Latchman. Eukariotic Transciption Factors
Подождите немного. Документ загружается.

system of the embryo. However, in moving from the 5’ to the 3’ end of the
cluster (i.e. from Hoxb-9 (2.5) to Hoxb-1 (2.9) in Fig. 7.11) each successive
gene is expressed earlier in development and also displays a more anterior
boundary of expression within the central nervous system (Figs 7.12 and 7.13).
Similar expression patterns have also been observed in Drosophila where each
successive gene in the Bithorax and Antennapedia clusters is expressed more
REGULATION OF TRANSCRIPTION FACTOR SYNTHESIS 221
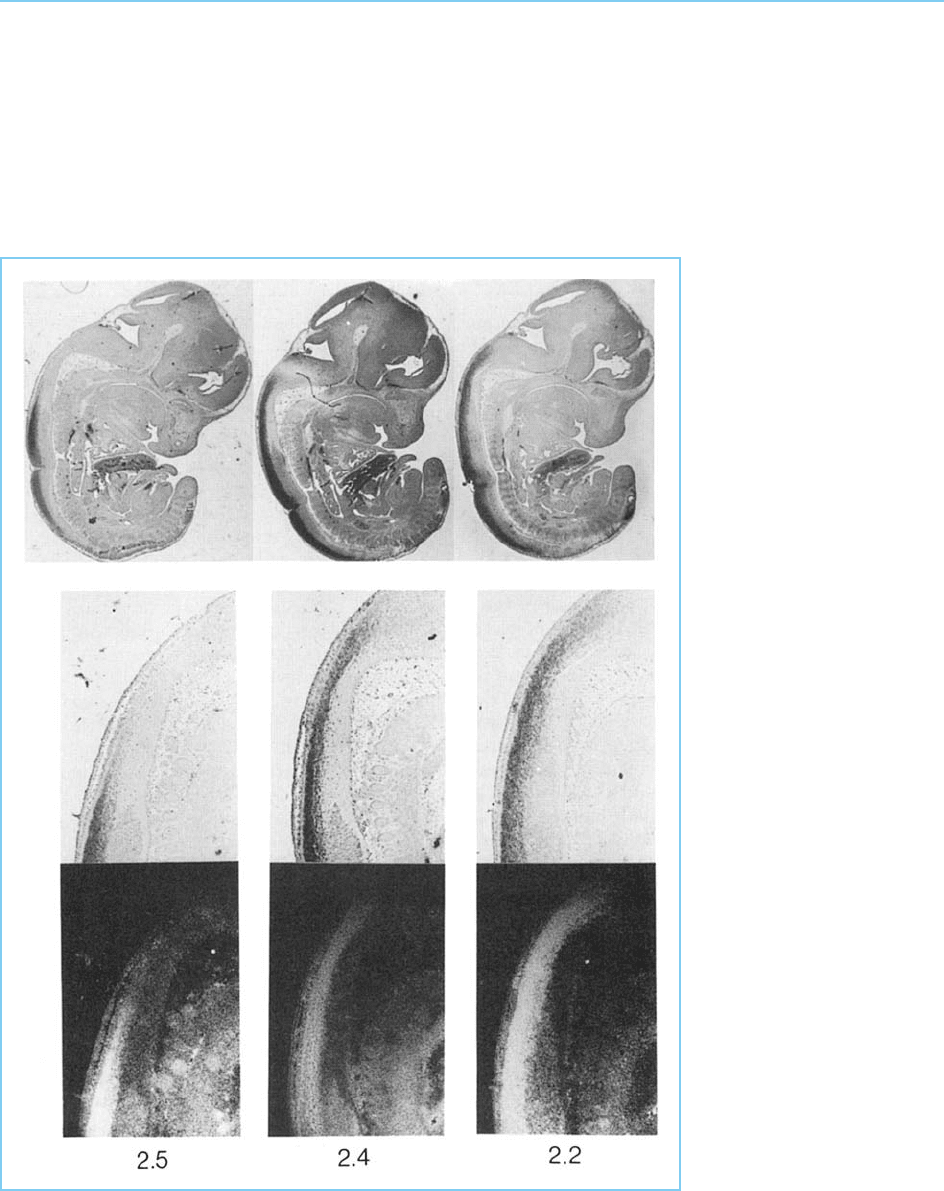
Figure 7.12
Comparison of the
expression pattern of the
Hoxb-9 (2.5), b-8 (2.4)
and b-6 (2.2) genes in
the 12.5-day mouse
embryo. The top panel
shows in situ
hybridization with the
appropriate gene probe to
a section of the entire
embryo while the middle
row shows a high power
view of the region in
which the anterior limit of
gene expression occurs.
In these panels, which
show the sections in
bright field, hybridization
of the probe and
therefore gene expression
is indicated by the dark
areas. In the lower panel,
which shows the same
area in dark field,
hybridization is indicated
by the bright areas. Note
the progressively more
anterior boundary of
expression of Hoxb-6
(2.2) compared to Hoxb-8
(2.4) and to Hoxb-9 (2.5)
and compare with their
positions in the Hoxb
(Hox 2) complex in Figure
7.11.
anteriorly and affects progressively more anterior segments when it is
mutated. Indeed, studies in which regulatory elements from the invertebrate
Amphioxus were tested in mouse and chick embryos have indicated that the
elements regulating homeobox gene expression have been highly conserved
in evolution with the Amphioxus elements functioning in these very different
species (Manzanares et al., 2000) .
In the case of the mouse genes a possible molecular mechanis m for the
differential expression pattern across a cluster is provided by the finding that
genes in the 3’ half of the Hoxb cluster are activated in cultured cells by
treatment with low levels of retinoic acid, whereas genes in the 5’ half of
the cluster require much higher levels of retinoic acid for their activa tion
(for review see Conlon, 1995; Tabin, 1995). Considerable eviden ce exists
that retinoic acid can act as a morphogen in vertebrate development and it
has been suggested that a gradient of retinoic acid concentration may exist
across the developing embryo (reviewed by Conlon, 1995; Tabin, 1995).
Hence, the observed difference in expression of the Hoxb genes could be
controlled by a retinoic acid gradient (Fig. 7.14). In turn, the Hoxb genes like
their Drosophila counterparts would switch on other genes required in cells at
222 EUKARYOTIC TRANSCRIPTION FACTORS
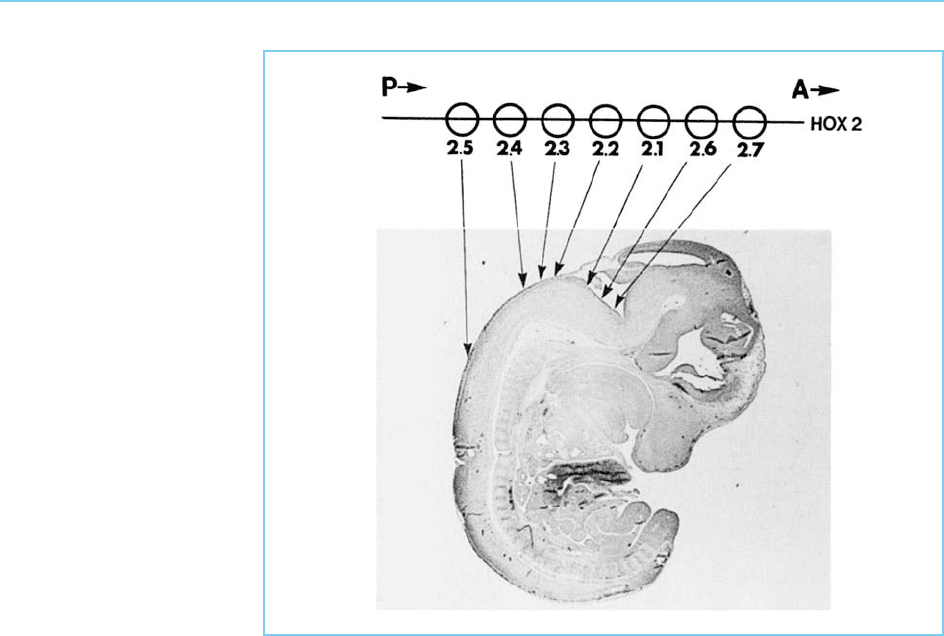
Figure 7.13
Summary of the anterior
boundary of expression of
the genes in the Hoxb (2)
complex indicated on a
section of a 12.5-day
mouse embryo and
compared to the position
of the gene in the Hoxb
(2) cluster. Note the
progressively more
anterior boundary of
expression from the 5’ to
the 3’ end of the Hoxb
(2) cluster.
particular positions in the embryo accounting for the morphogenetic effects
of retinoic acid.
Retinoic acid functions by binding to and activating specific recept ors
which are members of the steroid-thyroid hormone receptor super family
and which in turn bind to specific sequences within retinoic acid responsive
genes activating their expression (see Chapter 4, section 4.4, and Chapter 8,
section 8.2.2). Hence, the activation of regulatory genes and the initiation of a
regulatory cascade can be achieved by the activation of specific receptors-
transcription factors by an inducing stimulus.
This illustrates therefore how the synthesis of one set of transcription
factors (the homeobox proteins) can be regulated by the activation of another
set of transcription factors (the retinoic acid receptors). In agreement with
this idea, the treatment of mouse embryos with retinoic acid results, for
example, in changes in the expression pattern of the Hoxb-1 gene which
contains a retinoic acid response element in its 3’ regulatory region.
Moreover, the inactivation of this element so that it no longer binds the
retinoic acid receptors, abolishes expression of Hoxb-1 in the neuroectoderm
of the early embryo, providing direct evidence that the retinoic acid response
element is necessary to produce the expression pattern of this gene observed
in the developing embryo (Marshall et al., 1994; for review see Stern and
Foley, 1998).
REGULATION OF TRANSCRIPTION FACTOR SYNTHESIS 223
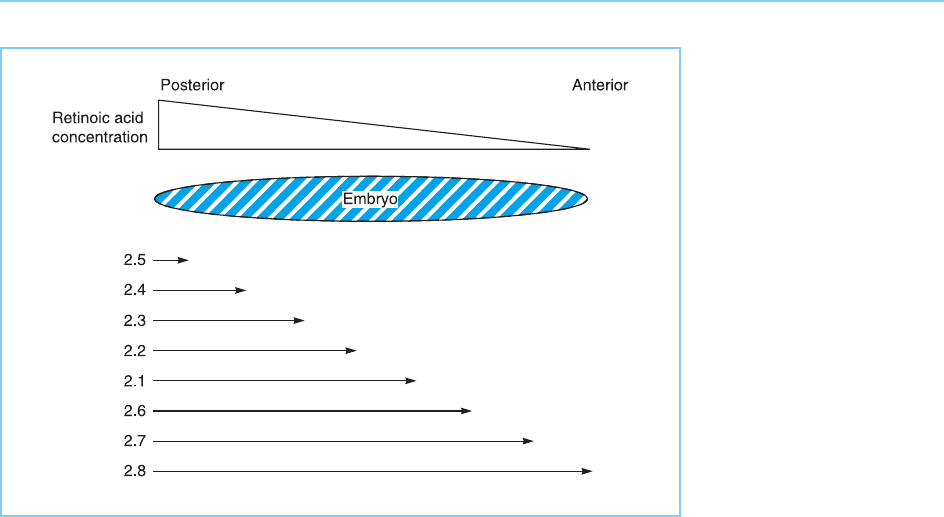
Figure 7.14
Model for the
progressively more
anterior expression of the
genes in the Hoxb (2)
cluster in which
expression is controlled
by a posterior to anterior
gradient in retinoic acid
concentration and the
increasing sensitivity to
induction by retinoic acid
which occurs from the 5’
to the 3’ end of the
cluster. Thus because
genes at the 3’ end of
the cluster are inducible
by very low levels of
retinoic acid they will be
expressed in anterior
points of the embryo
where the retinoic acid
level will be too low to
induce the genes at the
5’ end of the cluster
which require a much
higher level of retinoic
acid to be activated.
Interestingly, the regulation of Hox gene expression by such DNA response
elements located adjacent to the individual genes appears to interact with
other regulatory processes which operate over the whole gene cluster. Thus,
in experiments where individual Hox genes (with their adjacent control ele-
ments) were moved to a different position withi n the gene cluster, their
pattern of expression was altered so that they behaved similarly to genes
normally located at that position in the cluster for example, in terms of the
time at which they were switched on during development (van der Hoeven et
al., 1996) (Fig. 7.15).
In the case of the HoxD cluster, this effect appears to involve the order of
the genes relative to a distant enhancer element located at least 100 000 bases
away. Thus, in this cluster the first gene, HoxD13 is expressed most anteriorly
and at the highest level with each successive gene being exposed at lower
levels and more posteriorly. If Hox D13 is deleted, the next gene in the cluster
HoxD12 is now expressed in the manner typical of Hox D13 even though it
remains in its normal position (Kmita et al., 2002; for review see Zeller and
Deschamps, 2002). In this case therefore, the genes appear to compete to
interact with the distant enhancer element so that the closest gene is
expressed in a particular pattern and so on (Fig. 7.16). This effect is evidently
reminiscent of the locus control region (LCR) in the -globin gene cluster (see
Chapter 1, section 1.3.4) where the globin genes were expressed in a specific
order in development which is determined by their position relative to the
LCR.
The specific pattern of expression of individual homeobox genes, which is
determined by their position in the cluster is absolutely critical to their func-
tion. Indeed, it appears that it is the different patterns of regulation rather
224 EUKARYOTIC TRANSCRIPTION FACTORS
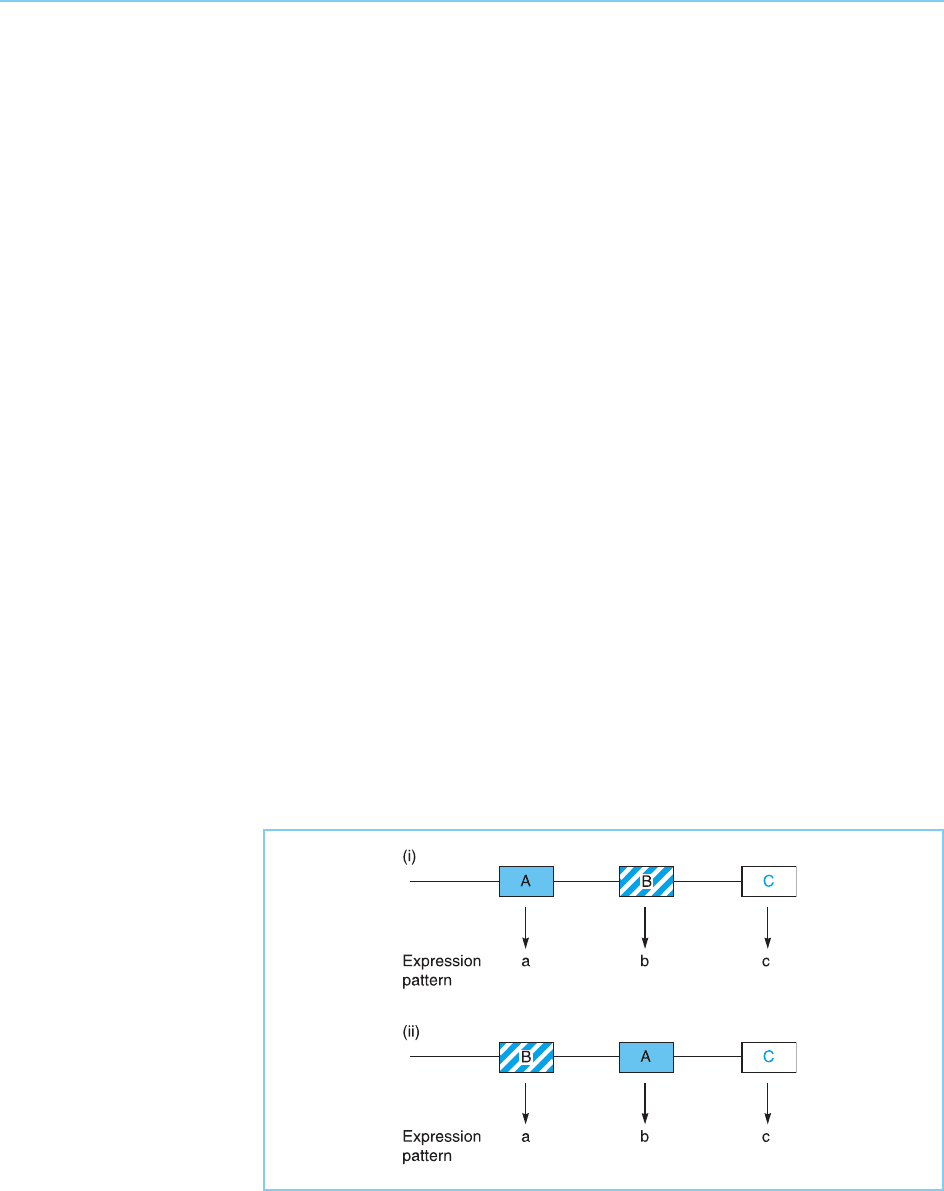
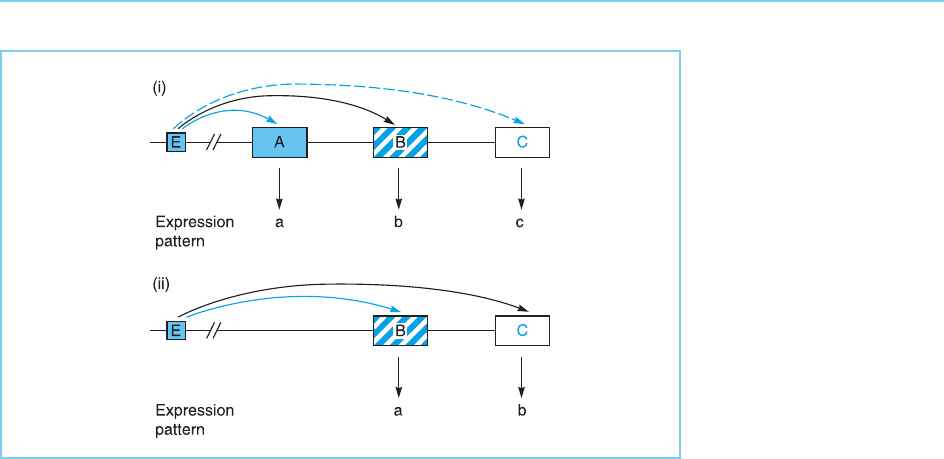
Figure 7.15
Each gene in a Hox
cluster has its own
specific expression
pattern (panel i). Moving
a particular gene to a
new position in the
cluster results in it having
the expression pattern of
the gene which is
normally located at that
position (panel ii).
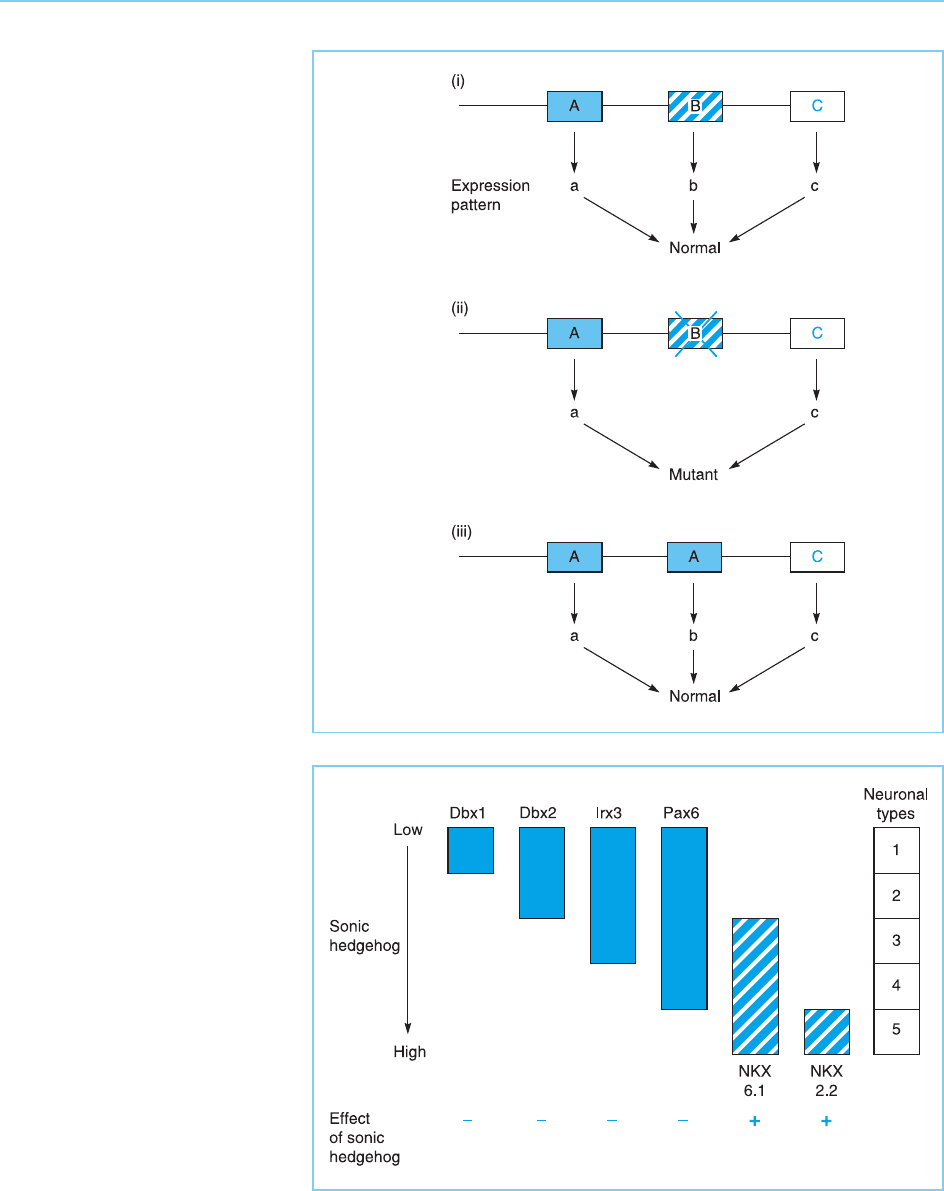
than the different proteins that they encode, that determine the different
roles of specific genes in a cluster. Thus, if an individual gene in a cluster is
deleted, the other genes in the cluster cannot substitute for it and an abnor-
mal anim al results (Fig. 7.17). However, if the deleted gene is replaced by a
further copy of another gene in the cluster, then a normal animal results (for
review see Dubou le, 2000) (Fig. 7.17). This occurs because the expression of
the inserted gene is now determined by its position in the cluster and it is
therefore express ed in the manner characteristic of the deleted gene. Hence,
the products of different genes in a cluster can functio nally substitute for one
another but only if they are expressed in the appropriate pattern, as deter-
mined by their position in the cluster. This illustrates the critical role of the
regulated synthesis of transcription factors in allowing them to produce their
functional effects.
The manner in which the regulated synthesis of multiple homeobox factors
can regulate the production of several different cell types has been analysed in
detail in the ven tral neural tube. In this case, the system is regulated by a
gradient in the concentration of a protein signalling molecule known as sonic
hedgehog (Shh) rather than via a retinoic acid gradient. The expression of
several homeobox factors (Dbx1, Dbx2, Irx3 and Pax6) is repressed by Shh,
but their sensitivity to such repression differs so that Pax6, for example, is
expressed at higher Shh concentrations than Irx3 and so on (Fig. 7.18). In
contrast, two oth er homeobox genes are activated by Shh but their sensitivity
differs so that Nkx6.1 is expressed at lower levels of Shh than Nkx2.2 (Fig.
7.18).
REGULATION OF TRANSCRIPTION FACTOR SYNTHESIS 225
Figure 7.16
In the HoxD cluster, the
expression of the genes
is affected by their order
relative to a distant
enhancer element (E)
(panel i). Deletion of
gene A results in gene B
being the closest to the
enhancer. It is therefore
expressed in the normal
pattern for gene A even
though its physical
location is unchanged
(panel ii).
226 EUKARYOTIC TRANSCRIPTION FACTORS
Figure 7.18
In the ventral neural tube, a
gradient of sonic hedgehog
regulates the expression of
several homeobox genes. Dbx1,
Dbx2, Irx3 and Pax6 are
repressed by sonic hedgehog
but differ in their sensitivity to
repression. Thus, Pax6, which
is the least sensitive to
repression, is expressed at
higher sonic hedgehog
concentrations than Irx3 and so
on. Conversely, Nkx6.1 and
Nkx2.2 are activated by sonic
hedgehog with Nkx6.1 being
activated at lower
concentrations than Nkx2.2.
Together these effects create a
homeodomain code in which
each region has a different
pattern of expression of the six
genes and hence different
neuronal types (1–5) form at
each point.
Figure 7.17
The normal expression pattern
of Hox genes results in the
production of a normal animal
(panel i). Inactivation of a
specific gene results in a
mutant animal being produced
(panel ii). However, if the
mutant gene is replaced with a
further copy of another gene in
the cluster, this gene is
expressed in the same way as
the deleted gene and a normal
animal results (panel iii).
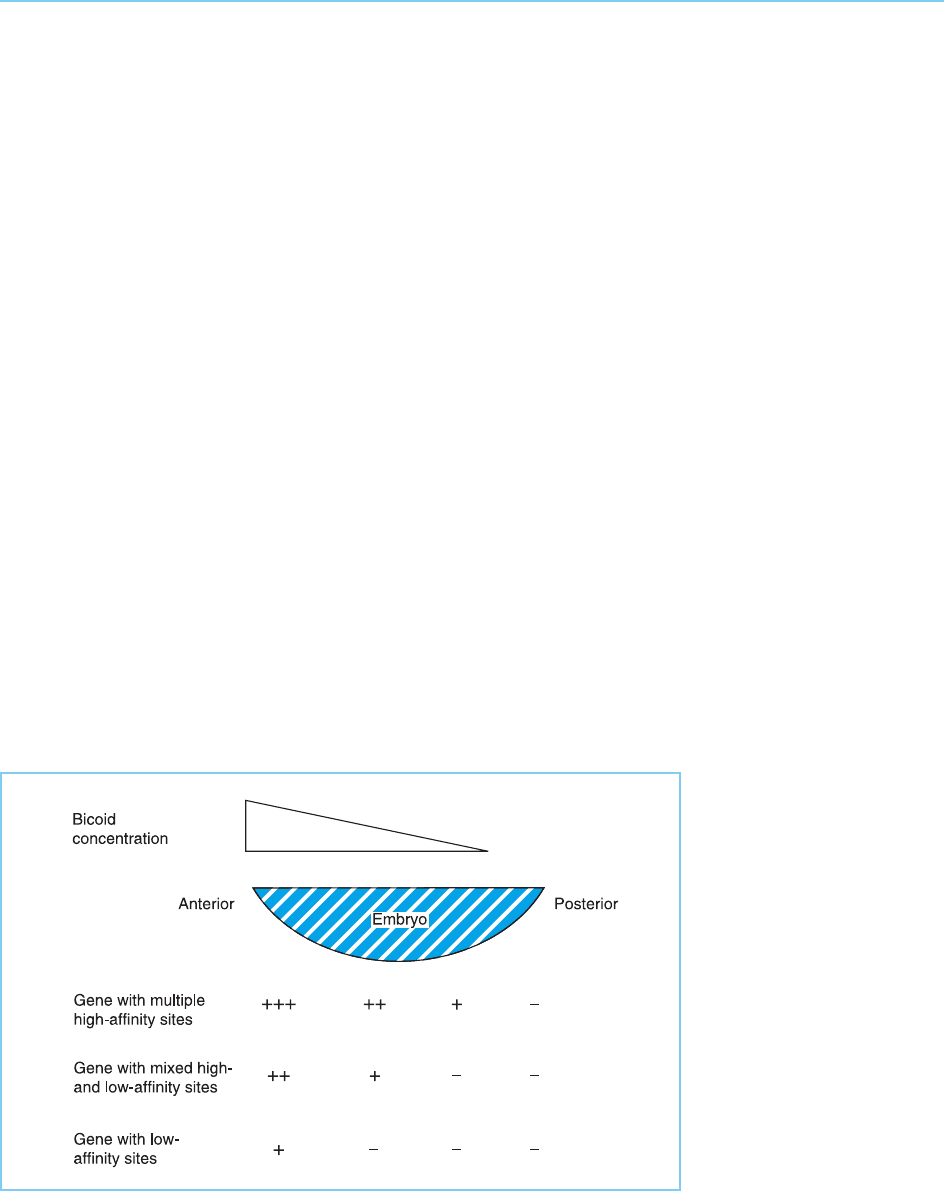
The different expression patterns of these genes, therefore, convert the
gradient of Shh expression into a homeobox code in which each region has
a unique pattern of expression of the different homeobox genes. In turn, this
results in five different neuronal types forming at different positions in the
ventral neural tube (Fig. 7.18) (Briscoe et al., 2000). Hence, in this case, the
precise combination of specific homeobox genes expressed in each position
controls the precise cell type that is formed (for review see Marquardt and
Pfaff, 2001).
In our discussion so far, it has been assumed that a homeobox factor is
either present in a particular cell or is entirely absen t. In fact, however, a
further level of complexity exists since many homeo box factors are not
expressed in a simple on/off manner but rather show a concentration gradi-
ent ranging from high levels in one part of the embryo via intermediate levels
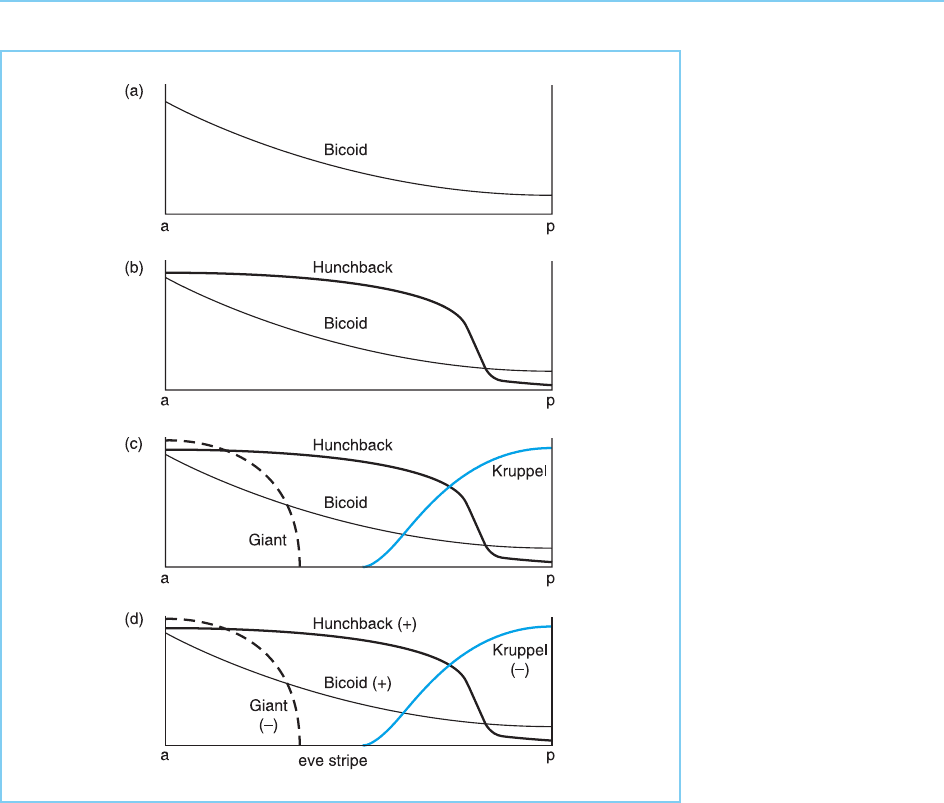
to low levels in another part. For example, in Drosophila, the bicoid protein
(bcd) whose absence leads to the development of a fly without head and
thoracic structures is found at high levels in the anterior part of the embryo
and declines progressively posteriorly, being absent in the posterior one third
of the embryo (Fig. 7.19).
Most interestingly, genes that are activated in response to bicoid contain
binding sites in their promoters which have either high affinity or low affinity
for the bicoid protein. If these sites are linked to a marker gene, it can be
demonstrated that genes with low affinity binding sites are only activated at
high concentrations of bicoid and are therefore expressed only at the extreme
anterior end of the embryo. In contrast, genes that have higher affinity binding
REGULATION OF TRANSCRIPTION FACTOR SYNTHESIS 227
Figure 7.19
The gradient in Bicoid
concentration from the
anterior to the posterior
point of the embryo
results in bicoid-
dependent genes with
only low affinity binding
sites for the protein being
active only at the extreme
anterior part of the
embryo, whereas genes
with high affinity binding
sites are active more
posteriorly. Note that, in
addition to the different
posterior boundaries in
the expression of genes
with high and low affinity
binding sites, genes with
high affinity binding sites
will be expressed at a
higher level than genes
with low affinity binding
sites at any point in the
embryo.

sites are active at much lower protein concentrations and will be active both at
the anterior end and more posteriorly. Moreover, the greater the number of
higher affinity sites the greater the level of gene expression that will occur at
any particular point in the gradient (Dr iever et al., 1989; Fig. 7.19).
The gradient in bicoid expression can be translated therefore into the
differential expression of various bicoid-dependent genes along the anterior
part of the embryo. Each cell in the anterior region will be able to ‘sense’ its
position within the embryo and respond by activating specific genes. One of
the genes activated by bicoi d is the homeobox-containing segmentation gene
hunchback. In turn, this protein regulates the expression of the gap gen es,
Kruppel and giant (Struhl et al., 1992). All four of these proteins then act on
the eve gene with bicoid and hunchback activating its expression while
Kruppel and giant repress it. The concentration gradients of these four fac-
tors thus result in the spatial localization of eve gene expression in a defined
region of the embryo where it exerts its inhibitory effects on gene express ion
(Small et al., 1991; Fig. 7.20). Hence, the gradient in bicoid gene expression
results in changes in the express ion of other genes encoding regulat ory pro-
teins leading to the activation of regulatory networks involving the controlled
synthesis of multiple transcription factors.
The bicoid factor therefore has all the properties of a morphogen whose
concentration gradient determines position in the anterior part of the
embryo. This idea is strongly supported by the results of genetic experiments
in which the bicoid gradient was artificially manipulated, cells containing
artificially increased levels of bicoid assuming a phenotype characteristic of
more anterior cells which normally contain the new level of bicoid and vice
versa (Driever and Nusslein-Volhard, 1988).
The anterior to posterior gradient in bicoid leve ls is required to produce
the opposite posterior to anterior gradient in the level of another protein,
caudal. However, the caudal mRNA is equally distributed throughout the
embryo indicating that the bicoid gradient does not regulate transcription
of the caudal gene. Rather, the bicoid protein binds to the caudal mRNA
and represses its translation into protein so that caudal protein is not pro-
duced when bicoid levels are high (reviewed by Carr, 1996; Chan and Struhl,
1997). As well as providing further evidence for the key role of the bicoid
factor, this finding also show s that homeodomain proteins can bind to RNA as
well as to DNA and that they may therefore act at the post-transcriptional level
as well as at transcription.
The bicoid case clearly illustrates therefore how the regulated synthesis of
an individual factor, resulting in a gradient in its concentration, can alter the
expression of a regulatory network of other genes and ultimately control the
differentiation of specific cells during development.
228 EUKARYOTIC TRANSCRIPTION FACTORS
7.3 MECHANISMS REGULATING THE SYNTHESIS OF
TRANSCRIPTION FACTORS
The cases discussed in the previous section illustrate therefore that where a
factor must be active in a particular cell type or at a spec ific point in devel-
opment, this is frequently achieved by the factor being present only in the
particular cells where it is required. Clearly, such regulated synthesis of a
specific transcription factor could be achieved by any of the methods that
are normally used to regulate the production of individual proteins, such as
the regulation of gene transcription, RNA splicing or translation of the mRNA
(Fig. 7.21, for review of the levels at which gene regulation can occur see
REGULATION OF TRANSCRIPTION FACTOR SYNTHESIS 229
Figure 7.20
Model illustrating how the
concentration gradients
of the activators bicoid
and hunchback and the
repressors Kruppel and
giant produce a stripe of
eve gene expression.
Note that the bicoid
gradient (a) affects
hunchback expression (b)
which in turn affects
giant and kruppel
expression (c). Eve gene
activation (+) by
hunchback and bicoid
and its repression (-) by
giant and Kruppel then
produces a specific stripe
or region of the embryo
in which eve is expressed
(d).
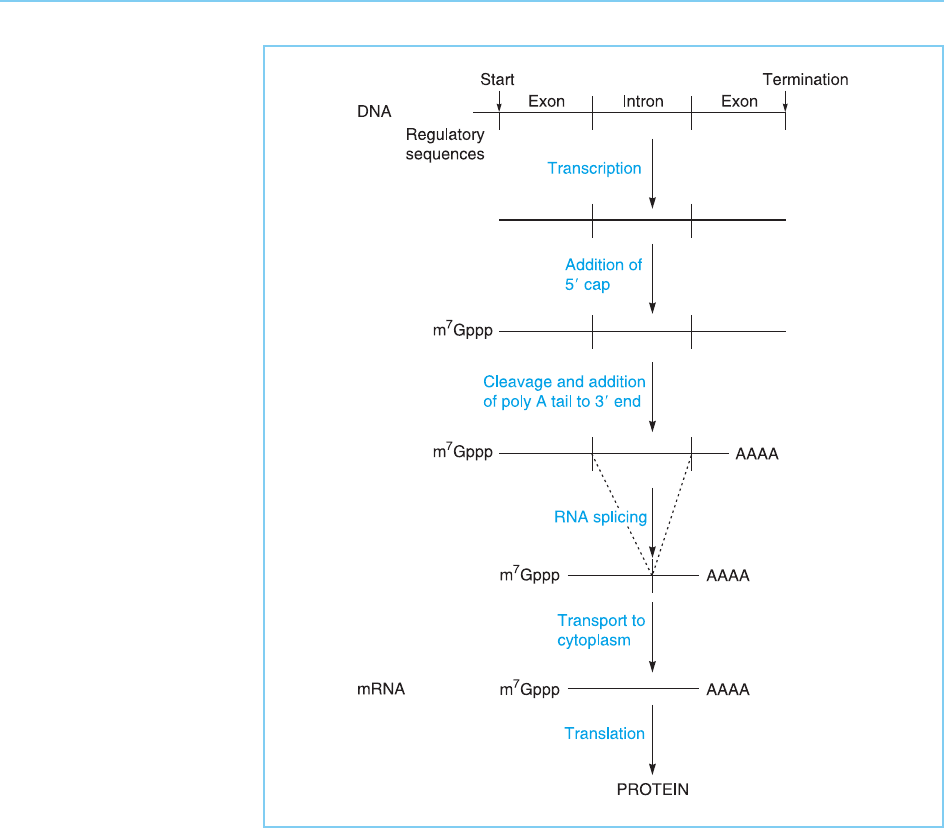
Latchman, 2002). Several of these mechanisms of gene regulation are utilized
in the case of individual transcription factors and these will be discussed in
turn.
7.3.1 REGULATION OF TRANSCRIPTION
As discussed above, a number of cases where the cell type-specific expression
of a transcription factor is paral leled by the presence of its corresponding
mRNA in the same cell type have now been described. In turn this cell type-
specific expression of the transcription factor mRNA is likely to result from
the regulated transcription of the gene encoding the transcriptio n factor.
230 EUKARYOTIC TRANSCRIPTION FACTORS
Figure 7.21
Potential regulatory
stages in the expression
of a gene encoding a
transcription factor.
