Jan Svoboda. Magnetic Techniques for the Treatment of Materials
Подождите немного. Документ загружается.


500 CHAPTER 6. INDUSTRIAL APPLICATIONS
Figure 6.35: Installation of Reading wet high-intensity magnetic separators
(courtesy of Roche Mining).
Heavy mineral
concentrate
Mags tailings
Low density
tailings
Mags
tailings
Feed to dry mill
Ilmenite
Mags
Mags
Non-mags
Non-mags
LIMS
WHIMS 1
WHIMS 2
Gravity separation
WHIMS 3
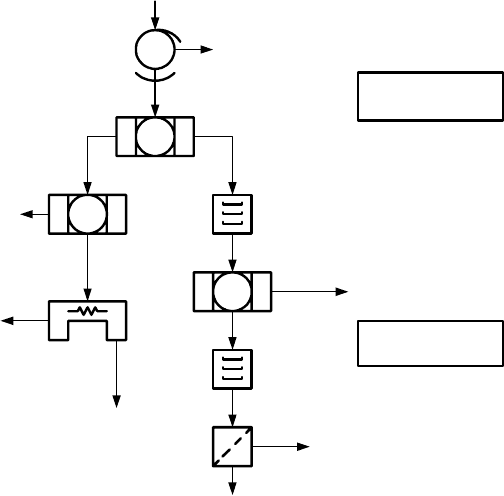
Figure 6.36: The dry mill feed preparation circuit at Richards Bay Minerals.
6.1. TREATMENT OF MINERALS 501
Mags
Non-mags
WHIMS 1
WHIMS 2
Ilmenite
Non-mags
Gravity
IMR
Gravity
Dry separation
Non-mags
Mags
Electrostatic
separation
Zircon
Rutile
Ilmenite
Tailings
Non-mags
Magnetic
tailings
Secondary
concentration
Mineral
separation
Figure 6.37: The concentration and separation circuits at Namakwa Sands
(South Africa).
85% of heavy minerals, is first passed through a low-intensity wet drum magnetic
separator to remove magnetite. The non-magnetic fraction from this stage is
re-treated with Reading wet high-intensity magnetic separators in a complex
rougher-cleaner circuit to produce a concentrate that consists largely of ilmenite
containing 46% TiO
2
. An installation of banks of Reading WHIMS machines is
shown in Fig. 6.35.
The non-magnetic fraction from WHIMS is again gravity-concentrated and
then further upgraded by the use of WHIMS to remove feebly magnetic material.
The resulting concentrate is dried and fed into the "dry mill", where high-tension
roll separators and induced magnetic separators are used to produce saleable
rutile and zircon products.
A diagram of this feed preparation circuit for the dry mill is shown in Fig.
6.36. A total of 31 Reading 16-pole WHIMS are used at RBM. The feed rate
into each WHIMS machine ranges from 23 to 31 t/h at pulp density of 40%
solids. The magnetic fraction from the WHIMS machines contains, in addition
to ilmenite, small quantities of weakly magnetic impurities. These are removed
prior to feeding the material to the smelter by dry magnetic separation, after
magnetizing roast in a fluidized bed.
502 CHAPTER 6. INDUSTRIAL APPLICATIONS
Feed
preparation
circuit
Ilmenite
circuit
Leucoxene
circuit
Ilmenite
roaster
Rutile
circuit
Wet zircon
circuit
Dry zircon
circuit
Heavy mineral
concentrate
Gravity tails
Non-mags, gravity
concentrate
Mags 1
Mags 2
N/Cond, tails
Mags
Mags, tails
Crude ilmenite
Ilmenite product
Leucoxene
N/cond
Tailings
Mags
N/cond, mags,
tails
Rutile product
Mags
Tailings
Mags, tailings
Conductors
Recycle to
rutile circuit
Zircon product
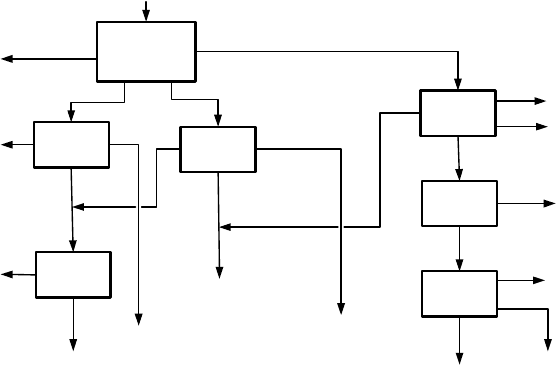
Figure 6.38: Schematic flow diagram of the Ginkgo (Broken Hill, Australia) min-
eral separation plant (adapted from [E8]).
Namakwa Sands, South Africa
Namakwa Sands (Anglo-American Corp.) produces heavy minerals from min-
eral sands from the Graauwduinen deposit in the Western Cape Province of
South Africa. This fairly new operation, that was commissioned in 1994, uses
a conventional concentration flowsheet based on gravity, magnetic and electro-
static separations. A simplified process flowsheet is shown in Fig. 6.37.
The primary concentration plant consists of a four-stage spiral circuit that
treats - 1 mm + 45 m feed and produces a heavy mineral concentrate assaying
90% of total heavy minerals. Low-intensity drum magnetic separators remove
magnetite from this concentrate in the secondary concentration plant and the
non-magnetic fraction is then processed in a four-stage wet high-intensity mag-
netic separator circuit. The Reading WHIMS machines produce a magnetic
fraction containing ilmenite and garnet and a non-magnetic fraction containing
zircon, rutile, leucoxene and other minerals. This fraction is further upgraded
in a spiral circuit.
The ilmenite concentrate and non-magnetic fraction from the secondary con-
centrate plant are further processed in the mineral separation plant. The il-
menite concentrate is produced using electrostatic separators while separation
of rutile and zircon takes place in a process consisting of magnetic, gravity and
electrostatic separation and acid cleaning circuits. Permanent magnetic rolls
and rare-earth drum magnetic separators are employed in this circuit.
6.1. TREATMENT OF MINERALS 503
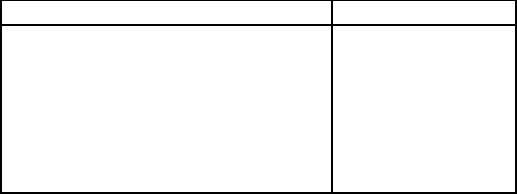
Table 6.7: Composition of Ginkgo (Australia) heavy mineral assemblage [E8].
Mineral Assay range [%]
Rutile (+ 95% TiO
2
) 8.8 - 12.5
Zircon 6.7 - 12.6
Primary ilmenite (? 54% TiO
2
) 0.7 - 21.2
Altered ilmenite (54% - 66% TiO
2
) 32.4 - 49.0
Leucoxene (66% - 85% TiO
2
) 4.5 - 12.6
Monazite 0.3 - 0.8
Chromite 0.7 - 1.1
Murray Basin, Australia
Extensive reserves of heavy minerals exist in the Murray Basin, an area covering
parts of South Australia, New South Wales and Victoria. Several of the deposits
are of potential economic size and of these the Ginkgo deposit has been identified
for establishment of the first commercial operation [E8].
The mineral assemblage within the Ginkgo ore body consists primarily of
ilmenite and its alteration products, rutile and zircon, with minor amounts of
other minerals. The mineral composition is shown in Table 6.7.
Extensive bench-scale and pilot-plant testwork has been carried out on sam-
ples of ore from the deposit. The results of the tests and mathematical modelling
of the beneficiation circuits resulted in a proposed design for the wet plant, min-
eral separation plant and the roaster.
As a result of wide mineralogical variations within the ore body, the wet
plant is based on a combination of roughing, scavenging and cleaning spiral
gravity separators and is expected to produce up to 130 t/h of heavy mineral
concentrate. This concentrate will be treated at the proposed mineral separation
plant to be located at Broken Hill. The block diagram of the flowsheet for this
plant is shown in Fig. 6.38.
This flowsheet consists of a number of discreet circuits with minimal in-
teraction between them. The feed preparation circuit is based on a two-stage
WHIMS circuit. The magnetic product will be the feed into the ilmenite and
leucoxene dry mill circuits while the non-magnetic fraction will be treated in
the zircon and rutile circuits.
In the ilmenite reduction roasting circuit the concentration of the chromite
contaminant in the ilmenite concentrate will be reduced from A 1.5% Cr
2
O
3
in
the unroasted ilmenite to ? 0.3% Cr
2
O
3
. The non-magnetic rejects from the
ilmenite roasting can be upgraded by magnetic and electrostatic separation to
yield a chromium-rich product with a grade similar to commercial chromite ores
[F32].
In the initial years of operation, 10.4 Mt of ore will be mined per annum
and 58 kt of zircon and 41.5 kt of rutile per annum are planned to be produced.
The scheduled date for the first shipment of product is January 2005 [E8].

504 CHAPTER 6. INDUSTRIAL APPLICATIONS
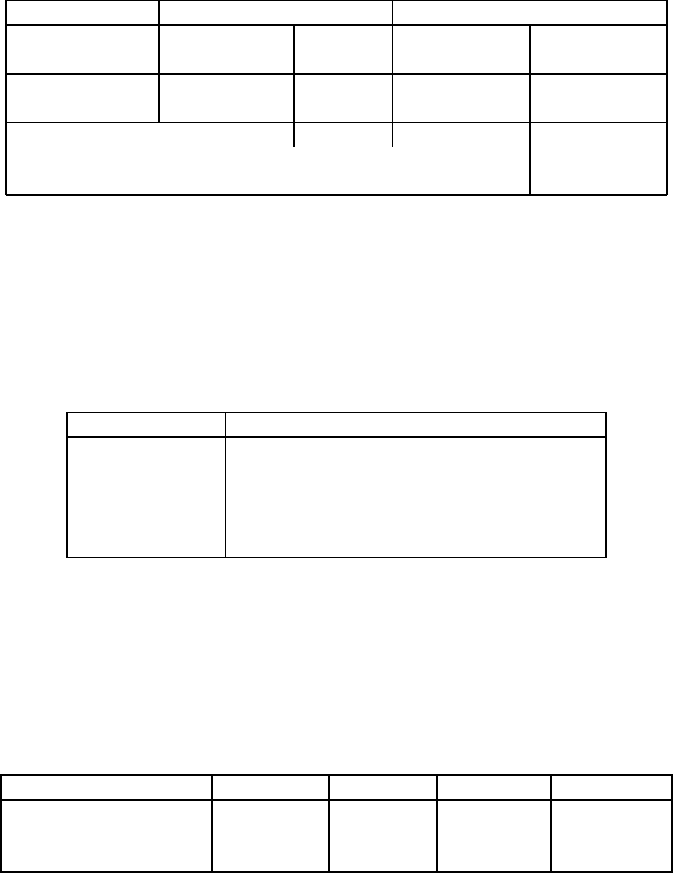
Table 6.8: Kaolin product specification [E9].
Specification Brightness
Premium Standard Filler
TAPPI brightness [%] 89.5 - 92.0 85.5 - 89.0 80.0 - 85.5
Particle size ? 2 m[%] 80 - 100 80 - 97.0 62.0 - 95.0
Particle size + 45 m 0.01 0.01 0.30 - 0.40
6.1.8 Industrial minerals
Kaolin beneficiation
One of the most successful applications of HGMS technology is in the bright-
ening of kaolin clay. Essentially all economically viable deposits of kaolin are
contaminated by small amounts (usually from 0.5% to 3%) of ferruginous min-
erals, such as iron oxides and rutile. Since these contaminants are deleterious
to clay brightness, considerable eort has been expended to remove them by
physical and chemical methods. Under favourable conditions chemical bleach-
ing usually removes less than half of the iron present in clays [I3]. Although
discolouring contaminants are usually very weakly magnetic and very fine (e.g.
80% smaller than 2 m), they can be e!ciently removed by HGMS, if a suf-
ficiently large magnetic field strength, fine matrix and low flow velocity of the
slurry are used [I6, L25, B35].
The high-gradient magnetic separation of kaolin clay became a practical
economic process that has been thoroughly proven on a large scale worldwide.
Moreover, magnetic beneficiation of kaolin became one of the rare production-
scale applications of superconductivity, with Eriez Magnetics and Outokumpu-
Carpco superconducting HGMS machines now well established in the minerals
industry. Iron-clad resistive solenoid separators or superconducting cyclic or
reciprocating magnet separators are used in several operations, with the oper-
ating magnetic induction ranging from 2 T to 5 T. [S18, R4, W5, R25].
A brief summary of the kaolin product specifications is given in Table 6.8,
while Tables 6.9 and 6.10 illustrate the metallurgical performance and technical
data of Cryofilter HGMS as applied to two dierent types of kaolin.
Limestone beneficiation
In the Gåsgruvan beneficiation plant of Svenska Mineral AB, Sweden, a two-
head carousel SALA HGMS is used to remove iron oxide impurities from the
limestone flotation concentrate. The quality of the non-magnetic concentrate
meets the market specifications for pigment and paper filler applications [W30].
Typical production results are listed in Table 6.11.
6.1. TREATMENT OF MINERALS 505
Table 6.9: Performance of Cryofilter HGMS as applied to the beneficiation of
two types of kaolin [E9].
Kaolin grade Feed specs Product (unbleached)
Grade %Solids Grade (gain) Throughput
(t/h)
Coating grade 54 to 84 ISO 23 to 28 67 (13) to 88
(4) ISO
5to40
Ceramic 77 ISO 23 81 (4) ISO 5
80 (3) ISO 12
79 (2) ISO 20
Table 6.10: Cryofilter HGMS performance in kaolin purification [E9].
Parameter Performance
Power input 0.1 kW/t
Throughput up to 100 t/h
Brightness gain 7 points on feed with 70 to 75 brightness
5 points on feed with 80 to 85 brightness
2 points on feed with + 90 brightness
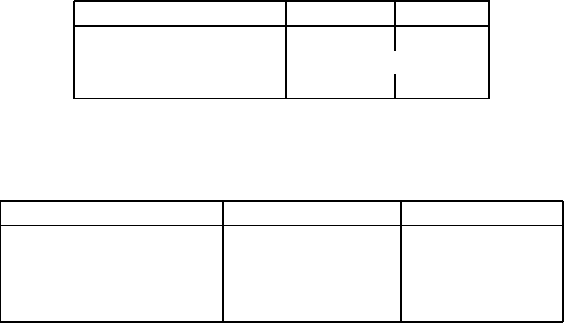
Table 6.11: Limestone beneficiation at Svenska Mineral AB [W30].
Product Yield [%] CaO [%] MgO [%] Fe
2
O
3
[%]
Feed 100 50.5 3.96 0.68
Flotation concentrate 85.5 53.4 1.90 0.24
HGMS concentrate 77.5 54.8 1.01 0.12
506 CHAPTER 6. INDUSTRIAL APPLICATIONS
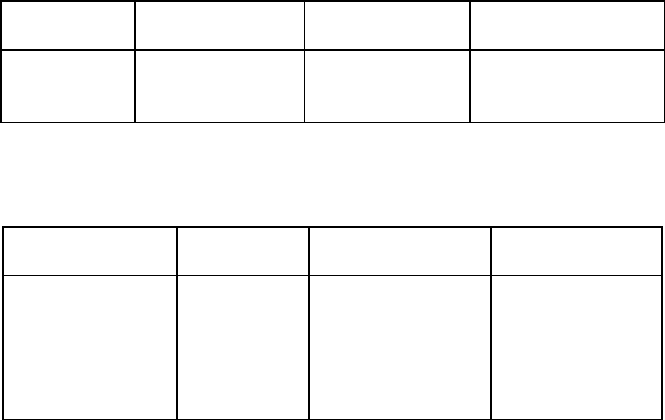
Table 6.12: Comparison of the performance of WHIMS and HGMS separators
in beneficiation of glass sand [S29].
Parameter WHIMS HGMS
Slurry velocity [cm/s] A 25 4.0
Target Fe
2
O
3
[%] ? 0.012
Achieved Fe
2
O
3
[%] 0.0172 0.0116
Table 6.13: Comparison of 1998 costs of glass sand beneficiation by WHIMS and
Eriez HI Filter HGMS [D8].
Parameter WHIMS HGMS
Capital cost £200k £220k
Running costs (power) 70 kW 10 kW
Matrix cost per month £500 - £1000 ? £50
Labour cost Manual operation Fully automatic
Glass sand beneficiation
Sand deposits exploited to supply the glass and ceramics industries, have, un-
til recently, been of a low iron content to enable production with little or no
processing of the sand. Screening and attrition techniques have been used, often
in combination with spiral gravity concentrators. With increasing demand from
the glass industry for high quality material and with diminishing high-quality
reserves, more sophisticated techniques are becoming more common to meet the
specifications of the glass making industry. Dry permanent magnetic rolls, wet
high-intensity magnetic separators and cyclic high-gradient magnetic separators
have been applied, on production scale, to beneficiation of glass sand.
Comparison of the performance of WHIMS and Eriez Magnetics HI Filter
HGMS machines as applied to the reduction of iron oxides, is shown in Table
6.12, while Table 6.13 compares the 1998 costs of these two types of magnetic
separators. Superior performance of the HGMS machine is a result of controlled
flow velocity of the pulp through the matrix. Since HGMS is a cyclic separator,
its throughput is limited, typically 10 t/h or less, while a WHIMS can operate
at throughputs of the order of 50 t/h or more. The problem of the HGMS being
a batch separator with low duty cycle (e.g. 50%) can be solved by installing
two units operating as a pair.
Dry magnetic separation using either induced magnetic roll or permanent
magnetic roll separators has been confined to relatively specialist grades pro-
duced in small quantities. Typical throughput of an installation of dry magnetic
separators for sand purification is 2 to 3 t/h per meter width of the roll [F33].
Table 6.14 illustrates the e!ciency of beneficiation of glass sand in a two-stage
dry high-intensity magnetic separation circuit [D17].
By incorporating spiral concentrators into the separation process, the non-
magnetic titanium and aluminium-bearing particles were removed even further.
6.1. TREATMENT OF MINERALS 507
Table 6.14: Comparison of glass sand ore material and a product from two-pass
dry high-intensity magnetic separation [D17].
Compound Glass sand ore Glass sand
product specs
Mag sep product
Fe
2
O
3
[%] 0.035 ? 0.010 0.007
TiO
2
[%] 0.105 ? 0.020 0.023
Al
2
O
3
[%] 0.852 ? 0.060 0.031
Table 6.15: Comparison of glass sand products from three process circuits with
capacity of 50 t/h [D17].
Parameter Mag sep
product
Spiral+mag sep Floatex+spiral
Fe
2
O
3
[%] 0.007 0.007 0.007
TiO
2
[%] 0.023 0.014 0.014
Al
2
O
3
[%] 0.031 0.023 0.020
Recovery [%] 94.3 93.1 93.8
Equipment cost
[k$], 2001
600 720 250
This improvement was, however, accompanied by higher capital and operating
costs, as the feed into the magnetic circuit had to be dried.
A process circuit comprised of a Floatex density separator and spiral con-
centrators provided equally e!cient metallurgical performance. However, this
all-wet gravity circuit produced a product equal to that obtained in the spiral-
magnetic process, with similar recovery and at less cost, as is shown in Table
6.15.
6.1.9 Magnetic beneficiation of coal
The emission levels of sulphur dioxide and ash particles from coal-burning fa-
cilities must meet stringent environmental standards. In order to reduce these
emissions, numerous techniques for coal cleaning, including magnetic methods,
have been tested over the last twenty years
Coals generally contain 1% to 5% of sulphur, of which over a third is car-
bonaceous in form and is referred to as organic sulphur. The rest is mineral
sulphur consisting of weakly paramagnetic pyrite or marcasite FeS
2
.Pyrite
found in coal often has a much higher magnetic susceptibility than the pure
mineral; this is presumably due to impurities or to its alteration to pyrrhotite.
On the other hand, organic sulphur is closely bonded to coal so that its re-
moval by physical methods is not possible. Consequently, virtually all work on
the desulphurization of coal has been directed towards the removal of inorganic
sulphur.
508 CHAPTER 6. INDUSTRIAL APPLICATIONS
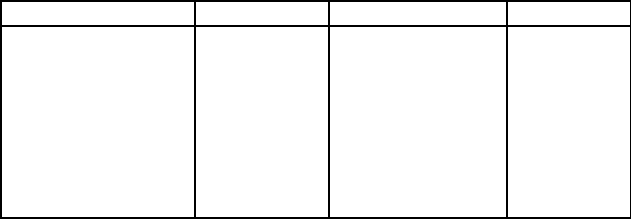
Table 6.16: Physical properties of coal constituents.
Property Coal Mineral matter Pyrite
Mohs hardness
[MHo]
2.2 - 2.5 2.0 - 4.5 6.0 - 6.5
Electrical conduc-
tivity [S/m]
10
6
-10
11
10
4
-10
6
1-10
2
Dielectric constant 2.0 - 2.5 4.5 - 7.8 5.2 - 8.6
Magnetic suscepti-
bility [m
3
/kg]·10
8
-0.5 20 - 200 0.34 - 1.5
Density [kg/m
3
] 1150 - 1500 2400 - 3900 4800 - 5000
Various techniques have been investigated for the extraction of pyrite, rang-
ing from froth flotation to electrostatic separation to microbiological desulphur-
ization, with varying degree of success. The physical techniques are based on of-
ten large dierences between the values of physical properties of coal and pyrite,
as is shown in Table 6.16. Magnetic separation has been recognized as a poten-
tially cost-eective and e!cient method of desulphurization and considerable
research and development work has been conducted in this area, particularly
with the advent of HGMS, OGMS and rare-earth-based permanent magnetic
separators.
Although it has been extensively demonstrated that both wet and dry mag-
netic separation methods are able remove up to 90% of pyritic sulphur, and a
significant amount of ash (up to 60%), at combustible recovery greater than
85%, no magnetic desulphurization process has proven to be su!ciently cost-
eective and e!cient, on an industrial scale, in removing pyrite and ash. Fur-
ther research into magnetic methods of desulphurization was considerably scaled
down in the nineteen nineties. This scale-down coincided with identification of
cleaning options for environmental control strategies by the US Department of
Energy, magnetic methods not being among them [B36].
WetHGMSofcoal
Wet high-gradient magnetic separation used to be a popular technique for labo-
ratory and pilot plant investigations into coal desulphurization. Numerous re-
search papers and reports were published in the seventies and eighties of the last
century. They are often associated with the names of Trindade [T8], Luborsky
[L14], Wechsler [W24] and Male [M35, M36]. The results show that the e!ciency
of sulphur and ash removal is strongly dependent on the origin of the coal and
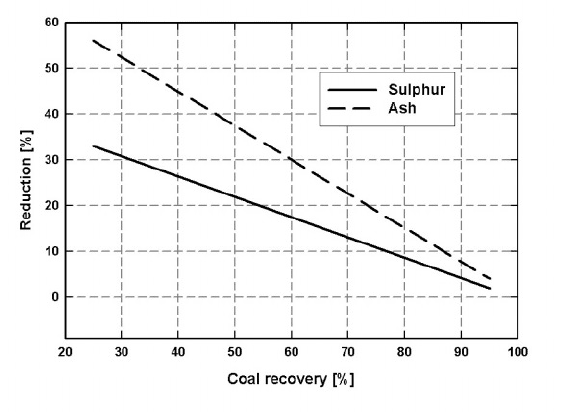
on the recovery of clean coal. It can be seen in Fig. 6.39 that with increasing
reduction of ash and sulphur concentrations, the losses of clean coal into the
magnetic fraction become unacceptably high. More recent reports [K29, D18]
on the subject confirmed the potential of HGMS to recover high percentage of
sulphur and ash, but at the cost of high losses of combustible matter.
Even though most studies suggest that desulphurization by wet HGMS is
6.1. TREATMENT OF MINERALS 509
Figure 6.39: The reduction in sulphur and ash as a function of the recovery of
clean coal (adapted from [L14]).
technically feasible, there are major problems that must be addressed before a
commercially viable system can be realized. The first is the cost of preparation
of the coal and subsequent dewatering and handling of the product. The second
is the non-selectivity of separation, causing an excessive amount of coal to be
lost in the magnetic fraction. Because of the high cost of the equipment and
loss of coal, wet HGMS does not appear economically feasible at present. A
detailed description of the research and development eort in coal beneficiation
by magnetic means, up to 1985, was given by Male [M36].
Dry magnetic separation of coal
Studies of dry HGMS of unclassified coal have generally shown that the sep-
aration of sulphur and ash from clean coal is non-selective, mainly due to the
presence of the fines. Classification and removal of fines, particularly of parti-
cles smaller than 10 m, considerably improved the performance of magnetic
separation [L14, H27].
More encouraging results have been obtained using rare-earth permanent
magnet roll separators. Feasibility tests on Western Kentucky (USA) coals
resulted in ash reduction up to 75% and total sulphur reduction up to 27%.
The recovery of the combustible matter ranged from 85% to 95% [P16]. Similar
reduction in the ash concentration was achieved in several UK coals [B37, S87],
although the technique proved again to be rather ine!cient in removing sulphur.
