Jacobson M.Z. Atmospheric Pollution
Подождите немного. Документ загружается.

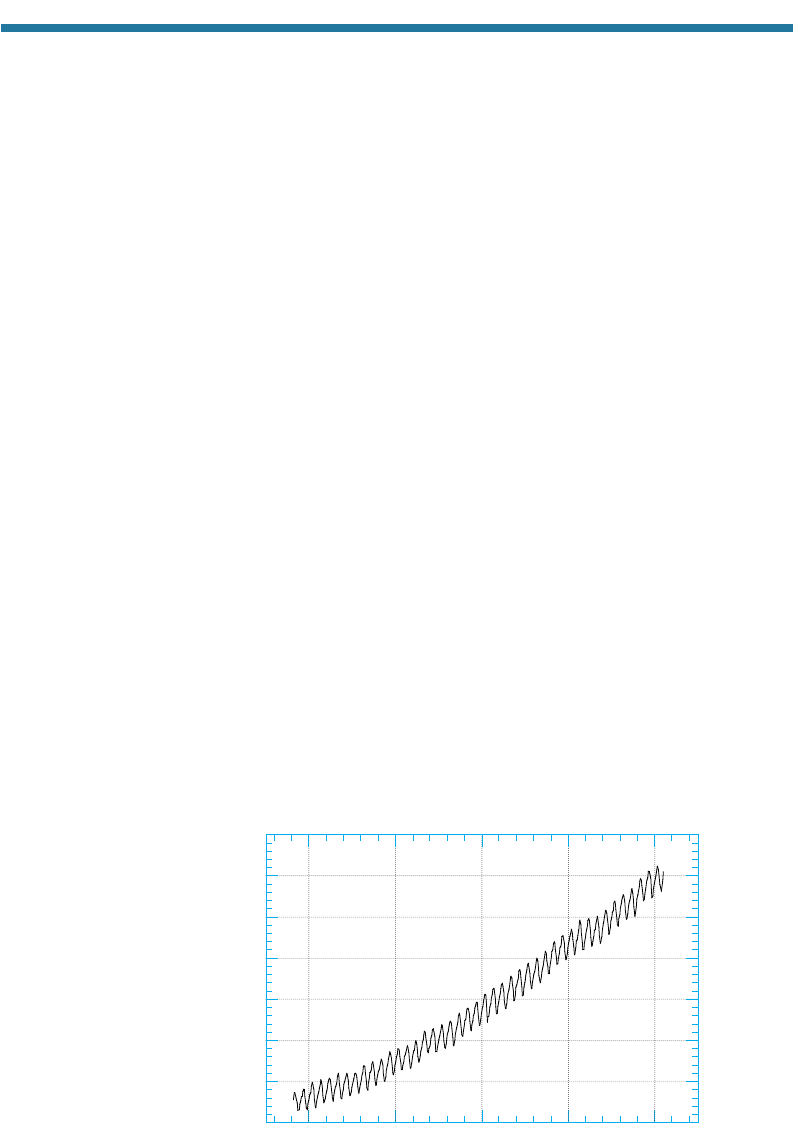
respiration and organic matter decomposition cause the partial pressure of CO
2
(g) to
be about 10 to 100 times that in the atmosphere (Brook et al., 1983). Thus, calcite is
broken down and CO
2
(g) is removed more readily within soils than at soil surfaces.
Dissolved calcium ultimately flows with runoff back to the oceans, where some of it is
stored and the rest of it is converted to shell material.
Mixing Ratios
Figure 3.11 shows how outdoor CO
2
(g) mixing ratios have increased steadily since
1958 at the Mauna Loa Observatory, Hawaii. Average global CO
2
(g) mixing ratios
have increased from approximately 280 ppmv in the mid-1800s to approximately 370
ppmv today. The yearly increases are due to increased CO
2
(g) emission from fossil-
fuel combustion. The seasonal fluctuation in CO
2
(g) mixing ratios is due to
photosynthesis and bacterial decomposition. When annual plants grow in the spring
and summer, photosynthesis removes CO
2
(g) from the air. When such plants die in the
fall and winter, their decomposition by bacteria adds CO
2
(g) to the air. Typical indoor
mixing ratios of CO
2
(g) are 700 to 2,000 ppmv, but can exceed 3,000 ppmv when
unvented appliances are used (Arashidani et al., 1996).
Health Effects
Outdoor mixing ratios of CO
2
(g) are too low to cause noticeable health problems.
In indoor air, CO
2
(g) mixing ratios may build up enough to cause some discomfort, but
those higher than 15,000 ppmv are necessary to af
fect human respiration. Mixing
ratios higher than 30,000 ppmv are necessary to cause headaches, dizziness, or nausea
(Schwarzberg, 1993). Such mixing ratios do not generally occur.
3.6.3. Carbon Monoxide
Carbon monoxide [CO(g)] is a tasteless, colorless, and odorless gas. Although CO(g)
is the most abundantly emitted variable gas aside from CO
2
(g) and H
2
O(g), it plays a
small role in ozone formation in urban areas. In the background troposphere, it plays a
68 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
310
320
330
340
350
360
370
380
1960 1970 1980 1990 2000
CO
2
(g) mixing ratio (ppmv)
Year
Figure 3.11. Yearly and seasonal fluctuations in carbon dioxide mixing ratio at Mauna Loa
Observatory, Hawaii, since 1958. Data for 1958–1999 from Keeling and Whorf (2000) and
for 2000 from Mauna Loa Data Center (2001).

larger role in ozone formation. CO(g) is not a greenhouse gas, but its emission and
oxidation to CO
2
(g) affect global climate. CO(g) is not important with respect to
stratospheric ozone reduction or acid deposition. CO(g) is an important component of
urban and indoor air pollution because it has harmful short-term health effects. CO(g)
is one of six pollutants, called criteria air pollutants (Section 8.1.5), for which U.S.
National Ambient Air Quality Standards (NAAQS) were set by the U.S.
Environmental Protection Agency (U.S. EPA) under the 1970 U.S. Clean Air Act
Amendments (CAAA70). CO(g) is now regulated in man
y other countries as well
(Section 8.2).
Sources and Sinks
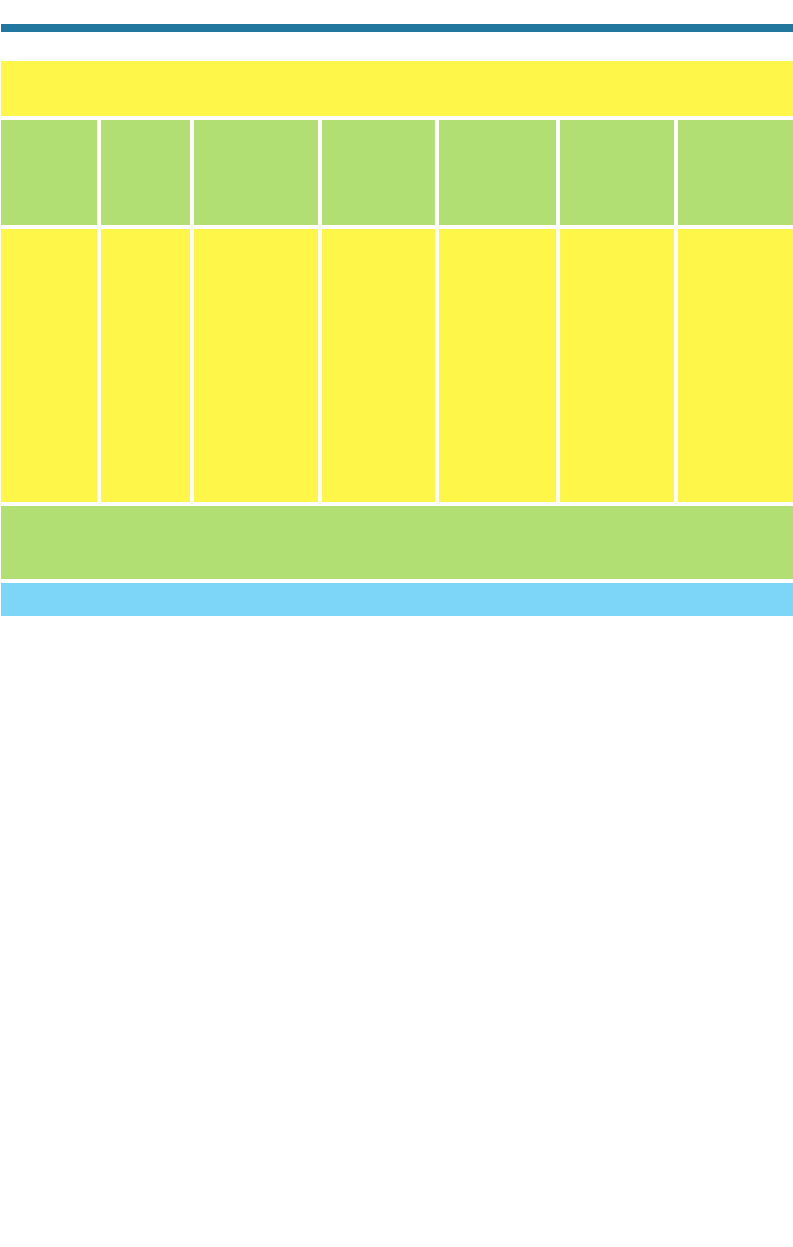
Table 3.8 summarizes the sources and sinks of CO(g). A major source of CO(g) is
incomplete combustion in automobiles, trucks, and airplanes. CO(g) emission sources
include wildfires, biomass burning, nontransportation combustion, some industrial
processes, and biological activity. Indoor sources of CO(g) include water heaters, coal
and gas heaters, and gas stoves. The major sink of CO(g) is chemical conversion to
CO
2
(g). It is also lost by deposition to soils and ice caps and dissolution in ocean
water. Because it is relatively insoluble, its dissolution rate is slow.
Table 3.9 shows that, in 1997, total emissions of CO(g) in the United States were
90 million short tons (1 metric ton 1.1023 short tons). The largest source was trans-
portation. CO(g) emissions decreased in the United States between 1988 and 1997 by
about 25 percent, primarily due to the increased use of the catalytic converter in vehi-
cles (Chapter 8).
Mixing Ratios
Mixing ratios of CO(g) in urban air are typically 2 to 10 ppmv. On freeways and in
traffic tunnels, they can rise to more than 100 ppmv. Typical CO(g) mixing ratios
inside automobiles in urban areas range from 9 to 56 ppmv (Finlayson-Pitts and Pitts,
1999). In indoor air, hourly average mixing ratios can reach 6–12 ppmv when a gas
stove is turned on (Samet et al., 1987). In the absence of indoor sources, CO(g) indoor
mixing ratios are usually less than are those outdoors (Jones, 1999). In the free tropo-
sphere, CO(g) mixing ratios vary from 50 to 150 ppbv.
Health Effects
Exposure to 300 ppmv of CO(g) for one hour causes headaches; exposure to 700
ppmv of CO(g) for one hour causes death, CO(g) poisoning occurs when it dissolves
in blood and replaces oxygen as an attachment to hemoglobin [Hb(aq)], an iron-con-
taining compound. The conversion of O
2
Hb(aq) to COHb(aq) (carboxyhemoglobin)
causes suffocation. CO(g) can also interfere with O
2
(g) diffusion in cellular mitochon-
dria and with intracellular oxidation (Gold, 1992). For the most part, the effects of
CO(g) are reversible once exposure to CO(g) is reduced. Following acute exposure,
STRUCTURE AND COMPOSITION OF THE PRESENT-DAY ATMOSPHERE 69
Table 3.8. Sources and Sinks of Atmospheric Carbon Monoxide
Fossil-fuel combustion Kinetic reaction to carbon dioxide
Biomass burning Transfer to soils and ice caps
Photolysis and kinetic reaction Dissolution in ocean water
Plants and biological activity in oceans
Sources Sinks
however, individuals may still express neurologic or psychologic symptoms for weeks
or months, especially if they become unconscious temporarily (Choi, 1983).
3.6.4. Methane
Methane [CH
4
(g)] is the most reduced form of carbon in the air. It is also the simplest
and most abundant hydrocarbon and organic gas. Methane is a greenhouse gas that
absorbs thermal-IR radiation 25 times more efficiently, molecule for molecule, than does
CO
2
(g), but mixing ratios of carbon dioxide, are much larger than are those of methane.
Methane slightly enhances ozone formation in photochemical smog, but because the
incremental ozone produced from methane is small in comparison with ozone produced
from other hydrocarbons, methane is a relatively unimportant component of photochem-
ical smog. In the stratosphere, methane has little effect on the ozone layer, but its
chemical decomposition provides one of the few sources of stratospheric water vapor.
Neither the emission nor ambient concentration of methane is regulated in any country.
Sources and Sinks
Table 3.10 summarizes the sources and sinks of methane. Methane is produced in
anaerobic environments, where methanogenic bacteria consume organic material and
excrete methane (Equation 2.4). Ripe anaerobic environments include rice paddies
(Fig. 3.12), landfills, wetlands, and the digestive tracts of cattle, sheep, and termites.
Methane is also produced in the ground from the decomposition of fossilized carbon.
The resulting natural gas, which contains more than 90 percent methane, often
leaks to the air or is harnessed and used for energy. Methane is also produced during
70 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
Table 3.9. Estimated Total Emissions and P
ercentage of T
otal Emissions b
y Source Categor
y
in the United States in 1997
Carbon CO(g) 90 6.9 5.5 76.6 11.0
monoxide
Nitrogen NO
(g) 24 3.9 45.4 49.2 1.5
oxides
Sulfur SO
2
(g) 20 8.4 84.7 6.6 0.3
dioxide
Particulate PM
10
(aq,s) 37 3.9 3.2 2.2 90.7
matter
10 m
a
Lead Pb(s) 0.004 74.1 12.6 13.3 0
Reactive ROGs 20 51.2 4.5 39.9 4.4
organic
gases
Industrial Fuel Transportation
Processes Combustion (mobile
Chemical Total Emissions (area sources; (point sources; sources; Miscellaneous
Formula (10
6
short tons percentage percentage percentage (percentage
Substance or Acronym per year) of total) of total) of total) of total)
a
PM
10
is particulate matter with diameter 10 m. Miscellaneous PM
10
sources include fugitive dust (57.9
percent of total PM
10
emissions), agricultural and forest emissions (14.0 percent), wind erosion (15.8 percent),
and other combustion sources (3.0 percent).
Source: U.S. EPA (1997).

biomass burning, fossil-fuel combustion, and atmospheric chemical reactions. Its sinks
include chemical reactions, transfer to soils, ice caps, the oceans, and consumption by
methanotrophic bacteria. The e-folding lifetime of methane due to chemical reaction is
about 8 to 12 years, which is slow in comparison with the lifetimes of other organic
gases. Because methane is relatively insoluble, its dissolution rate into ocean water is
slow. Approximately 80 percent of the methane in the air today is biogenic in origin;
the rest originates from fuel combustion and natural gas leaks
Mixing Ratios
Methane’s average mixing ratio in the troposphere is near 1.8 ppmv, which is an
increase from about 0.8 ppmv in the mid-1800s (Ethridge et al., 1992). Its tropospheric
mixing ratio has increased steadily due to increased biomass burning, fossil-fuel com-
bustion, fertilizer use, and landfill development. Mixing ratios of methane are relatively
constant with height in the troposphere, but decrease in the stratosphere due to chemical
loss. At 25 km, methane’s mixing ratio is about half that in the troposphere.
STRUCTURE AND COMPOSITION OF THE PRESENT-DAY ATMOSPHERE 71
Table 3.10. Sources and Sinks of Atmospheric Methane
Methanogenic bacteria (lithotrophic autotrophs) Kinetic reaction
Natural gas leaks during fossil-fuel mining Transfer to soils and ice caps
and transport Methanotrophic bacteria (conventional
Biomass burning heterotrophs)
Fossil-fuel combustion
Kinetic reaction
Sources Sinks
Figure 3.12. Rice paddies, such as this one in Sundarbans, West Bengal, India, produce not
only an important source of food, but also methane gas. Photo by Jim Welch, available from
the National Renewable Energy Laboratory.

Health Effects
Methane has no harmful human health effects at typical outdoor or indoor mixing
ratios.
3.6.5. Ozone
Ozone [O
3
(g)] is a relatively colorless gas at typical mixing ratios. It appears faintly
purple when its mixing ratios are high because it weakly absorbs green wavelengths of
visible light and transmits red and blue, which combine to form purple. Ozone exhibits
an odor when its mixing ratios exceed 0.02 ppmv. In urban smog or indoors, it is con-
sidered an air pollutant because of the harm that it does to humans, animals, plants,
and materials. In the United States, it is one of the six criteria air pollutants that
requires control under CAAA70. It is also regulated in many other countries. In the
stratosphere, ozone’s absorption of UV radiation provides a protective shield for life
on Earth. Although ozone is considered to be “good” in the stratosphere and “bad” in
the boundary layer, ozone molecules are the same in both cases.
Sources and Sinks
Table 3.11 summarizes the sources and sinks of ozone. Ozone is not emitted. Its
only source into the air is chemical reaction. Sinks of ozone include reaction,
transfer
to soils and ice caps, and dissolution in ocean waters. Because ozone is relatively
insoluble, its dissolution rate is relatively slow.
Mixing Ratios
In the free troposphere, ozone mixing ratios are 20 to 40 ppbv near sea level and
30 to 70 ppbv at higher altitudes. In urban air, ozone mixing ratios range from less
than 0.01 ppmv at night to 0.50 ppmv (during afternoons in the most polluted cities
world wide), with typical values of 0.15 ppmv during moderately polluted afternoons.
Indoor ozone mixing ratios are almost always less than are those outdoors. In the strat-
osphere, peak ozone mixing ratios are around 10 ppmv.
Health Effects
Ozone causes headaches at mixing ratios greater than 0.15 ppmv, chest pains at
mixing ratios greater than 0.25 ppmv, and sore throat and cough at mixing ratios greater
than 0.30 ppmv. Ozone decreases lung function for people who exercise steadily for
more than an hour while exposed to concentrations greater than 0.30 ppmv. Symptoms
of respiratory problems include coughing and breathing discomfort. Small decreases
in lung function affect people with asthma, chronic bronchitis, and emphysema. Ozone
may also accelerate the aging of lung tissue. At levels greater than 0.1 ppmv, ozone
affects animals by increasing their susceptibility to bacterial infection. It also interferes
with the growth of plants and trees and deteriorates organic materials, such as rubber,
72 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
Table 3.11. Sources and Sinks of Atmospheric Ozone
Chemical reaction of O(g) with O
2
(g) Photolysis
Kinetic reaction
Transfer to soils and ice caps
Dissolution in ocean water
Sources Sinks

textile dyes and fibers, and some paints and coatings (U.S. EPA, 1978). Ozone increases
plant and tree stress and their susceptibility to disease, infestation, and death.
3.6.6. Sulfur Dioxide
Sulfur dioxide [SO
2
(g)] is a colorless gas that exhibits a taste at levels greater than
0.3 ppmv and a strong odor at le
vels greater than 0.5 ppmv. SO
2
(g) is a precursor to
sulfuric acid [H
2
SO
4
(aq)], an aerosol particle component that affects acid deposition,
global climate, and the global ozone layer. SO
2
(g) is one of the six air pollutants
for which NAAQS standards are set by the U.S. EPA under CAAA70. SO
2
(g) is now
regulated in many countries.
Sources and Sinks
Table 3.12 summarizes the major sources and sinks of SO
2
(g). Some sources include
coal-fired power plants, automobile tailpipes, and volcanos. SO
2
(g) is also produced
chemically in the air from biologically produced dimethylsulfide [DMS(g)] and hydro-
gen sulfide [H
2
S(g)]. SO
2
(g) is removed by chemical reaction,
dissolution in water, and
transfer to soils and ice caps. SO
2
(g) is relatively soluble. SO
2
(g) emissions decreased
in the U.S. between 1988 and 1997 by about 12 percent. Table 3.9 shows that, in 1997,
the total mass of emitted SO
2
(g) in the United States was 20 million short tons. Between
1988 and 1997, SO
2
(g) emissions decreased in the United States by 17 percent.
Mixing Ratios
In the background troposphere, SO
2
(g) mixing ratios range from 10 pptv to 1 ppbv.
In polluted air, they range from 1 to 30 ppbv. SO
2
(g) levels are usually lower indoors
than outdoors. The indoor to outdoor ratio of SO
2
(g) is typically between 0.1:1 to 0.6:1
in buildings without indoor sources (Jones, 1999). In one study, indoor mixing ratios
were found to be 30 to 57 ppbv in homes equipped with kerosene heaters or gas stoves
(Leaderer et al., 1984, 1993).
Health Effects
Because SO
2
(g) is soluble, it is absorbed in the mucous membranes of the nose and
respiratory tract. Sulfuric acid [H
2
SO
4
(aq)] is also soluble, but its deposition rate into
the respiratory tract depends on the size of the particle in which it dissolves (Maroni et
al., 1995). High concentrations of SO
2
(g) and H
2
SO
4
(aq) can harm the lungs (Islam and
Ulmer, 1979). Bronchiolar constrictions and respiratory infections can occur at mixing
ratios greater than 1.5 ppmv. Long-term exposure to SO
2
(g) from coal burning is asso-
ciated with impaired lung function and other respiratory ailments (Qin et al., 1993).
People exposed to open coal fires emitting SO
2
(g) are likely to suffer from breathlessness
and wheezing more than are those not exposed to such fires (Burr et al., 1981).
STRUCTURE AND COMPOSITION OF THE PRESENT-DAY ATMOSPHERE 73
Table 3.12. Sources and Sinks of Atmospheric Sulfur Dioxide
Oxidation of DMS(g) Kinetic reaction to H
2
SO
4
(g)
Volcanic emission Dissolution in cloud drops and ocean water
Fossil-fuel combustion Transfer to soils and ice caps
Mineral ore processing
Chemical manufacturing
Sources Sinks

3.6.7. Nitric Oxide
Nitric oxide [NO(g)] is a colorless gas and a free radical. It is important because it is a
precursor to tropospheric ozone, nitric acid [HNO
3
(g)], and particulate nitrate [NO
3
].
Whereas NO(g) does not directly affect acid deposition, nitric acid does. Whereas
NO(g) does not affect climate, ozone and particulate nitrate do. Natural NO(g) reduces
ozone in the upper stratosphere. Emissions of NO(g) from jets that fly in the strato-
sphere also reduce stratospheric ozone. Outdoor levels of NO(g) are not regulated in
any country.
Sources and Sinks
Table 3.13 summarizes the sources and sinks of NO(g). NO(g) is emitted by
microbes in soils and plants during denitrification, and it is produced by lightning,
combustion,
and chemical reaction. Combustion sources include aircraft, automobiles,
oil refineries, and biomass burning. The primary sink of NO(g) is chemical reaction.
Mixing Ratios
A typical sea-level mixing ratio of NO(g) in the background troposphere is 5 pptv.
In the upper troposphere, NO(g) mixing ratios are 20 to 60 pptv. In urban regions,
NO(g) mixing ratios reach 0.1 ppmv in the early morning,
but may decrease to zero by
midmorning due to reaction with ozone.
Health Effects
Nitric oxide has no harmful human health effects at typical outdoor or indoor mix-
ing ratios.
3.6.8. Nitrogen Dioxide
Nitrogen dioxide [NO
2
(g)] is a brown gas with a strong odor. It absorbs short (blue
and green) wavelengths of visible radiation, transmitting the remaining green and all
red wavelengths, causing NO
2
(g) to appear brown. NO
2
(g) is an intermediary between
NO(g) emission and O
3
(g) formation. It is also a precursor to nitric acid, a component
of acid deposition. Natural NO
2
(g), like natural NO(g), reduces ozone in the upper
stratosphere. NO
2
(g) is one of the six criteria air pollutants for which ambient stan-
dards are set by the U.S. EP
A under CAAA70. It is now regulated in many countries.
Sources and Sinks
Table 3.14 summarizes sources and sinks of NO
2
(g). Its major source is oxidation
of NO(g). Minor sources are fossil fuel combustion and biomass burning. During
74 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
Table 3.13. Sources and Sinks of Atmospheric Nitric Oxide
Denitrification in soils and plants Kinetic reaction
Lightning Dissolution in ocean water
Fossil-fuel combustion Transfer to soils and ice caps
Biomass burning
Photolysis and kinetic reaction
Sources Sinks

combustion or burning, NO
2
(g) emissions are about 5 to 15 percent those of NO(g).
Indoor sources of NO
2
(g) include gas appliances, kerosene heaters, woodburning
stoves, and cigarettes. Sinks of NO
2
(g) include photolysis, chemical reaction, dissolu-
tion into ocean water, and transfer to soils and ice caps. NO
2
(g) is relatively insoluble
in water. Table 3.9 shows that in 1997,
24 million short tons of NO
2
(g) were emitted in
the United States.
Mixing Ratios
Mixing ratios of NO
2
(g) near sea level in the free troposphere range from 20 to
50 pptv. In the upper troposphere, mixing ratios are 30 to 70 pptv. In urban regions,
they range from 0.1 to 0.25 ppmv. Outdoors, NO
2
(g) is more prevalent during mid-
morning than during midday or afternoon because sunlight breaks down most
NO
2
(g) past midmorning. In homes with gas-cooking stoves or unvented gas space
heaters, weekly average NO
2
(g) mixing ratios can range from 21 to 50 ppbv,
although peak mixing ratios may reach 400–1,000 ppbv (Spengler, 1993; Jones
et al., 1999).
Health Effects
Although exposure to high mixing ratios of NO
2
(g) harms the lungs and increases
respiratory infections (Frampton et al., 1991), epidemiologic evidence indicates that
exposure to typical mixing ratios of NO
2
(g) has little effect on the general population.
Children and asthmatics are more susceptible to illness associated with high NO
2
mix-
ing ratios than are adults (Li et al., 1994). Pilotto et al. (1997) found that levels of
NO
2
(g) greater than 80 ppbv resulted in increased reports of sore throats, colds, and
absences from school. Goldstein et al. (1988) found that exposure to 300 to 800 ppbv
NO
2
(g) in kitchens reduced lung capacity by about 10 percent. NO
2
(g) may trigger
asthma by damaging or irritating and sensitizing the lungs, making people more sus-
ceptible to allergic response to indoor allergens (Jones, 1999). At mixing ratios
unrealistic under normal indoor or outdoor conditions, NO
2
(g) can result in acute
bronchitis (25 to 100 ppmv) or death (150 ppmv).
3.6.9. Lead
Lead [Pb(s)] is a gray-white, solid heavy metal with a low melting point that is present
in air pollution as an aerosol particle component. It is soft, malleable, a poor conductor
of electricity, and resistant to corrosion. It was first regulated as a criteria air pollutant
in the United States in 1976. Many countries now regulate the emission and outdoor
concentration of lead.
Sources and Sinks
Table 3.15 summarizes the sources and sinks of atmospheric lead. Lead is emitted
during combustion of leaded fuel, manufacture of lead-acid batteries, crushing of lead
STRUCTURE AND COMPOSITION OF THE PRESENT-DAY ATMOSPHERE 75
Table 3.14. Sources and Sinks of Atmospheric Nitrogen Dioxide
Photolysis and kinetic reaction Photolysis and kinetic reaction
Fossil-fuel combustion Dissolution in ocean water
Biomass burning Transfer to soils and ice caps
Sources Sinks

ore, condensation of lead fumes from lead-ore smelting, solid-waste disposal, uplift of
lead-containing soils, and crustal weathering of lead ore. Between the 1920s and the
1970s, the largest source of atmospheric lead was automobile combustion.
In December 1921, General Motors researcher Thomas J. Midgley Jr. (1889–
1944; Fig. 3.13) discovered that tetraethyl lead was a useful fuel additive for reducing
engine knock, increasing octane levels, and increasing engine power and efficiency in
automobiles. (Midgley later discovered chlorofluorocarbons, CFCs, the precursors to
stratospheric ozone destruction–Section 11.5.1.)
Although Midgley also found that ethanol/benzene blends reduced knock in
engines, he chose to push tetraethyl lead, and it was first marketed in 1923 under the
name “Ethyl gasoline”. That same year, Midgley and three other General Motors
laboratory employees experienced lead poisoning. Despite his personal experience
and warnings sent to him from leading experts on the poisonous effects of lead,
Midgley countered, “The exhaust does not contain enough lead to worry about, but
no one knows what legislation might come into existence fostered by competition
and fanatical health cranks” (Kovarik, 1999,). Between September 1923 and April
1925, 17 workers at du Pont, General Motors, and Standard Oil died and 149 were
injured due to lead poisoning during the processing of leaded gasoline. Five of the
workers died in October 1924 at a Standard Oil of New Jersey refinery after they
became suddenly insane from the cumulative exposure to high concentrations of
tetraethyl lead. Despite the deaths and public outcry, Midgley continued to defend
his additive. In a paper presented at the American Chemical Society conference in
April 1925, he stated
…[T] etraethyl lead is the only material available which can bring about these
[antiknock] results, which are of vital importance to the continued economic
use by the general public of all automotive equipment, and unless a grave and
inescapable hazard exists in the manufacture of tetraethyl lead, its abandon-
ment cannot be justified. (Midgley, 1925)
Midgley’s claim about the lack of antiknock alternatives contradicted his own work
with ethanol/benzene blends, iron carbonyl, and other mixes that prevented knock.
In May 1925, the U.S. Surgeon General (head of the Public Health Service) put
together a committee to study the health effects of tetraethyl lead. The Surgeon
General argued that because no regulatory precedent existed, the committee would
have to find striking evidence of serious and immediate harm for action to be taken
against lead (Kovarik, 1999). Based on measurements that showed lead contents in
fecal pellets of typical drivers and garage workers lower than those of lead-industry
workers, and based on the observations that drivers and garage workers had not
76 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
Table 3.15. Sources and Sinks of Atmospheric Lead
Leaded-fuel combustion Deposition to soils, ice caps,
Lead-acid battery manufacturing and oceans
Lead-ore crushing and smelting Inhalation
Dust from soils contaminated with lead-based paint
Solid-waste disposal
Crustal physical weathering
Sources Sinks

experienced direct lead poisoning, the Surgeon General concluded that there were “no
grounds for prohibiting the use of ethyl gasoline” (U.S. Public Health Service, 1925).
He did caution that further studies should be carried out (U.S. Public Health Service,
1925). Despite the caution, more studies were not carried out for thirty years, and
effective opposition to the use of leaded gasoline
ended.
By the mid-1930s, 90 percent of U.S. gasoline was
leaded. Industrial backing of lead became so strong
that in 1936, the U.S. Federal Trade Commission
issued a restraining order forbidding commercial
criticism of tetraethyl lead, stating that it is
entirely safe to the health of (motorists) and to
the public in general when used as a motor fuel,
and is not a narcotic in its effect, a poisonous
dope, or dangerous to the life or health of a cus-
tomer, purchaser, user or the general public.
(Federal Trade Commission, 1936)
Only in 1959 did the Public Health Service
reinvestigate the issue of tetraethyl lead. At that
time, they found it “regrettable that the investiga-
tions recommended by the Sur
geon General
’s
Committee in 1926 were not carried out by the
Public Health Service” (U.S. Public Health Service,
1959). Despite the concern, tetraethyl lead was not
regulated as a pollutant in the United States until
1976. In 1975, the catalytic converter, which
reduced emission of carbon monoxide, hydrocar-
bons, and eventually oxides of nitrogen from cars,
was invented. Because lead deactivates the catalyst
in the catalytic converter, cars using catalytic con-
verters could run only on unleaded fuels. Thus, the
required use of the catalytic converter in new cars
inadvertently provided a convenient method to phase
out the use of lead. The regulation of lead as a crite-
ria air pollutant in the United States in 1976 due to
its health effects also hastened the phase out of lead
as a gasoline additive. Between 1970 and 1997, total
lead emissions in the United States decreased from
219,000 to 4,000 short tons per year. Table 3.9
shows that, in 1997, only 13.3 percent of total lead
emissions originated from transportation. Today, the
largest sources of atmospheric lead in the United
States are lead-ore crushing, lead-ore smelting, and
battery manufacturing. Since the 1980s, leaded
gasoline has been phased out in many countries,
although it is still an additive to gasoline in several
others.
STRUCTURE AND COMPOSITION OF THE PRESENT-DAY ATMOSPHERE 77
Figure 3.13. Thomas J. Midgley, Jr.
(1889–1944). Inventor of leaded gasoline and
chlorofluorocarbons (CFCs), Midgley was born
in Beaver Falls, Pennsylvania in 1889. He
grew up in Dayton and Columbus, Ohio, and
graduated from Cornell University with a
degree in Mechanical Engineering in 1911. In
1916, he joined the Dayton Engineering
Laboratories Company (DELCO) as a
researcher. Delco became the main research
laboratory for General Motors in 1919. In
1921, Midgley invented leaded gasoline,
which he named Ethyl. In 1923, he became
vice president of the Ethyl Gasoline
Corporation, a subsidiary of General Motors
and Standard Oil. In 1924, he was forced to
step down due to management problems. He
returned to research on synthetic rubber at
the Thomas and Hochwalt Laboratory in
Dayton, Ohio, with funding from General
Motors. In 1928, Midgley and two assistants
invented chlorofluorocarbons (CFCs) as a sub-
stitute refrigerant for ammonia. Midgley
moved on to became vice president of Kinetic
Chemicals, Inc. (1930), dir
ector and vice
president of the Ethyl-Dow Chemical Company
(1933) and director and vice president of the
Ohio State University Research Foundation
(1940–1944). In 1940, he became afflicted
with polio, which became so severe that he
lost a leg and designed a system of ropes to
pull himself out of bed. On November 2,
1944, he died of strangulation in the rope
system. Some consider his death a suicide.
