Lennarz W.J., Lane M.D. (eds.) Encyclopedia of Biological Chemistry. Four-Volume Set . V. 4
Подождите немного. Документ загружается.

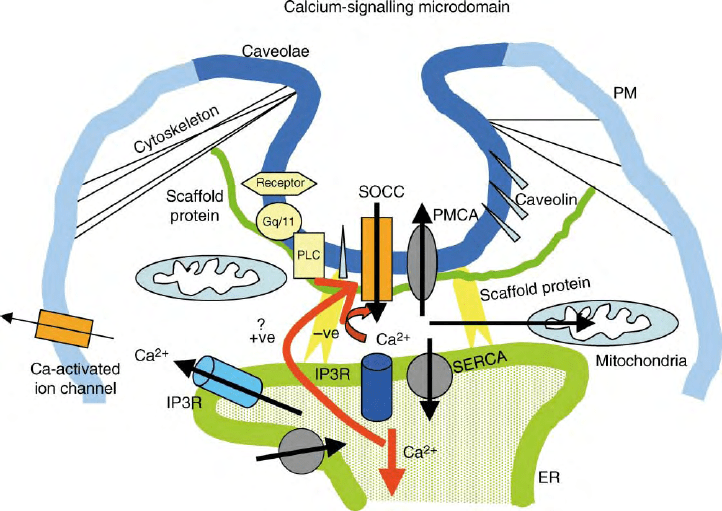
spatially restricted microdomains. During Ca
2þ
influx-
dependent refill of internal Ca
2þ
stores there is minimal
diffusion of Ca
2þ
in the subplasma membrane region
due to rapid uptake into the ER by the SERCA pump,
indicating close apposition between the ER and plasma
membrane at the site of Ca
2þ
influx. SERCA, mitochon-
dria, and PMCA together determine the efficiency of
SOCC by removing Ca
2þ
from the vicinity of the
channel, thereby decreasing Ca
2þ
-dependent feedback
inhibition of the channel. PMCA, mitochondria, and
SERCA have been functionally localized to the micro-
domain where SOCE is occurring. Furthermore, PMCA
and SERCA have been shown to be immunoprecipitated
with TRPC channels. Thus, the architecture of such
Ca
2þ
signaling microdomains facilitates direct physical,
or functional, coupling between the molecular com-
ponents that are involved in regulating plasma mem-
brane SOCC. Current models support the idea that
SOCC and functionally associated proteins are strategi-
cally localized by the action of scaffolding proteins
which allow dynamic regulation of cellular function
(Figure 3).
A candidate for such a microdomain is caveolar lipid
raft domains which have been shown to be involved in
the regulation of SOCE in several cell types. These
domains are functionally and biochemically distinct
microdomains formed by the lateral packing of glyco-
sphingolipids and cholesterol within the membrane
bilayer. It has been proposed that arrangement of the
lipids and scaffolding proteins within LRD forms a
platform for the assembly of a number of proteins into
signaling complexes. This compartmentalization of the
signaling molecules can increase the rate of interactions
and enhance crosstalk networks. Importantly, key
protein and nonprotein molecules involved in the Ca
2þ
signaling cascade, such as PIP
2
,G
a
q/11
, PMCA, several
TRPC proteins, and IP
3
R-like protein, and Ca
2þ
signaling events such as receptor-mediated turnover of
PIP
2
have been localized to caveolar microdomains in
the plasma membrane. Studies have also revealed that
(1) agonist-stimulated Ca
2þ
signal in endothelial cells
originates in specific areas of the PM that are enriched in
caveolin-1, and (2) intact lipid rafts are required for
activation of SOCE. Thus, it has been proposed that
caveolae might regulate the spatial organization of Ca
2þ
signaling by contributing to the localization of Ca
2þ
signaling complex as well as the site of Ca
2þ
entry.
It is now well recognized that Ca
2þ
signaling proteins
are assembled in multiprotein complexes. This finding is
again supported by studies carried out with TRP
proteins. It was earlier shown that the well-studied
TRP prototype, the Drosophila TRP, is assembled in a
signaling complex by the scaffolding action of INAD, a
multi-PDZ domain containing protein. Mammalian
TRPC channels are also assembled in multimeric
protein complexes that are associated with key Ca
2þ
signaling proteins such as G
a
q/11
,IP
3
R, PLC
b
, PMCA,
and SERCA as well as scaffolding proteins such as
FIGURE 3 Proposed calcium-signaling microdomain: Functionally associated proteins are localized in distinct structures and assembled together
by scaffolding proteins as well as cytoskeletal interactions. The architecture of the region enables the coupling between ER and PM that is involved
in activation of SOCE. Protein components are labeled in the figure.
STORE-OPERATED MEMBRANE CHANNELS: CALCIUM 121
caveolin-1, homer, and NHERF. Accessory proteins have
been identified not only for TRPC channels, but also for
PMCA, SERCA, and IP
3
Rs. These proteins encompass a
variety of functions such as trafficking, phosphorylation,
dephosphorylation, scaffolding, etc. Thus, further infor-
mation regarding the protein components and assembly
of the mammalian Ca
2þ
-signaling complex will provide
a better understanding of SOCE. Such knowledge will
impact several important areas of physiology and
pathophysiology.
SEE ALSO THE FOLLOWING ARTICLES
Calcium Signaling: Cell Cycle † Lipid Rafts † Plasma-
Membrane Calcium Pump: Structure and Function †
Voltage-Sensitive Ca
2þ
Channels
GLOSSARY
Ca
21
channels Protein(s) that mediate passive, electrochemical
gradient driven, flux of Ca
2þ
.
Ca
21
pump Protein(s) that utilize energy of ATP hydrolysis to drive
Ca
2þ
flux against its gradient.
Ca
21
signaling Events that initiate elemental changes in cellular
levels of Ca
2þ
which are then decoded into a physiological
response.
cellular microdomains Morphological and functionally distinct sub-
cellular domains.
neurotransmitter Chemical messengers secreted by nerve endings.
FURTHER READING
Berridge, M. J. (1995). Capacitative Ca
2þ
entry. Biochem. J. 312,
1–11.
Isshiki, M., and Anderson, R. G. W. (2003). Function of caveolae in
Ca
2þ
entry and Ca
2þ
-dependent signal transduction. Traffic 4,
717–723.
Montell, C., Birnbaumer, L., and Flockerzi, V. (2002). The TRP
channels, a remarkable functional family. Cell 108, 595– 598.
Muallem, S., and Wilkie, T. M. (1999). G-protein dependent Ca
2þ
signaling complexes in polarized cells. Cell Ca
2þ
26, 173–180.
Parekh, A. B. (2003). Store-operated Ca
2þ
entry: Dynamic interplay
between endoplasmic reticulum, mitochondria, and plasma mem-
brane. J. Physiol. 547, 333–348.
Putney, J. W., Jr., Broad, L. M., Braun, F.-J., Lievremont, J.-P., and Bird,
G. J., St. (2001). Mechanisms of capacitative Ca
2þ
entry. J. Cell Sci.
114, 2223– 2229.
Venkatachalam, K., von Rossum, D. B., Patterson, R. L., Ma, H.-T.,
and Gill, D. L. (2002). The cellular and molecular basis of store-
operated Ca
2þ
influx. Nat. Cell Biol. 4, 263– 272.
BIOGRAPHY
Indu Ambudkar is Chief of Secretary Physiology Section at the
National Institute of Dental and Craniofacial Research, NIH,
in Bethesda, Maryland. She holds both Masters and Ph.D. degrees in
Biochemistry from Lucknow and Madurai Kamaraj Universities in
India and received postdoctoral training at the University of Maryland,
School of Medicine. Her primary research interest is regulation of
cellular Ca
2þ
homeostasis and more recently store-operated Ca
2þ
influx in salivary epithelial cells. Her work has contributed signifi-
cantly to the understanding of SOCE, and the role of TRPC proteins
in this process.
122 STORE-OPERATED MEMBRANE CHANNELS: CALCIUM

Substrate Binding, Catalysis, and
Product Release
W. Wallace Cleland
University of Wisconsin, Madison, Wisconsin, USA
Enzymes catalyze reactions by forming complexes with their
substrate(s), accelerating the chemical reaction by lowering the
activation energy for the reaction, and then releasing
the product(s). The enzyme has to have sufficient affinity for
a substrate to form a complex with it at the physiological
concentration of this molecule. Conversely, however, too great
an affinity for the substrate usually results in too great an
affinity for the product, so that product release becomes rate
limiting. The maximum rate of an enzyme-catalyzed reaction
is limited, in fact, by the relationship between the equilibrium
constant and the kinetic constants called the Haldane
relationship, and one element of this restriction is the affinity
of the substrate.
The Haldane Relationship
As noted above, the Haldane relationship allows
calculation of the maximum rate of an enzyme-catalyzed
reaction. For the simple conversion of substrate A into
product P, the initial rate of the forward reaction in the
absence of P is
v
f
¼ 2d½A
dt ¼ V
1
½A
ðK
a
þ½AÞ ½1
and that in the back reaction is
v
r
¼ 2d½P
dt ¼ V
2
½P
ðK
p
þ½PÞ ½2
where V
1
and V
2
are maximum velocities in forward and
reverse reaction, and K
a
and K
p
are Michaelis constants
for A and P (i.e., apparent dissociation constants in the
steady state).
The Haldane for this mechanism is the ratio of the
V=K values in forward and reverse directions:
K
eq
¼½P
eq
½A
eq
¼ðV
1
=K
a
Þ
ðV
2
=K
p
Þ
¼ V
1
K
p
ðV
2
K
a
Þ½3
V=K; with units of reciprocal time, is the apparent first-
order rate constant for reaction at low substrate
concentration, so eqn. [3] is analogous to the one for a
nonenzymatic reaction where K
eq
would equal k
1
/k
2
,
i.e., the ratio of rate constants in forward and reverse
directions.
To optimize the turnover number of an enzyme
(V
1
/E
t
) in this case, also known as k
cat
, with units of
reciprocal time, we can raise the two V/K values in
constant ratio until one V/KE
t
value (with units of
M
21
s
21
) reaches the limit set by diffusion. V/KE
t
(or k
cat
/K) is a second-order rate constant for productive
combination of enzyme and substrate and subsequent
reaction to give product, and the combination of enzyme
and substrate can only go as fast as these two can diffuse
together. This limit is , 10
9
M
21
s
21
for small sub-
strates, and somewhat less for larger ones like ATP.
Once the V
1
/K
a
E
t
value in eqn. [3] is as high as it can
go, the only way to increase V
1
/E
t
is to increase K
a
as
well. But K
a
cannot exceed the physiological level of A,
or the enzyme will not be at least half-saturated. Thus
V
1
/E
t
has an upper limit, and enzymes that are part of
metabolic pathways are optimized to operate at this
limit. The actual turnover numbers vary, depending on
K
eq
and the physiological level of the substrate, but
evolution has brought these enzymes to the limit set by
the Haldane relationship. On the other hand, enzymes
that are involved in control, as opposed to ones in
metabolic pathways, often sacrifice speed for control
characteristics, and do not have turnovers numbers at
the Haldane limit.
Catalysis
The catalytic process on an enzyme starts once the
substrate is bound. As noted above, the enzyme has to
have sufficient affinity to bind the substrate at its
physiological concentration. The enzyme then has to
lower the activation energy of the reaction sufficiently
for it to go at a rate approaching the maximum given by
the Haldane relationship. It is commonly said that the
enzyme accomplishes this by binding the transition state
structure more tightly than the ground state of the
substrate, but this is really a definition of catalysis.
Molecules resembling the transition state structure are
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 123
often bound much more tightly than the substrate (see
entry on transition state analogues). This is either
because the geometry of the transition state is a better
fit, or includes inherently tighter interactions (stronger
hydrogen bonds, for example; see entry on low barrier
hydrogen bonds). Alternatively, the differential binding
results because the substrate is destabilized when it is
bound (sterically deformed, placed in an unfavorable
electrostatic environment, etc.), but this destabilization
is relieved in the transition state. Of course, destabiliza-
tion in one part of the substrate must be matched by
tight binding in another part, or the substrate will not
have sufficient affinity for the enzyme at its physiological
level. OMP decarboxylase is a classic example, where a
substrate analogue with –COO
2
replaced by – O
2
binds 10
5
-fold more tightly to the enzyme. The
difference is ascribed to charge repulsion between
the carboxyl group of the substrate and an aspartate
on the enzyme.
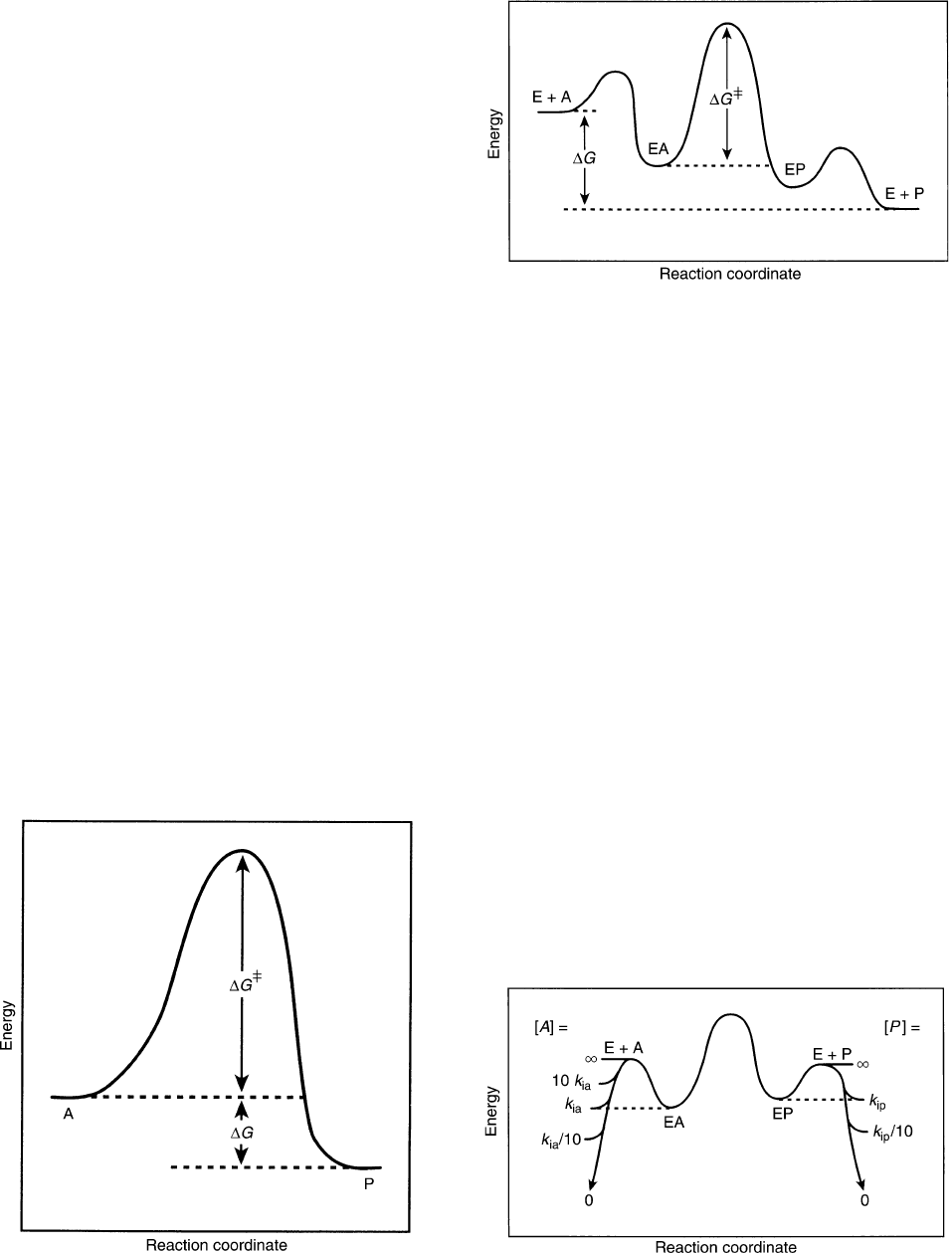
Free-Energy Profiles
The energetics of what happens during an enzymatic
reaction is illustrated by a free-energy profile. Figure 1
shows the free-energy profile at equilibrium for the
nonenzymatic reaction of A to P. The activation energy,
DG
‡
, corresponds to the height of the barrier, and DG
for the reaction ð¼ 2RT ln K
eq
Þ is the difference
between the levels marked A and P. For a corresponding
enzymatic reaction, one must decide what levels of A
and P to use. Figure 2 shows an equilibrium free-energy
profile where the concentration of A is above its
dissociation constant, so that formation of EA has an
equilibrium constant greater than unity, but where P is
less than its dissociation constant, so that EP dis-
sociation is favorable. DG
‡
is given by the height of
the barrier above the level of EA, not E þ A. The
turnover number (V/E
t
or k
cat
) is determined by DG
‡
.
If one picks different levels of A or P, the difference
between E þ A and EA, or E þ P and EP, will differ as
shown in Figure 3. The dissociation level of A (K
ia
)is
convenient, but then the level of P is determined by K
eq
if
an equilibrium free-energy profile is plotted, and will not
usually match K
ip
, its dissociation constant. Free-energy
profiles may, of course, be plotted for nonequilibrium
concentrations of reactants, but in any case it is critical
to state the concentrations of reactions that are assumed
in plotting the profile. When there are two or more
substrates or products involved in the reaction, one must
pick suitable levels of all reactants (and state them!) in
order to plot a free-energy profile.
So far we have considered only simple free-
energy profiles with a single transition state connecting
FIGURE 1 Free-energy profile at equilibrium for an uncatalyzed
reaction. DG
‡
is the activation energy and DG ¼ 2RT ln K
eq
; the
energy difference between product and substrate.
FIGURE 2 Free-energy profile at equilibrium for an enzymatic
reaction. The symbols have the same meaning as in Figure 1.
FIGURE 3 Free-energy profile showing the difference in the levels of
(E þ A) or (E þ P) as the concentrations of A or P are changed. K
ia
and
K
ip
are the dissociation constants of A from EA and of P from EP.
124 SUBSTRATE BINDING, CATALYSIS, AND PRODUCT RELEASE
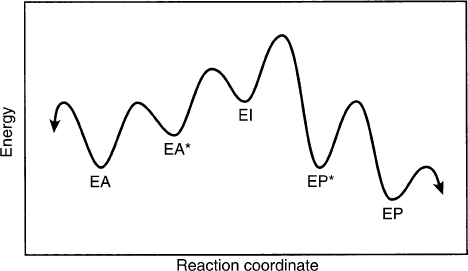
EA and EP. In practice, enzymes have different confor-
mations at the various stages of the catalytic cycle. Thus,
they have open forms where the reactants are free to
bind and dissociate, and closed forms in which catalysis
takes place. The chemistry may also take place in more
than one step, so that intermediates are present. Figure 4
shows a profile where a conformation change converts
the open EA form to a closed, precatalytic EA
p
form.
This undergoes a catalytic reaction to give an EI
intermediate complex, which then is further converted
to the closed EP
p
form. A final conformation change
gives the open EP form, from which P can dissociate.
Rate-Limiting Step for V/K
The two independent kinetic constants for an enzymatic
reaction are the maximum velocity, V, and the ratio of V
and the Michaelis constant, V/K. There is a separate V/K
for each substrate. Each of these parameters varies
separately with pH, ionic strength, temperature, and the
concentrations of other substrates, products, or inhibi-
tors. The Michaelis constant, K, while it measures the
apparent dissociation constant of the substrate in
the steady state, is not an independent constant, but
just the ratio of V and V/K. Thus when one speaks of
“rate-limiting steps” one must specify whether one is
referring to V or V/K.
The rate-limiting step for V/K is the one with the
highest barrier in the free-energy profile. V/K involves
the combination of enzyme and substrate and reaction
through the first irreversible step, which normally is
release of the first product. Later steps that involve
further conformation changes in the enzyme and release
of other products do not affect V/K. Thus the slow
release of NADH from dehydrogenases, which often
limits the maximum velocities of these enzymes, has no
effect on V/K. As a result, the V/K value often is limited
more by the chemistry of the reaction than V,and
isotope effects on the chemistry are more fully expressed
(see entry on kinetic isotope effects). Except for some
slow mutants, full rate-limitation by the chemical
reaction is unusual, however, and the conformation
changes that precede and follow the chemical
reaction are usually partly rate-limiting. The equation
for an isotope effect on V/K is:
x
ðV=KÞ¼ð
x
k þ c
f
þ
x
K
eq
c
r
Þ
ð1 þ c
f
þ c
r
Þ½4
where x defines the isotope effect (D, T, 13, 15, 18 for
deuterium, tritium,
13
C,
15
N,
18
O) and the leading
superscript indicates the ratio of the parameters for light
and heavy isotopes.
x
k is the intrinsic isotope effect on
the chemical step, and
x
K
eq
the equilibrium isotope
effect on the reaction. The constants c
f
and c
r
are
forward and reverse commitments to catalysis and are
the ratio of the rate constant for the isotope sensitive
step to the net rate constant for release from the enzyme
of either the substrate (for c
f
) or the first product (for c
r
).
For the simple mechanism
E þ A O
k
1
k
2
EA O
k
3
k
4
EA
p
O
k
5
k
6
EPQ
p
O
k
7
k
8
EPQ
!
k
9
EQ þ P !
k
11
E þ P þ Q
½5
c
f
¼ðk
5
=k
4
Þð1 þ k
3
=k
2
Þ c
r
¼ðk
6
=k
7
Þð1 þ k
8
=k
9
Þ½6
The commitments thus consist of partition ratios of
intermediates and these ratios correspond to the
differences in barrier heights in a free-energy profile. In
the profile shown in Figure 4, c
f
and c
r
will be small if the
isotope effect measured involves reaction of EI to EP
p
because the barrier between EI and EP
p
is the highest
one. But if the isotope effect measured is on reaction of
EA
p
to EI, c
f
will be small, but c
r
will be large. Thus one
will see largely the equilibrium isotope effect, as the EA
p
to EI step approaches equilibrium.
In the definition of c
f
in eqn. [5], k
5
/k
4
is the internal
part of the commitment (c
f-in
) and k
3
k
5
/(k
2
k
4
) is the
external part (c
f-ex
). A sticky substrate is one where the
net rate constant for reaction of the initial collision
complex to give products is faster than the rate constant
for dissociation (k
2
). This ratio is called the stickiness
ratio (S
r
) and is given by S
r
¼ c
f-ex
=ð1 þ c
f-in
þ c
r
Þ in
terms of the parameters of eqn. [5].
Rate-Limiting Steps for V
The rate-limiting step for the maximum velocity depends
on the definition of rate limiting. One definition is the
“slowest” step, or the one with the highest individual
barrier in the forward direction. Another definition is the
“least-conductive” step, which is the one that sees the
highest total barrier to reach an irreversible step. A third
definition is the “most-sensitive” step. This is the one
FIGURE 4 Free-energy profile showing conformation changes in the
EA and EP complexes, as well as an intermediate between the two
activated complexes. The levels of (E þ A) and (E þ P) are not shown.
SUBSTRATE BINDING, CATALYSIS, AND PRODUCT RELEASE 125
which causes the greatest percentage change in the
turnover number for a given percent change in
the forward rate constant. An isotope effect on this
step is more fully expressed than one on any other step.
The isotope effect on V for mechanism 3 is
x
V ¼ð
x
k þ c
v
f
þ
x
K
eq
c
r
Þ
ð1 þ c
v
f
þ c
r
Þ½7
where
x
k;
x
K
eq
; and c
r
have the same meaning as in eqn.
[5], but
c
v
f
¼½k
3
k
5
=ðk
3
þ k
4
Þ½1=k
3
þð1=k
7
Þð1 þ k
8
=k
9
Þ
þ 1=k
9
þ 1=k
11
½8
Note that c
v
f
; unlike c
f
, does not consist of partition
ratios. Rather k
5
, reduced by the factor k
3
=ðk
3
þ k
4
Þ; is
compared to k
3
, and to each of the net rate constants
after the chemical step. A low value of k
11
, for example,
can result in a large value of c
v
f
; and a greatly reduced
expression of the isotope effect on V.
The “slowest,” “least-conducting,” and “most-sensi-
tive” steps may be the same in an enzymatic reaction,
but need not be, so one must define what one means
when using the term “rate-limiting” for the maximum
velocity.
SEE ALSO THE FOLLOWING ARTICLES
Enzyme Inhibitors † Enzyme Kinetics † Enzyme
Reaction Mechanisms: Stereochemistry † Kinetic Iso-
tope Effects † Low Barrier Hydrogen Bonds † Meta-
bolite Channeling: Creatine Kinase Microcompartments
GLOSSARY
Haldane relationship An equation relating the equilibrium constant
to the kinetic constants of an enzymatic reaction.
isotope effect The effect of isotopic substitution, expressed as the
ratio of the parameter for the light isotope to that for the heavy one.
Michaelis constant The concentration of a substrate that gives half of
the maximum velocity of an enzymatic reaction. It is the apparent
dissociation constant in the steady state.
substrate A molecule that is converted to a product during an
enzyme-catalyzed reaction.
turnover number The number of substrate molecules converted to
product per enzyme molecule per unit time. The units are usually
reciprocal seconds.
FURTHER READING
Cleland, W. W. (1982). An analysis of haldane relationships. Meth.
Enzymol. 87, 366–369.
Cleland, W. W. (1986). Enzyme kinetics as a tool for determination of
enzyme mechanisms. In Investigation of Rates and Mechanisms of
Reactions (C. F. Bernasconi, ed.) 4th edition, Part 1, pp. 791–870.
John Wiley & Sons, New York.
Cleland, W. W., and Northrop, D. B. (1999). Energetics of substrate
binding, catalysis, and product release. Meth. Enzymol. 308, 3–27.
BIOGRAPHY
W. Wallace Cleland is M. J. Johnson Professor of Biochemistry at the
University of Wisconsin and a co-director of the Institute for Enzyme
Research. His research interest is in the use of kinetic methods for
the determination of enzyme mechanisms, and in particular the use of
isotope effects. He has a Ph.D. from the University of Wisconsin and
was a postdoctoral Fellow at the University of Chicago. He is a
member of the National Academy of Sciences and the American
Academy of Arts and Sciences.
126 SUBSTRATE BINDING, CATALYSIS, AND PRODUCT RELEASE

Sugar Nucleotide Transporters
Carlos B. Hirschberg
Boston University School of Dental Medicine, Boston, Massachusetts, USA
Sugar nucleotide transporters are proteins in the membrane
of the Golgi apparatus. Their role is to translocate, from the
cytosol into the Golgi lumen, nucleotide substrates for the
glycosylation of proteins and lipids. Mutants in the above
transporters have biochemical and developmental pheno-
types, resulting in diseases such as leukocyte adhesion
deficiency II.
A Requirement for Sugar
Nucleotide Transport into
the Golgi Apparatus Lumen
Approximately half of all proteins in eukaryotic
cells are secreted or membrane-bound. These proteins
are processed through the secretory pathway where
80% undergo post-translational modifications such
as glycosylation, sulfation, and phosphorylation in
the lumen of the Golgi apparatus. Intact nucleotide
sugars, nucleotide–sulfate, and ATP, the substrates
for these reactions, enter the lumen of the Golgi
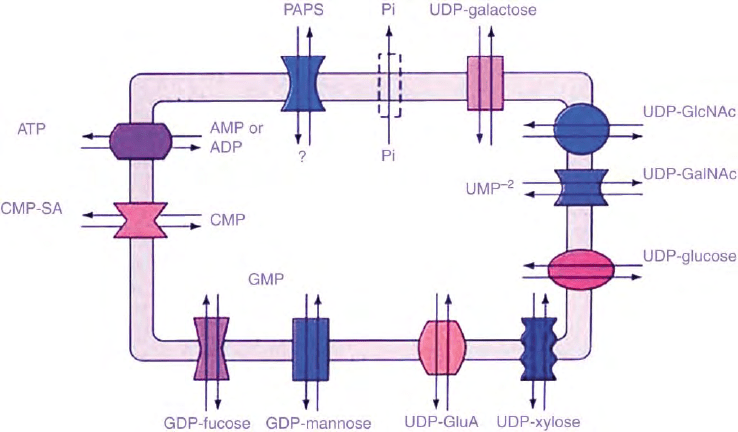
apparatus via specific transporter proteins (Figure 1).
These are multi-transmembrane spanning proteins
which function as antiporters with the corresponding
nucleoside monophosphates and thereby concentrate
the nucleotide derivatives in the Golgi lumen relative
to their concentration in the cytosol (Figure 1).
Evidence for this mechanism has been obtained
through biochemical studies with rat liver-derived
Golgi vesicles as well as with different species of
yeast. In the latter, gene disruption of a Golgi lumenal
GDPase, which generates GMP (the antiporter
molecule coupled to entry of GDP-mannose into the
yeast Golgi lumen), results in severe undermannosyla-
tion of proteins and lipids in vivo, reduced transport
of the GDP-mannose into Golgi vesicles in vitro,
as well as in a failure to make hyphae in Candida
albicans.
Mutants in Nucleotide Sugar
Transport—Biochemical
and Developmental Phenotypes
Including Diseases
Mutant mammalian cells grown in tissue culture have
been described as having 95% reduced transport of
specific nucleotide sugars into their Golgi apparatus,
while their proteins and lipids have a drastic reduction in
the specific sugar transported by the corresponding
nucleotide derivative transporters. Multicellular orga-
nisms such as C. elegans, Drosophila melanogaster,
plants, and humans that have mutations in the above
proteins also have distinct morphological phenotypes
affecting development of limbs, wings, brain, etc.
Studies of these mutants have shown that some of the
transporters can translocate more than one nucleotide
sugar, whereas other transporters retain high substrate
specificity such as differentiating between sugar epimers.
Mutants in nucleotide sugar transport have also been
described in Leishmania donovani and in yeasts such as
S. cerevisiae, K. lactis,andC. albicans.
Structure of Golgi Nucleotide
Sugar Transporters
So far the primary amino acid sequence, together with
the substrate specificity of approximately two dozen
Golgi membrane nucleotide sugar transporters, has
been determined. In most cases this was done by
correcting the phenotypes of mutant cells or mutant
multicellular organisms with libraries containing wild-
type DNA or cDNA from homologous or heterologous
organisms. In most cases after phenotypic correction,
the specific DNA (or cDNA) was expressed in yeast
or mammalian cells, Golgi vesicles were isolated, and
transport of different nucleotide sugars assayed
in vitro. Yeast is a particularly attractive system for
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 127
heterologous expression of nucleotide sugar transporter
genes, because it has few endogenous nucleotide sugar
transport activities. This approach led to the identifi-
cation of multisubstrate nucleotide sugar transporters
from C. elegans. In some instances a particular cDNA
was also tested for its ability to correct the phenotype
of mutant cells where the substrate specificity of the
defective nucleotide sugar is known.
The fact that the above approaches for determining
the substrate specificity of particular transporters have
worked is based on the following points:
1. the transporters DNA (or cDNA) can express the
proteins in a heterologous system;
2. the targeting signals allowing the protein to
localize to the Golgi apparatus are functional in different
cell types; and
3. the targeted transporter is active once it reaches
the Golgi apparatus of the recipient cell and can
therefore affect phenotypic correction.
To date, the relationship of amino acid sequences and
substrate specificities of nucleotide sugar transporters
can be summarized as follows:
1. substrate specificities of nucleotides sugar trans-
porters cannot be inferred even when amino acid
sequences are , 50% identical;
2. only sequence identities of over 80% allow
accurate predictions of substrate specificity; and
3. transporters from different organisms that have
the same substrate specificity may have only 20%
overall amino acid sequence identity.
Due to the above reasons the reader is cautioned that
annotations about the identity (substrate specificity) of
nucleotide sugar transporters in databases should be
looked at very carefully!
Topography of Nucleotide
Sugar Transporters in
the Golgi Membrane
So far the only detailed topographic study of a
nucleotide sugar transporter has been done with that
for CMP-sialic acid in mammalian cells. Using a
combination of tagged epitopes and immunocyto-
chemistry, the transporter was found to have ten trans-
membrane domains and both its amino and carboxy
termini facing the cytosol. A study on the topography
of the Kluyveromyces lactis UDP-acetylglucosamine
transporter using membrane accessibility of a peptide
antibody and amino and carboxy termini epitope
tags also found that both termini face the cytosol
and that this transporter has either six or eight trans-
membrane domains. It is important to stress that none
of the existing algorithms in the literature are designed
specifically to address the issue of protein topography
in the Golgi apparatus membrane which is somewhat
thinner than the plasma membrane; most algorithms
are designed for predicting topography in the
plasma membrane.
FIGURE 1 Golgi membrane nucleotide sugar, PAPS, and ATP transporters have been detected in mammals, yeast, protozoa, nematodes, insects,
and plants. They are antiporters with the corresponding nucleoside monophosphate, except for PAPS, where the antiporter is not known, and for
ATP, where it is either AMP or ADP or both.
128 SUGAR NUCLEOTIDE TRANSPORTERS
Regulation of Macromolecular
Glycosylation by Nucleotide
Sugar Transporters
Studies with mutant mammalian cells in nucleotide
sugar transporters grown in tissue culture as well as with
fibroblasts and lymphoblasts from patients with leuko-
cyte adhesion deficiency (LAD) syndrome II showed that
decreased concentrations of nucleotide sugar in the
Golgi lumen result in selective hypoglycosylation of
proteins rather than across the board reduction. Thus,
mutant MDCK cells which have a 95% reduction in
transport of UDP-galactose into their Golgi lumen have
a corresponding decrease in galactosylation of bulk
glycoproteins, glycolipids, and keratan sulfate but not in
heparan and chondroitin sulfates. LAD II patients
appear to have normal O-fucosylation of Notch protein,
whereas N-fucosylation of bulk proteins is drastically
reduced. Although the detailed mechanism for these
biochemical phenotypes is not understood, an attractive
hypothesis is that when supply of nucleotide sugars is
limited due to impairment in their transport, glycosyla-
tion reactions with lower K
m
s would proceed, whereas
those with higher K
m
s would not.
SEE ALSO THE FOLLOWING ARTICLES
Glycosylation, Congenital Disorders of † Golgi
Complex † Protein Glycosylation, Overview
GLOSSARY
glycosylation The covalent addition of sugars to each other or to
proteins and lipids.
Golgi apparatus A membrane-enveloped organelle in the cytoplasm
of all eukaryotes composed of several cisternae and vesicles;
virtually all membrane-bound and secreted proteins of eukaryotes
traverse through this organelle in route to their final location within
or outside the cell.
nucleotide sugar Phosphodiesters, usually between nucleoside 5
0
diphosphates and carbon 1 of the sugar.
phenotype The visual or physical characteristics of individual cells or
a multicellular organism.
FURTHER READING
Abeijon, C., Mandon, E. C., and Hirschberg, C. B. (1997).
Transporters of nucleotide sugars, nucleotide sulfate and ATP in
the Golgi apparatus. Trends Biochem. Sci. 22, 203–207.
Hirschberg, C. B., Robbins, P. W., and Abeijon, C. (1998).
Transporters of nucleotide sugars, ATP and nucleotide sulfate in
the endoplasmic reticulum and Golgi apparatus. Ann. Rev.
Biochem. 67, 49 –69.
Lodish, H., Berk, A., Zipusky, S. L., Matsudaira, P., Baltimore, D.,
and Darnell, J. (2000). Molecular Cell Biology. W.H. Freeman,
New York.
Varki, A., Cummings, R., Esko, J., Freeze, H., Hart, G., and Marth, J.
(1999). Essentials of Glycobiology. Cold Spring Harbor Labora-
tory Press, New York.
BIOGRAPHY
Carlos B. Hirschberg is a Professor and Founding Chairperson of the
Department of Molecular and Cell Biology at the Boston University
Goldman School of Dental Medicine. He holds a Ph.D. in Chemistry
from the University of Illinois at Urbana-Champaign and received his
postdoctoral training at the Harvard Medical School and MIT. His
principal research interests are mechanisms of posttranslational
modifications in eukaryotes. His laboratory co-workers discovered
the transport/antiport system for nucleotide derivatives in the Golgi
apparatus and endoplasmic reticulum membranes and were the first
to purify and clone proteoglycan sulfotransferases.
SUGAR NUCLEOTIDE TRANSPORTERS 129

SUMO Modification
Frauke Melchior and Andrea Pichler
Max-Planck Institute of Biochemistry, Martinsried, Germany
Small ubiquitin-related modifier, SUMO-1, and its homol-
ogues are 10–12 kDa eukaryotic proteins that serve to regulate
protein function. Like their relative ubiquitin, they are
covalently coupled to many different target proteins in the
cell. Specific enzymes required for the formation or cleavage of
isopeptide bonds between SUMO and its targets ensure
specificity and dynamics of this posttranslational modification.
Functional consequences reach from changes in protein-
protein or protein–DNA interactions, alteration in subcellular
localization, or enhanced stability, to changes in biological
activity.
The SUMO Family
EXPRESSION
Proteins of the small ubiquitin-related modifier (SUMO)
family appear to exist in all eukaryotic cells. For
nomenclature see Table I. The number of distinct family
members varies from one (e.g., in baker’s and fission
yeasts, nematodes, and fruit fly) to several (three in
mammals; eight in Arabidopsis thaliana).Where present,
different family members appear to have at least
partially distinct functions. Interestingly, mammalian
SUMO1 is mainly conjugated to substrates, while
mammalian SUMO2/3 primarily exists in its free form
under normal growth conditions, but is conjugated
rapidly after stress stimuli. Whether SUMO proteins are
differentially expressed, and their expression levels are
regulated, is currently unknown. Available knockout
data suggest that reversible SUMO conjugation is
essential for life in most organisms. Fission yeast
grows without a functional SUMO gene, but the cells
are severely impaired.
STRUCTURE
SUMO proteins are small acidic proteins with distant
homology to ubiquitin. At primary amino acid sequence,
they are 10–20% identical to ubiquitin. On a structural
level, their relatedness is much more pronounced.
As shown in Figure 1, ubiquitin and SUMO share
the classical ubiquitin-superfold. Characteristic for
all members of the SUMO family, and absent from
ubiquitin or other ubiquitin-related proteins, is an
N-terminal flexible extension of 10–30 amino acids.
The function of this extension is however currently
unknown.
All SUMO proteins are expressed as precursors that
require trimming of their C-terminus. Proteolytic
removal of several amino acids, which is accomplished
by specific enzymes (SUMO isopeptidases), results in
exposure of a C-terminal glycine–glycine motif. Matu-
ration is rapid, and there is currently no evidence for
regulation of this process.
Enzymology
Covalent interaction between SUMO and its targets is a
reversible, often dynamic, process. This is accomplished
by formation and subsequent cleavage of an isopeptide
bond between the carboxy-terminus of mature SUMO
and a lysine of the acceptor protein. Isopeptide bond
formation requires ATP and involves several (two or
three) distinct enzymes (see Figure 2). In contrast,
cleavage is accomplished by a single enzyme and does
not require energy.
CONJUGATION
Overview
The first step towards isopeptide bond formation is
activation of the SUMO carboxy-terminus. This is
accomplished by ATP-dependent thioester bond for-
mation between a cysteine residue in the E1 activating
enzyme and the C-terminal glycine residue in SUMO.
Subsequently, SUMO is transferred to a cysteine residue
in the E2 conjugating enzyme and again the resulting
bond is a thioester. Finally, an isopeptide bond is formed
by transferring SUMO to the 1-amino group in a lysine
of the acceptor protein. Depending on the specific
acceptor protein, this step can be carried out by the E2
conjugating enzyme alone, or it may require an
additional component, a so-called E3 ligase.
Encyclopedia of Biological Chemistry, Volume 4. q 2004, Elsevier Inc. All Rights Reserved. 130
