is
plausible to assume that conjugation can lead to
masking of binding sites, generation of novel binding
interphases, or conformational changes in the modified
protein. Examples have been reported for changes in
protein–protein or protein –DNA interactions, altera-
tion in subcellular localization, enhanced stability
through antagonizing ubiquitin/proteasome-mediated
degradation, and changes in enzymatic activity.
REGULATION
While some SUMO targets appear to be modified
constitutively, others are sumoylated only during a
specific period of the cell cycle, upon stress, or upon a
specific extracellular signal. Examples are sumoylation
of yeast septins during mitosis, sumoylation of topoi-
somerase upon treatment with DNA-damaging agents,
or Dictyostelium MEK1 sumoylation in response to
chemoattractant. While this suggests the existence of
elaborate regulatory mechanisms, current knowledge is
rather poor. Increased modification of a specific target
may for example be due to changes in the target,
activation of a specific E3 ligase, or relocalization of a
specific isopeptidase. Evidence for cell-cycle-dependent
regulation of E3 ligases and isopeptidases is currently
only available in yeast: (1) fission yeast ulp1 resides at
the NPC during interphase, but is nuclear during
mitosis; (2) baker’s yeast E3 ligase Siz1 is intranuclear
during interphase, but partially relocalizes to the bud
neck in mitosis.
SEE ALSO THE FOLLOWING ARTICLES
Cysteine Proteases † JAK-STAT Signaling Paradigm †
Nuclear Pores and Nuclear Import/Export † Ubiquitin
System † Ubiquitin-Like Proteins † Zinc Fingers
SUMO MODIFICATION 133
GLOSSARY
cysteine protease Protease that requires a cysteine for activity.
isopeptide bond Any amide bond formed between a carboxyl group
of one amino acid and an amino group of another amino acid with
the exception of the conventional peptide bond that is formed
between amino- and carboxy- groups in
a
-position.
nuclear pore complex A large multiprotein complex embedded in the
nuclear envelope that mediates both active transport of macro-
molecules and passive diffusion of small components to and from
the nucleus.
RING finger proteins A family of structurally related zinc-binding
proteins containing the RING consensus sequence: CX
2
CX
(9-39)
CX
(1-3)
HX
(2-3)
C/HX
2
CX
(4-48)
CX
2
C. The cysteines and histidines
represent the zinc-binding sites, whereby the first, second, fifth, and
sixth of these complex the first zinc ion, and the third, fourth,
seventh, and eighth complex the second.
FURTHER READING
Bernier-Villamor, V., Sampson, D. A., Matunis, M. J., and Lima, C. D.
(2002). Structural basis for E2-mediated SUMO conjugation
revealed by a complex between ubiquitin-conjugating enzyme
Ubc9 and RanGAP1. Cell 108, 345–356.
Freiman, R. N., and Tjian, R. (2003). Regulating the regulators: Lysine
modifications make their mark. Cell 112, 1–17.
Goettsch, S., and Bayer, P. (2002). Structural attributes in the
conjugation of ubiquitin, SUMO and RUB to protein substrates.
Front Biosci. 7, a148–a162.
Johnson, E. S. (2002). Ubiquitin branches out. Nat. Cell Biol. 4,
E295–E298.
Kim, K. I., Baek, S. H., and Chung, C. H. (2002). Versatile protein tag,
SUMO: Its enzymology and biological function. J. Cell Physiol.
191, 257– 268.
Kurepa, J., Walker, J. M., Smalle, J., Gosink, M. M., Davis, S. J.,
Durham, T. L., Sung, D. Y., and Vierstra, R. D. (2003). The small
ubiquitin-like modifier (SUMO) protein modification system in
Arabidopsis. Accumulation of SUMO1 and -2 conjugates is
increased by stress. J. Biol. Chem. 278, 6862– 6872.
Melchior, F. (2000). SUMO – nonclassical ubiquitin. Annu. Rev. Cell
Develop. Biol. 16, 591– 626.
Melchior, F., Schergaut, M., and Pichler, A. (2003). SUMO: ligases,
isopeptidases and nuclear pores. Trends Biochem. Sci. 28,
612–618.
Muller, S., Hoege, C., Pyrowolakis, G., and Jentsch, S. (2001). SUMO,
ubiquitin’s mysterious cousin. Nat. Rev. Mol. Cell Biol. 2, 202–210.
Schwartz, D. C., and Hochstrasser, M. (2003). A superfamily of
protein tags: ubiquitin, SUMO and related modifiers. Trends
Biochem. Sci. 28, 321– 328.
Seeler, J. S., and Dejean, A. (2003). Nuclear and unclear functions of
SUMO. Nat. Rev. Mol. Cell Biol. 4, 690–699.
Wilson, V. G., and Rangasamy, D. (2001). Viral interaction with the
host cell sumoylation system. Virus Res. 81, 17–27.
BIOGRAPHY
Frauke Melchior is a group leader at the Max-Planck Institute for
Biochemistry in Martinsried, Germany. Her principal research interests
are nucleocytoplasmic transport, and ubiquitin-related proteins of the
SUMO family. She holds a Ph.D. from the University of Marburg and
received her postdoctoral training at the Max-Planck Institute for
Biophysical Chemistry in Go
¨
ttingen and the Scripps Research Institute
in La Jolla. She participated in the discovery of SUMO during her
postdoctoral time, and has since contributed to the field with her own
group.
Andrea Pichler obtained her Ph.D. from the University of Vienna and
received a postdoctoral training at the Novartis Forschungsinstitut of
Vienna. Since 2000 she is a member of Dr. Frauke Melchior’s group at
the Max-Planck Institute of Biochemistry in Martinsried.
134 SUMO MODIFICATION

Superoxide Dismutase
Irwin Fridovich
Duke University Medical Center, Durham, North Carolina, USA
A small fraction of the O
2
consumed by living cells is
converted to superoxide O
2
2
. This free radical, and its
progeny, can damage a variety of biomolecules. This is the
cause of oxidation stress. Defenses are provided by the
superoxide dismutases that catalytically eliminate O
2
2
and by
the catalases and peroxidases that do the same for hydroper-
oxides. The varieties, distributions, and mode of action of the
superoxide dismutases are presented herein.
Introduction
Molecular oxygen (O
2
), while essential for the life of
aerobes, is potentially toxic. This toxicity is due to the
propensity of O
2
for reduction by a univalent pathway.
This facile univalent pathway of O
2
reduction generates
intermediates that lie between one O
2
and its four
electron reduction products – two molecules of water –
and it is the reactivity of these intermediates that is
responsible for the toxicity of O
2
. In order of their
production, these intermediates are the superoxide
anion radical (O
2
2
), hydrogen peroxide (H
2
O
2
), and
the hydroxyl radical (HO·). Superoxide dismutases
(SODs) catalyze the conversion of O
2
2
into H
2
O
2
plus
O
2
, i.e.,
O
2
2
þ O
2
2
þ 2H
þ
!
SOD
H
2
O
2
þ O
2
and in so doing provide an important defense. H
2
O
2
,in
turn, is eliminated by the catalases and peroxidases. The
concerted action of the SODs with the catalases and
peroxidases prevents the formation of the very reactive
HO·. This is the case because HO· production in vivo
requires both O
2
2
and H
2
O
2
.O
2
2
oxidizes the [4Fe–4S]
clusters of dehydratases, such as the aconitases,
causing release of Fe (II); the freed Fe (II) then reduces
H
2
O
2
to OH
2
plus HO· in a reaction known as the
Fenton reaction.
The SOD Family of Enzymes
The first truly photosynthetic organisms were the
cyanobacteria and the oxygen they produced oxyge-
nated a previously anaerobic biosphere. This imposed a
common selection pressure on what must have been a
varied anaerobic biota. It is not surprising that several
different SODs were then evolved to deal with the
toxicity of the accumulating O
2
and that these persist to
this day. Thus, there are SODs based on Cu (II) plus
Zn (II), Mn (III), Fe (III), and Ni (II). They all catalyze
the dismutation of O
2
2
into H
2
O
2
þ O
2
.
All SODs work by a similar mechanism in which the
metal at the active site is reduced by one O
2
2
and then
reoxidized by the next O
2
2
. The active site metal thus
acts as a mediator passing an electron from one O
2
2
to
the next. In this way the electrostatic repulsion, which
would prevent close approach of one O
2
2
to another, is
bypassed by the SODs. All the SODs are very efficient
catalysts and operate at close to the theoretical diffusion
limit. Their rate constants for interaction with O
2
2
are
, 3 £ 10
9
M
21
s
21
.
THE CU, ZN SODS
These SODs have been found in the cytosols of
eukaryotic cells, the intermembrane space of mitochon-
dria, the periplasm of gram-negative bacteria, and
in chloroplasts. The Cu (II) is linked to the Zn (II) at
the active site by the imidazolate moiety of a histidine
residue and this bridging imidazolate functions as a
proton supply during the catalytic cycle. Thus, when
the Cu (II)) is reduced by O
2
2
, the Cu (I) releases the
imidazolate that then protonates to imidazole. When
the Cu (I) is reoxidized to Cu (II), the imidazole
provides a proton to the nascent O
2
¼
converting it
to HO
2
2
, as the bridging to the Zn (II) is reestablished.
The HO
2
2
that leaves the active site protonates in the
water to H
2
O
2
. This mechanism can be depicted
as follows:
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 135
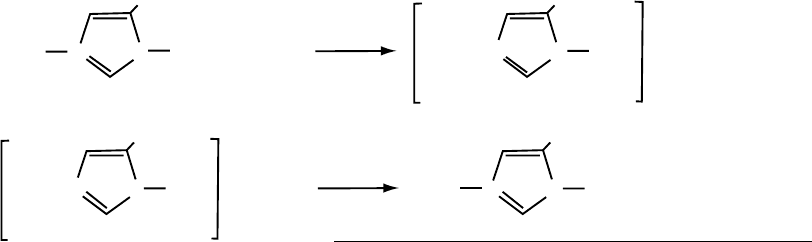
Both of these half-reactions of the catalytic cycle proceed
with a rate constant of , 3 £ 10
9
M
21
s
21
. The enzyme
surface contains charged groups that provide an
electrostatic funnel guiding the O
2
2
toward the active
site crevice. This electrostatic facilitation contributes to
the efficiency of these enzymes.
The Cu, Zn SODs found in eukaryotic cytosols are
homodimeric proteins with subunit weights , 16 kDa.
The corresponding enzyme from the periplasm of
Escherichia coli is monomeric. There is an extracellular
Cu, Zn SOD found in higher animals. It is usually
homotetrameric and glycosylated and has a subunit
weight of , 23 kDa.
THE MN SODS
These enzymes, whether from the matrix of mitochon-
dria or bacteria, exhibit marked sequence similarity,
reflecting a close evolutionary history and supporting
an endosymbiotic origin for mitochondria. The bac-
terial enzyme is usually a homodimer, whereas the
mitochondrial enzyme is a homotetramer. The subunit
weight is , 23 kDa. Some bacterial Mn SODs are
tetrameric. This is the case in Cryptococcus neofor-
mans. In E. coli the biosynthesis of Mn SOD is under
the control of the soxRS regulon, which coordinately
up-regulates the expression of a number of genes in
response to O
2
2
. The constitutively expressed SOX R
protein is transcriptionally inactive in its reduced form.
It can be oxidized by O
2
2
and then activates the
expression of the SOX S protein, which in turn
activates all the genes in the regulon. Thus, Mn SOD
is not measurable in extracts of anaerobically grown
E. coli, but exposure of cultures to aerobic conditions
elicits production of Mn SOD. Increasing production
of O
2
2
, by raising pO
2
, or by adding compounds such as
viologens, which can mediate enhanced production of
O
2
2
, increases the level of Mn SOD. It has been
possible to force E. coli to produce Mn SOD to 7%
of its soluble protein by aerobic exposure to the
viologen paraquat.
The nectar of tobacco flowers has been found to
contain a stable Mn-protein named nectarin that
appears to be an Mn SOD.
THE FE SODS
These SODs, which are highly homologous to the
Mn SODs, are found in bacteria and in plants. Although
usually homodimeric, homotetrameric Fe SOD has been
found in Rhodococcus bronchialis and Mycobacterum
tuberculosis. The Fe SOD of E. coli is constitutive and
thus is found even in anaerobically grown cells. It can
thus be viewed as a standby defense against O
2
2
, which is
always maintained to protect in the event of a sudden
exposure to O
2
.
It is possible to reversibly remove the metals from
SODs. The apo enzymes so prepared are inactive but
can be reactivated by restoring the active site metal. It
is striking, that in spite of sequence and structural
homologies, the Fe SOD is inactive when made to
contain Mn in place of Fe and the Mn SOD is inactive
when similarly reconstituted with the non-native metal
Fe. There are, however, SODs that are active with
either Mn or Fe at their active sites. These SODs,
termed cambialistic SODs, are usually found in anae-
robes such as Propionibacterium shermanii or Strepto-
coccus mutans. These organisms produce the SOD
with iron when grown anaerobically but, when
exposed to low levels of O
2
, produce the same SOD
but with Mn. It is clear that even anaerobes have
need of a defense against the O
2
2
during transient
exposure to O
2
. Ferrous salts are soluble, and thus
available anaerobically, but become insoluble when
oxidized to the ferric state by O
2
. Manganous salts
remain soluble under both conditions – hence the
wisdom of a cambialistic SOD for an anaerobe. An
iron SOD has also been reported in the protozoan
Tetrahymena pyriformis.
Deletions and Consequences
E.COLI
Mutants of E. coli lacking both the Mn SOD and
the Fe SOD (SodA SodB) have been prepared.
Anaerobically they grow as well as the parental strain.
However, aerobically they grow slowly, exhibit a
high rate of mutagenesis, and require branched-chain,
R
N
N Zn(II)
+
O
2
–
+
O
2
–
+H
+
Cu(I)
NH
R
N Zn(II)
+
O
2
Cu(I)
HN
R
N Zn(II)
Cu(II) N
R
N Zn(II)
+
HO
Cu(II)
–
2
136 SUPEROXIDE DISMUTASE
sulfur-containing, and aromatic amino acids. The null
mutants are also hypersensitive towards paraquat. All
of these phenotypic deficits disappear when a functional
gene encoding any active SOD is inserted. The slow
growth of the null mutant can be understood in terms
of the energy devoted to repairing or replacing
oxidatively damaged molecules and to the inactivation
of such O
2
2
sensitive enzymes as aconitase. The high rate
of O
2
-dependent mutagenesis reflects oxidative DNA
damage, and repair, which is error-prone. The nutri-
tional auxotrophies exhibited by the null mutant have
several explanations. The need for branched amino
acids arises because the penultimate step in the
biosynthesis of these amino acids is catalyzed by a
dihydroxy acid dehydratase that contains a [4Fe –4S]
cluster that is rapidly oxidized, and inactivated, by O
2
2
.
The need for sulfur-containing amino acids evidently
reflects leakiness of the cell envelope towards sulfite,
which is an intermediate on the pathway from sulfate
to cysteine. Finally, the aromatic amino acid auxotro-
phy reflects the inability to make erythrose-4-phos-
phate, which is one of the starting materials on the
pathway leading to these amino acids. Erythrose-4-
phosphate, in turn, depends on the sequential actions of
transketolase and transaldolase, and the transketolase
intermediate 1, 2-dihydroxyethylthiamine pyropho-
sphate is rapidly oxidized by O
2
2
. The varied expla-
nations for these phenotypic deficits reflect the variety
of damage that can be caused by O
2
2
and the multiple
targets that are protected by the SODs.
YEAST
Mutants of the yeast Saccharomyces cerevisia lacking
either the cytosolic Cu, Zn SOD or the mitochondrial
Mn SOD have been prepared. Their phenotypic deficits,
which are all oxygen-dependent, include slow growth,
hypersensitivity towards paraquat or quinones, vacuolar
fragmentation, sensitivity towards oxygen, and, in the
case of the Cu, Zn SOD-null, lysine auxotrophy. All of
these problems can be explained on the basis of O
2
2
damage, as was the case for the E. coli SOD-null mutants.
MICE
Murine mutants lacking Mn SOD are severely affected
and those that survive to term are low-birth-weight and
only survive for 4–14 days. Their problems can be seen
as a deficit of mitochondrial functions and that in turn
can be explained on the basis of damage to mitochon-
drial components by O
2
2
. The heterozygotes that have
half the normal level of Mn SOD are viable but have
been shown to be hypersensitive towards oxidative
inactivation of aconitase and towards release of
cytochrome c from mitochondria when challenged
with tertiary butyl hydroperoxide. The deficit imposed
by halving the Mn SOD was also seen as a greater
susceptibility to reperfusion injury.
In contrast to the embryonic and neonatal fatality
imposed by Mn SOD deletion, the homozygous Cu, Zn
SOD-null mice were apparently normal under labora-
tory conditions. They are reported to be relatively
intolerant of neuronal injury and prone to develop
hepatoma at . 9 months of age. It seems likely that
there is some redundancy in scavenging O
2
2
in the
cytosol, so that the lack of Cu, Zn SOD may be
compensated by up-regulation of another O
2
2
scaven-
ger, perhaps a superoxide reductase, such as has been
found in anaerobes.
SOD and Amyotrophic
Lateral Sclerosis
ALS or Lou Gherig’s disease is a late onset, progressive,
and fatal paralytic disease due to death of motor
neurons. There are sporadic and familial types of ALS
and these are not distinguishable on the basis of clinical
symptoms. Approximately 20% of the familial cases of
ALS have been associated with point mutations causing
single amino acid replacements in Cu, Zn SOD.
Transgenic mice expressing the wild-type human Cu,
Zn SOD are normal, but those expressing one of the
ALS-associated mutant Cu, Zn SODs develop progress-
ive paralysis at , 3 months of age. Thus, the mutations
in the SOD cause the death of motor neurons because
of some as yet unknown gain of function, rather than
because of a loss of SOD activity. This is certainly the
case because: most of the mutant SODs retain full SOD
activity; the transgenic mice retain the active murine
Cu, Zn SOD; the disease is genetically dominant, i.e.,
heterozygotes develop the disease; and finally, Cu, Zn
SOD knockout mice do not become paralyzed. The
transgenic mice provide a useful model of the human
disease and are being used to explore both the toxic
gain of function of the mutant Cu, Zn SOD and
treatment modalities.
Mimics
O
2
2
has been found to play causative roles in numerous
inflammatory diseases and reperfusion injuries. For
this reason non-enzymic catalysts of the dismutation
of O
2
2
are being explored as pharmaceutical agents and
appear to have promise.
SEE ALSO THE FOLLOWING ARTICLES
DNA Oxidation † Free Radicals, Sources and Targets
of: Mitochondria † Iron–Sulfur Proteins
SUPEROXIDE DISMUTASE 137
GLOSSARY
apo enzyme Enzyme whose prosthetic group has been removed.
Cu, Zn SOD Superoxide dismutases with copper and zinc at the
active site.
Fe SOD Superoxide dismutases with iron at active site.
HO· The hydroxyl radical.
homodimer Protein composed of two identical subunits.
homotetramer Protein composed of four identical subunits.
Mn SOD Superoxide dismutases with manganese at an active site.
O
2
2
The superoxide anion radical.
FURTHER READING
Hassan, H. M. (1997). Cytotoxicity by oxyRadicals and the evolution
of superoxide dismutases. In Lung Biology in Health and Disease
(L. B. Clerch and D. J. Massaro, eds.) Vol 105, pp. 27–47. Marcel
Dekker, New York.
Maier, C. M., and Chan, R. H. (2002). Role of superoxide dismutases
in oxidative damage and neurodegenerative disorders. Neuroscien-
tist 8, 323–334.
McCord, J. M. (2002). Superoxide dismutases in aging and disease.
Methods Enzymol. 349, 331 –341.
Melov, S. (2002). Therapeutics against mitochondrial oxidative
stress in animal models of aging. Ann. N.Y. Acad. Sci. 959,
330–340.
Storz, G., and Imlay, J. A. (1999). Oxidative stress. Curr. Opin.
Microbiol. 2, 188–194.
BIOGRAPHY
Irwin Fridovich is a James B. Duke Professor of Biochemistry,
Emeritus. He is a member of the National Academy of Science and
of the National Academy of Arts and Sciences. He and his students
discovered and characterized the superoxide dismutase.
138 SUPEROXIDE DISMUTASE

Syk Family of Protein
Tyrosine Kinases
Andrew C. Chan
Genentech, Inc., San Francisco, California, USA
The Syk (spleen tyrosine kinase) family of protein tyrosine
kinases (PTKs) encode essential signaling components required
for normal immunity. Their functions have been most intensely
studied within mammalian immune cells. While not found
within the Caenorhabditis elegans genome, this family of
PTKs is represented earlier within the phylogenetic tree, in the
hydra vulgaris, as a single gene product expressed in epithelial
cells and plays an important function in the recognition of
foreign cells. In mammalian systems, this family of PTKs
appears to have evolved from a gene duplication event to give
rise to its two family members – ZAP-70 and Syk in
mammalian cells. Genetic studies in humans and mice have
demonstrated their essential roles in the function and devel-
opment of T cells, B cells, mast cells, monocytes/macrophages,
and the lymphatic system. Studies further underscore their
importance in T cell antigen receptor (TCR), B cell antigen
receptor (BCR), IgG and IgE receptors (FcRs) and integrin
receptor signaling. This review will discuss our current
understanding of this family of PTKs in mammalian immune
cell function.
Zeta-Chain-Associated Protein
of 70K Mr (ZAP-70)
ZAP-70 was initially identified as a 70K Mr tyrosine
phosphorylated protein that associates with the TCR
following receptor activation. Biochemical and molecu-
lar characterization revealed that ZAP-70 is a PTK with
the characteristic Syk-family signature of tandem Src-
homology (tSH2) domains at its amino (N) terminus and
a carboxy (C)-terminal catalyticdomain. The domainbet-
ween the two SH2 domains has been termed Interdomain
A, while Interdomain B spans between the C-terminal
SH2 and the C-terminal kinase domain (Figure 1).
INTERACTION OF ZAP-70
WITH THE TCR ITAM
Binding studies and, ultimately, the solution of the crystal
structure encoding the tandem SH2 domains revealed
that both SH2 domains of ZAP-70 cooperate to bind the
doubly phosphorylated immunoreceptor tyrosine based
activation (dP-ITAM) motifs (Figure 2). The ITAMs
consist of the consensus sequence D/E x x Y x x L/I X
6–8
Y x x L/I that is represented in the cytoplasmic domains
of integrins as well as each of the signaling subunits
of the TCR, BCR, and FcRs. Both tyrosine residues
within the ITAM are phosphorylated by Src-family PTKs
that, in turn, provide the high-avidity binding sites for the
tSH2 domains of ZAP-70. While the C-terminal SH2
domain binds the N-terminal ITAM tyrosine, the
C-terminal SH2 domain forms a portion of the pocket
for the N-terminal SH2 domain that correspondingly
binds the C-terminal ITAM tyrosine residue. This inter-
dependence of the two SH2 domains explains the rigid
requirements for the tandem SH2 domains of ZAP-70 as
well as the little flexibility of spacing between the two
tyrosine residues within the ITAM sequence in binding
ZAP-70. Mutation of either SH2 domains or mutation of
either ITAM tyrosine results in . 100-fold decrease in
binding avidities and, in turn, a non-functional TCR.
While ZAP-70 was initially found to be inducibly
associated with the TCR in Jurkat T cells, subsequent
studies in human and murine thymocytes and peripheral
T cells revealed that the TCR
z
-subunit is already
phosphorylated in its basal unactivated state, though the
degree of phosphorylation is further augmented follow-
ing TCR activation. In turn, in resting thymocytes and
peripheral T cells, ZAP-70 is constitutively associated
with the TCR, though the degree of association appears
to be augmented concomitant with the degree of
z
-phosphorylation following receptor activation.
A model of sequential phosphorylation of the
z
-chain
was proposed based on observations in the 3.L2 T cell
hybridoma, but this sequence does not appear to apply
to thymocytes nor normal peripheral T cells. In addition
to localizing ZAP-70 to the TCR complex, the tSH2:I-
TAM interaction has also been proposed to regulate
ZAP-70 enzymatic activity. In vitro binding of ZAP-70
to a dpITAM peptide results in enhanced ZAP-70
activity. This model of ZAP-70 activation, however, is
at odds with the pre-existing ZAP-70:ITAM interaction
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 139
described in normal human thymocytes, murine thymo-
cytes, and peripheral T cells.
In contrast to the classical tSH2:dPITAM interaction,
the crystal structure and NMR studies of the tSH2
domains in the absence of an ITAM peptide reveals two
highly flexible independent SH2 domains. These
additional forms of interaction may provide the struc-
tural basis for more non-classical interactions. For
example, the N-terminal SH2 domain alone in conjunc-
tion with Interdomain A of both ZAP-70 and Syk bind
the NXXY motifs within the cytoplasmic domain of the
b
3 integrin. Of note, tyrosine phosphorylation of the
NXXY motif inhibits binding to the SH2 domain. These
more non-classical interactions may, in part, explain
immunofluorescence studies that demonstrate targeting
of ZAP-70 to the T cell cortex independent of the tSH2
domains. In contrast, studies utilizing fluorescence
imaging and immunofluorescence of green fluorescent
protein-tagged ZAP-70 is more consistent with a
cytosolic distribution of ZAP-70 in resting cells with
redistribution to the plasma membrane following
TCR engagement.
SH2
SH2
Kinase
SH2
SH2
Kinase
SH2 SH2
Kinase
ZAP-70
Syk
SykB
492
493
292
315
319
525
526
Vav
PLCg
Lck
Cbl
323
348
352
Vav
PLCg
Cbl
323
348
352
Vav
PLCg
Cbl
Interdomain A Interdomain B
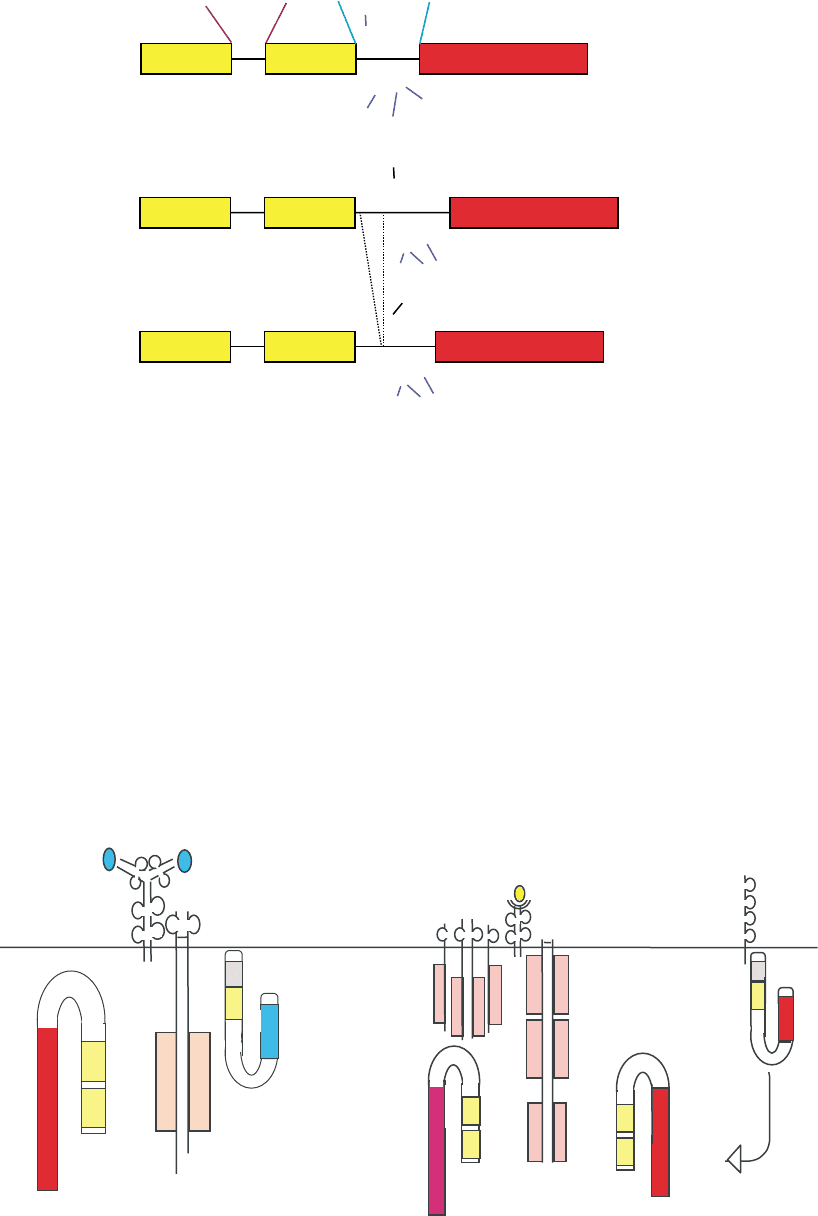
FIGURE 1 Schematic representation of ZAP-70 and Syk PTKs. Schematic structural representation of the structural domains within the
Syk PTKs. T-loop tyrosine residues are represented in red; positive regulatory Interdomain B tyrosine residues are represented in blue;
negative regulatory Interdomain B tyrosine residues are represented in black. Numbering utilizes the human protein sequences for both ZAP-70
and Syk.
a /b
mIg
Syk
I
T
A
M
YPO4
YPO4
2
2
2
3
Src-PTKs
ZAP-70
YPO4
YPO4
YPO4
YPO4
Syk
2
2
I
T
A
M
YPO4
YPO4
ab
z
d
ee
g
2
2
YPO4
YPO4
YPO4
Lc k
CD4
2
3
BCR
TCR
FIGURE 2 Schematic representation of T and B cell antigen receptors with Src- and Syk-PTKs.
140 Syk FAMILY OF PROTEIN TYROSINE KINASES
