Kamal A.A. 1000 Solved Problems in Classical Physics: An Exercise Book
Подождите немного. Документ загружается.

428 10 Heat and Matter
∴ m =
2kT
v
2
mp
=
2 × 1.38 × 10
−23
× 1200
(850)
2
= 4.584 ×10
−26
kg
=
4.584 × 10
−26
1.66 × 10
−27
amu = 27.6amu
Therefore the gas is N
2
.
In 5 mol of gas total number (N) of gas molecules will be
N =
5
28
× 6.02 × 10
23
= 1.075 ×10
23
Number of molecules N(v) dv in the interval v and v + dv will be
N(v)dv = 4πN
m
2πkT
3/2
v
2
exp
−
mv
2
2kT
dv (Maxwellian distribution)
(2)
The mean value of the interval is v =
800 + 900
2
= 850 m/s
which happens to be identical with v
mp
found in (a). In this case (2) is
reduced to a simpler form
N(v)dv =
4N dv
√
π e v
mp
Number of N
2
molecules in 5 mol will be
N =
5
28
× 6.02 × 10
23
= 1.075 ×10
23
The speed interval
dv = 900 − 800 = 100 m/s
Thus the required number of molecules is
N(v)dv =
4 × 1.075 × 10
23
× 100
√
π ×2.718 × 850
= 1.05 ×10
22
10.3 Solutions 429
10.5 f (v) = 4π
m
2πkT
3/2
v
2
e
−mv
2
/2kT
d f (v)
dv
= const.
d
dv
v
2
e
−mv
2
/2kT
= 0
∴ e
−mv
2
/2kT
2v −
m
kT
v
3
= 0
whence v = 0, ∞,
2kT
m
The most probable speed is v
mp
=
2kT
m
10.6
(i)
¯
E =
3
2
kT =
3
2
× 1.38 × 10
−23
× 300 = 6.21 × 10
−21
J
(ii)
v
2
=
3RT
M
=
3 × 8.31 × 300
44 × 10
−3
= 412.3m/s
(iii) v
mp
=
2RT
M
=
2 × 8.31 × 300
44 × 10
−3
= 336.6m/s
(iv) v
av
=
8RT
π M
=
8 × 8.31 × 300
44 × 10
−3
π
= 380.0m/s
10.7
(a) Assumptions:
(i) The molecules of a gas behave like hard, smooth spheres and of neg-
ligible size compared to that of the container.
(ii) The molecules are in random motion undergoing collisions with one
another and with the walls of the container for negligible duration.
(iii) Newton’s laws of motion are applicable and the number of molecules
is large so that statistics may be applied.
(b)
P
1
V
1
T
1
=
P
2
V
2
T
2
(from the gas equation)
∴ V
2
=
P
1
P
2
·
T
2
T
1
V
1
=
1
0.5
×
(273 − 40)
(273 + 27)
× 2 = 3.1m
3
10.8
(a)
n
V
=
P
RT
=
9.8 × 10
5
8.31 × 400
= 294.8mol/m
3
(b)
n
V
=
⎡
⎢
⎢
⎣
P + a
n
2
V
2
RT
⎤
⎥
⎥
⎦
1 −
bn
V
=
9.8 × 10
5
+ 0.448 × (294.8)
2
8.31 × 400
(1 − 4.29 × 10
−5
× 294.8)
= 302.66 mol/m
3
430 10 Heat and Matter
10.3.2 Thermal Expansion
10.9 Let the length of the bars be L
0
, each at 0
◦
C when they are straight. With the
rise of temperature T , their lengths will be (Fig. 10.2)
L
1
= θ R
1
= L
0
(1 + α
1
T ) (1)
L
2
= θ R
2
= L
0
(1 + α
2
T ) (2)
Subtracting (2) from (1)
θ(R
1
− R
2
) = θd = (α
1
− α
2
)L
0
T (3)
∵ R
1
− R
2
= d
Adding (1) and (2)
θ(R
1
+ R
2
) = 2L
0
+ (α
1
+ α
2
)L
0
T 2L
0
(4)
∵ (α
1
+ α
2
)T << 2
R =
R
1
+ R
2
2
=
L
0
θ
=
d
(α
1
− α
2
)T
(5)
where we have used (3).
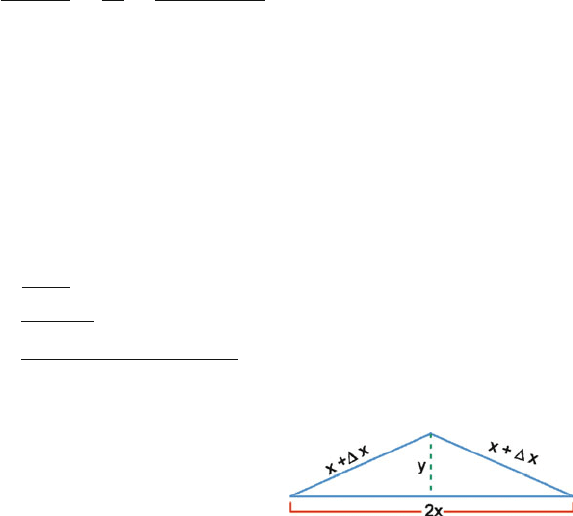
10.10 Let the initial length of the rod be 2x and the final total length be 2(x +x).
Let the centre of the buckled rod be raised by y, then
x = αxT
From the geometry of Fig. 10.6,
y =[(x +x)
2
− x
2
]
1/2
=[2x x +(x)
2
]
1/2
√
2xx (∵ x << 2x)
=
2αx
2
T
=
2 × 12 × 10
−6
× 20
2
× 30 = 0.5367 m
= 53.67 cm
Fig. 10.6 Buckling of rail
10.11 Let L
0
(S) and L
0
(Cu) be the l engths of steel and copper rod at 0
◦
C, respec-
tively. Let the respective lengths be L(S) and L(Cu) at temperature T
◦
C.
Then

10.3 Solutions 431
L(S) = L
0
(S)(1 + α
s
T ) (1)
L(Cu) = L
0
(Cu)(1 + α
Cu
T ) (2)
Subtracting (2) from (1)
L(S) − L(Cu) = L
0
(S) − L
0
(Cu) +[L
0
(S)α
s
− L
0
(Cu)α
Cu
]T (3)
Now in the RHS, L
0
(S) – L
0
(Cu) is constant. If L(S) – L(Cu) is to remain
constant, then [L
0
(S)α
s
− L
0
(Cu)α
Cu
]=0, at any temperature T .This
gives us
L
0
(S)
L
0
(Cu)
=
α
cu
α
s
=
1.7 × 10
−5
1.1 × 10
−5
=
17
11
(4)
Furthermore, L
0
(S) − L
0
(Cu) = 4cm. (5)
Solving (4) and (5) we obtain
L
0
(S) = 11.33 cm; L
0
(Cu) = 7.33 cm
10.12 Let the volume of mercury in the flask be V
0
cm
3
and that of glass flask
1000 cm
3
at initial temperature T
1
. At a higher temperature T
2
the volume of
glass will be
V
g
= 1000(1 +γ
g
T ) (1)
where T = T
2
− T
1
. The volume of mercury will be
V = V
0
(1 + γT ) (2)
The volume of air inside the flask at temperature T
2
will be
V
g
− V = 1000(1 +γ
g
T ) − V
0
(1 + γT )
= 1000 − V
0
+ (1000γ
g
− V
0
γ)T (3)
The RHS will be constant if
1000γ
g
− V
0
γ = 0(4)
for any value of T . Therefore,
V
0
= 1000
γ
g
γ
=
1000 × 27 × 10
−6
1.8 × 10
−4
= 150 cm
3
wherewehaveusedγ
g
= 3α
g
.
432 10 Heat and Matter
10.13
Y =
F/A
L/L
(1)
or F =
YAL
L
=
YAL
L
0
(1 + αT )
YAL
L
0
(2)
(∵ αT << 1)
But L = α L
0
T
∴ F = YAαT = 2.1 × 10
10
× 0.5 × 10
−6
× 12 × 10
−6
× 20 = 2.52 N
where we have used SI units.
10.14 The coefficient of apparent expansion of a liquid
A = γ − g = γ − 3α = 11 × 10
−4
− 3 × 8 × 10
−6
= 1.076 ×10
−3
Apparent expansion =
mass expelled
(mass left) (temperature rise)
A =
W
W
0
T
=
50 − W
0
W
0
T
∴ W
0
=
50
1 + AT
=
50
1 + 1.076 × 10
−3
× 80
= 46 g
Gas Laws
10.15 PV = nRT(gas equation)
∴ R =
PV
nT
=
(1.0129 × 10
5
)(22.4 × 10
−3
)
(1.0)(273)
= 8.31 J/mol/K
10.16 P
0
= 0.76 ×13, 600 × 9.8 = 1.0129 × 10
5
Pa
Pressure at depth 30 m, P = 30 ×1000 × 9.8 = 2.94 × 10
5
Pa.
∴ Total pressure inside the bubble, P
1
= P
0
+ P
= (1.0129 +2.94) × 10
5
= 3.9529 ×10
5
Pa
P
2
V
2
= P
1
V
1
(Boyle’s law)
∴ P
2
(3V
1
) = P
1
V
1
∴ P
2
= P
1
/3 = 1.376 × 10
5
Pa
This corresponds to a water depth equivalent of 1.3176 × 10
5
− 1.0129 ×
10
5
= 0.3047 ×10
5
Pa.
Therefore water depth =
30 × 0.3047 × 10
5
2.94 × 10
5
= 3.11 m
10.3 Solutions 433
10.17
ρ
1
T
1
P
1
=
ρ
2
T
2
P
2
M
1
= Vρ
1
, M
2
= V ρ
2
∴
M
1
M
2
=
ρ
1
ρ
2
=
P
1
T
2
P
2
T
1
∴ M
2
=
P
2
P
1
·
T
1
T
2
M
1
=
50
76
×
273
263
× 175
= 119.5kg
10.18 Let n moles be total mass of air in the two bulbs.
Initially, T = 273 + 20 = 293 K, P = 76 cm of Hg, V = V
1
+ V
2
=
100 + 500 = 600 cc.
n =
PV
RT
=
70 × 600
293R
Finally, let n
1
and n
2
moles be the mass of air in the small and large bulb,
respectively. Under new conditions
n
1
=
P
1
V
1
RT
1
=
100P
1
293R
n
2
=
P
2
V
2
RT
2
=
500P
2
293R
(∵ P
2
= P
1
)
But n = n
1
+ n
2
=
100P
1
293R
+
500P
1
373R
=
70 × 600
293R
Cancelling off R, we find P
1
= 85.23 cm of Hg.
10.3.3 Heat Transfer
10.19
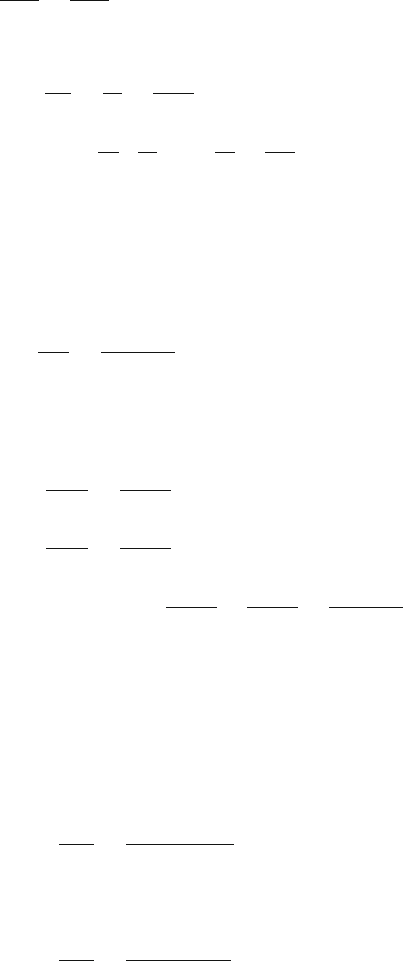
(a) In the first slab, heat flow is given by
−
dQ
1
dt
=
k
1
A (T
1
− T )
d
1
(1)
In the second slab, heat flow is given by
−
dQ
2
dt
=
k
2
A(T − T
2
)
d
2
(2)
Now the continuity of heat flow requires that heat flow must be the same
in both the slabs (Fig. 10.7). Thus
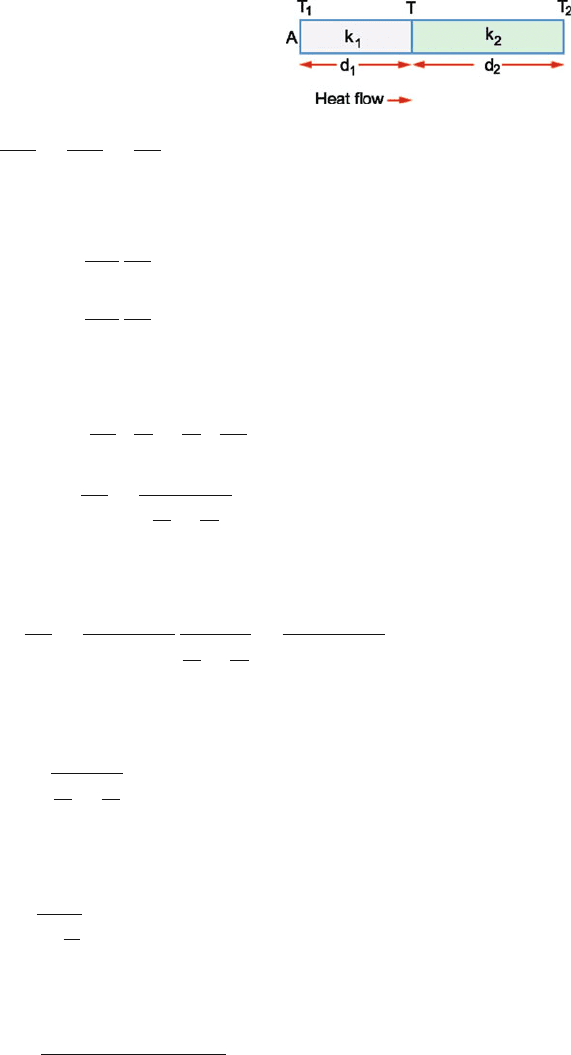
434 10 Heat and Matter
Fig. 10.7 Heat flow in the
composite slab made of two
slabs in series
dQ
1
dt
=
dQ
2
dt
=
dQ
dt
(3)
Using (3) in (1) and (2)
T
1
− T =
−d
1
k
1
A
dQ
dt
(4)
T − T
2
=
−d
2
k
2
A
dQ
dt
(5)
Adding (4) and (5)
T
1
− T
2
=
−1
A
d
1
k
1
+
d
2
k
2
dQ
dt
(6)
or −
dQ
dt
=
A(T
1
− T
2
)
d
1
k
1
+
d
2
k
2
(7)
(b) Rewriting (7)
−
dQ
dt
=
A(T
1
− T
2
)
d
d
d
1
k
1
+
d
2
k
2
=
A(T
1
− T
2
)k
d
(8)
with d = d
1
+ d
2
, and the equivalent conductivity
k
eq
=
d
1
+ d
2
d
1
k
1
+
d
2
k
2
(9)
Formula (9) can be generalized to any number of slabs in series.
k =
d
i
d
i
k
i
(10)
(c) Eliminating dQ/dt between (4) and (5)
T =
(
k
1
T
1
/d
1
)
+
(
k
2
T
2
/d
2
)
(
k
1
/d
1
)
+
(
k
2
/d
2
)
(11)
10.3 Solutions 435
10.20
(a) The rate of flow of heat through the composite slab (Fig. 10.8)is
given by
−
dQ
dt
=
(T
1
− T
2
)
d
n
l
k
i
A
i
(1)
(b) Rewriting (1)
−
dQ
dt
=
(T
1
− T
2
)
d
n
1
A
i
k
i
A
i
A
i
(2)
The equivalent conductivity of the system is
k
eq
=
k
i
A
i
A
i
(3)
Fig. 10.8 Heat flow in a
composite slab made of n
slabs in parallel
10.21
T =
(
k
1
T
1
/d
1
)
+
(
k
2
T
2
/d
2
)
(
k
1
/d
1
)
+
(
k
2
/d
2
)
as d
1
= d
2
T =
k
1
T
1
+ k
2
T
2
k
1
+ k
2
=
92 × 100 + 16 × 0
92 + 16
= 85.18
◦
C
10.22 Heat transferred/second
−
dQ
dt
= kA
(θ
2
− θ
1
)
d
= kπr
2
(θ
2
− θ
1
)
d
= 90π(0.01)
2
(100 − 0)
0.2
= 14.137 J/s
Heat required to melt 0.05 kg of ice
= 0.05 ×8 ×10
4
= 4000 cal = 16720 J
Time required = 16720/14.137 = 1183 s.
436 10 Heat and Matter
10.23 Heat flow
dQ = A
dT
dx
k = AaT
dT
dx
or AaT dT = dQ dx
Integrating Aa
T
1
T
2
T dT =
Q
0
dQ
L
0
dx
∴
Aa
2
T
2
1
− T
2
2
= QL
or Q =
aA
2L
T
2
1
− T
2
2
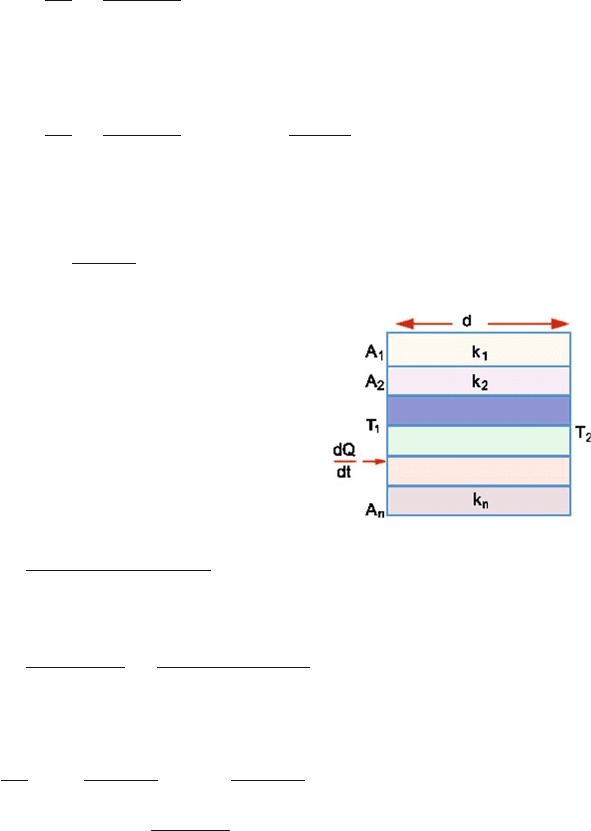
10.24
dQ
dt
=−kA
dT
dr
∴ dT =−
1
k
dQ
dt
dr
4πr
2
When steady state is reached, dQ/dt will be independent of r and is constant
(Fig. 10.9). Integrating
T
2
T
1
dT =−
1
4πk
dQ
dt
r
2
r
1
dr
r
2
=−
1
4πk
1
r
2
−
1
r
1
dQ
dt
∴ T
2
− T
1
=
1
4πk
(
r
1
−r
2
)
r
1
r
2
dQ
dt
or T
1
− T
2
=
1
4πk
(
r
2
−r
1
)
r
1
r
2
dQ
dt
∴
dQ
dt
= 4π k
r
1
r
2
r
2
−r
1
(
T
1
− T
2
)
Fig. 10.9 Radial flow of heat
through two concentric
spheres
10.25 Rate of flow of heat
dQ
dt
=−kA
dT
dr
(1)

10.3 Solutions 437
Neglecting the area of the faces, area of the cylinder A = 2πrL. For steady
state, dQ/dt = constant. We can then write (1) as
dr
r
=−
2π Lk
dQ/dt
dT
Integrating
r
2
r
1
dr
r
= ln(r
2
/r
1
) =−
2π Lk
dQ/dt
(T
2
− T
1
)
or dQ/dt =
2π Lk
ln(r
2
/r
1
)
(T
1
− T
2
)
10.26
dQ/dt = kA(T
1
− T
2
)/d
= (0.59A) ×
10
0.01
= 590 A J/s(1)
Let x m/s ice be added at the bottom of the layer.
Mass of ice formed per second
M = ρ × A (2)
The required energy per second
E = ρ × AL (3)
Equating (1) and (3), ρ × AL = 590 A
∴ x =
590A
ρ AL
=
590
917 × 333 × 10
3
= 1.932×10
−6
m/s = 0.00695 m/h
10.27
−
dθ
dt
= C(θ −θ
0
) (Newton’s law of cooling)
θ
1
− θ
2
t
= C
θ
1
+ θ
2
2
− θ
0
(C = constant)
85 − 75
2
= C
85 + 75
2
− 30
∴ C = 0.1
55 − 45
t
= 0.1
55 + 45
2
− 30
∴ t = 5min
