Jacobson M.Z. Atmospheric Pollution
Подождите немного. Документ загружается.


Earth’s gravitational field. As a result of these two loss processes (solar wind stripping
and gravitational escape), the ratios of H and He to other elements in the Earth’s
atmosphere today are less than are the corresponding ratios in the sun.
2.3.1. Solid-Earth Formation
The rock-forming elements that reached the Earth reacted to form compounds with
different melting points, densities, and chemical reactivities. Dense compounds and
compounds with high melting points, including many iron- and nickel-containing
compounds, settled to the center of the Earth, called the Earth’s core. Table 2.2 shows
that the total Earth contains more than 34 percent iron and 2 percent nickel by mass,
but the Earth’s crust (its top layer) contains less than 7 percent iron and 0.1 percent
nickel by mass, supporting the contention that iron and nickel settled to the core. Low-
density compounds and compounds with low melting points, including silicates of
aluminum, sodium, and calcium, rose to the surface and are the most common com-
pounds in the Earth’s crust. Table 2.2 supports this contention. Some moderately dense
and moderately high-melting-point silicates, such as those containing magnesium or
iron, settled to the Earth’s mantle, which is a layer of Earth’s interior between its crust
and its core.
During the formation of the Earth’s core, between 4.5 and 4.0 b.y.a., temperatures
in the core were hotter than they are today, and the only mechanism of heat escape to
the surface was conduction, the transfer of energy from molecule to molecule. Because
conduction is a slow process, the Earth’s internal energy could not dissipate easily, and
its temperature increased until the entire body became molten.
At that time, the Earth
’s
surface consisted of magma oceans, a hot mixture of melted rock and suspended crys-
tals. When the Earth was molten, convection, the mass movement of molecules,
became the predominant form of energy transfer between the core and surface.
Convection occurred because temperatures in the core were hot enough for core materi-
al to expand and float to the crust, where it cooled and sank down again. This process
enhanced energy dissipation from the Earth’s center to space. After sufficient energy
dissipation (cooling), the magma oceans solidified, creating the Earth’s crust. The crust
is estimated to have formed 3.8 to 4.0 b.y.a., but possibly as early as 4.2 to 4.3 b.y.a.
(Crowley and North, 1991). The core cooled as well, but its outer part, now called the
outer core, remains molten. Its inner part, now called the inner core, is solid.
Figure 2.7 shows temperature, density, and pressure profiles inside the Earth today.
The Earth’s crust extends from the topographical surface to about 10 to 75 km below
continents and to about 8 km below the ocean floor. The crust itself contains low-
density, low-melting-point silicates. The continental crust contains primarily granite,
whereas the ocean crust contains primarily basalt. Granite is a type of rock composed
mainly of quartz [SiO
2
(s)] and potassium feldspar [KAlSi
3
O
8
(s)]. Basalt is a type of
rock composed primarily of plagioclase feldspar [[NaAlSi
3
O
3
-CaAl
2
Si
2
O
8
(s)] and
pyroxene (multiple compositions). The densities of both granite and basalt are about
2,800 kg m
3
.
Below the Earth’s crust is its mantle, which consists of an upper and lower part,
both made of iron–magnesium–silicate minerals. The upper mantle extends from
the crust down to about 700 km. At that depth, a density gradation occurs due to a
change in crystal packing. This gradation roughly defines the base of the upper mantle
and the top of the lower mantle. Below 700 km, the density gradually increases to the
mantle–core boundary at 2,900 km.
38 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
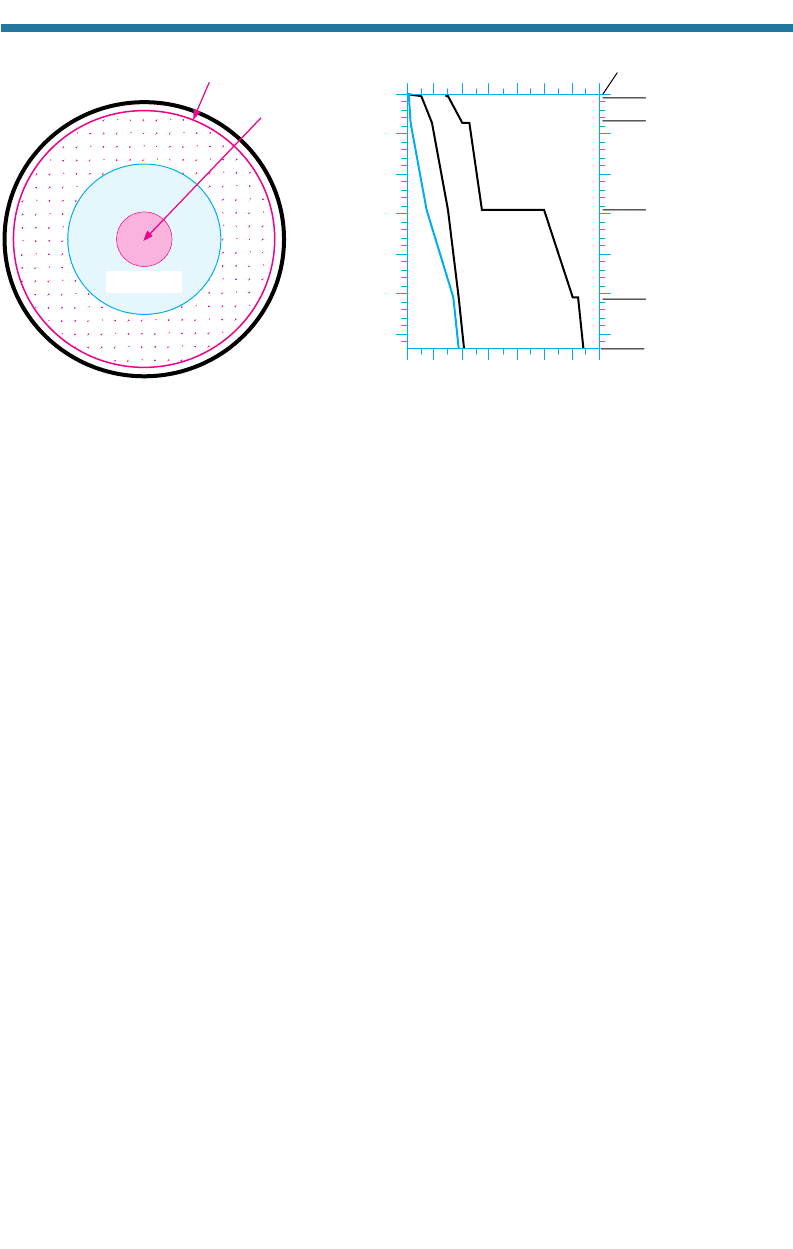
The outer core extends from 2,900 km down to about 5,100 km. This region con-
sists of liquid iron and nickel, although the top few hundred kilometers contain liquids
and crystals. The inner core extends from 5,100 km down to the Earth’s center and is
solid, also consisting of iron and nickel, but packed at a higher density. Temperatures,
densities, and pressures at the center of the Earth are estimated to be 4,300C, 13,000
kg m
3
, and 3,850 kbar, respectively (1 kilobar 1,000 bar 10
8
N m
2
10
8
Pa – for comparison, surface air pressures are about 1 bar).
2.3.2. Prebiotic Atmosphere
Earth’s second atmosphere evolved as a result of outgassing from the Earth’s mantle. As
temperatures increased during the molten stage, hydrogen and oxygen, bound in crustal
minerals as hydroxyl molecules (OH), became detached, forming the gas-phase hydroxyl
radical. The hydroxyl radical then reacted with reduced gases, such as molecular hydro-
gen [H
2
(g)], methane [CH
4
(g)], ammonia [NH
3
(g)], molecular nitrogen [N
2
(g)], and
hydrogen sulfide [H
2
S(g)], to form oxidized gases, such as water [H
2
O(g)], carbon
monoxide [CO(g)], carbon dioxide [CO
2
(g)], nitrogen dioxide [NO
2
(g)], and sulfur dioxide
[SO
2
(g)]. As the molten rock rose to the Earth’s surface during convection, oxidized and
reduced gases were ejected into the air by volcanos, fumaroles, steam wells, and geysers.
After the crust and mantle solidified, outgassing continued. The resulting second-
ary atmosphere contained no free elemental oxygen. All oxygen was tied up in
oxidized molecules. Indeed, if any free oxygen did exist, it would have been removed
by chemical reaction.
Most outgassed water vapor in the air condensed to form the oceans. The oceans
are a critical part of today’s hydrologic cycle. In this cycle, ocean water evaporates, the
vapor is transported and recondenses to clouds, rain precipitates back to land or ocean
surfaces, and water on land flows gravitationally back to the oceans. Sizable oceans
have been present during almost all of the Earth’s history (Pollack and Yung, 1980). In
1955, geochemist William W. Rubey (1898–1974) calculated that all the water in the
Earth’s oceans and atmosphere could be accounted for by the release of water vapor
from volcanos that erupted throughout the Earth’s history.
THE SUN, THE EARTH, AND THE EVOLUTION OF THE EARTH’S A
TMOSPHERE
39
Crust
Upper mantle
Lower mantle
Outer core
Inner core
0 4,000 8,000 12,000
0
0 4,000 8,000 12,000
1,000
2,000
3,000
4,000
5,000
6,000
Depth below surface (km)
Density (kg m
-3
)
Temperature (°C)
Pressure (kbar)
Inner core
(5,100–6,371 km)
Outer core
(2,900–5,100 km)
Lower mantle
(400–2,900 km)
Upper mantle (50–700 km)
Crust (0–50 km)
(b)(a)
Figure 2.7. (a) Diagram of the Earth’s interior and (b) variation of pressure, temperature, and density in the
Earth’s interior.
Living Organism Term Used
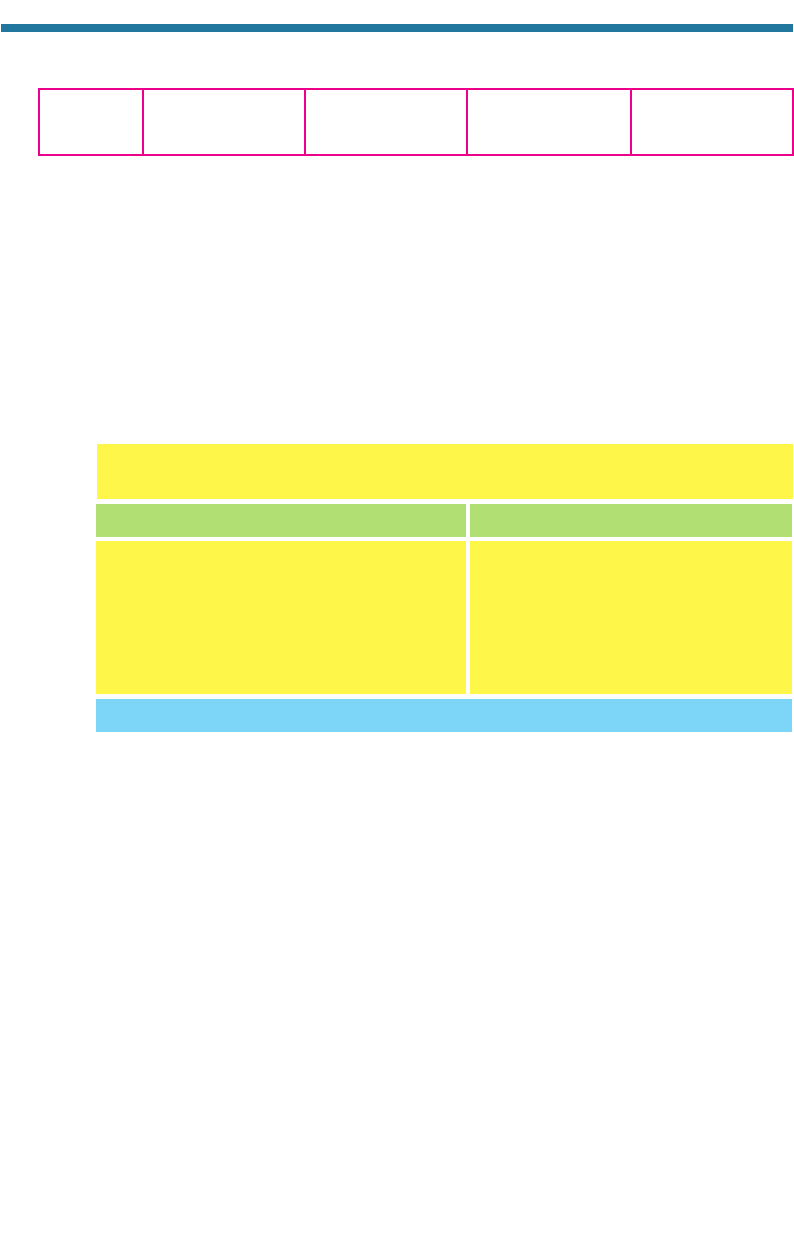
2.3.3. Biotic Atmosphere Before Oxygen
Figure 2.8 shows an approximate timeline of important steps during the e
volution of
the Earth’s atmosphere. Living organisms have been responsible for most of the
changes.
Living organisms can be classified according to their energy and carbon sources.
Table 2.3 shows that energy sources for organisms include sunlight and both inorganic
and organic compounds. Organisms that obtain their ener
gy from sunlight are
pho-
totrophs. Organisms that obtain their energy from oxidation of inorganic compounds
(e.g., carbon dioxide [CO
2
(g)], molecular hydrogen [H
2
(g)], hydrogen sulfide [H
2
S(g)],
the ammonium ion [NH
4
], the nitrite ion [NO
2
]) are lithotrophs. Organisms
that obtain their energy from oxidation of organic compounds are conventional
heterotrophs.
The two sources of carbon for organisms include carbon dioxide and organic com-
pounds. Organisms that obtain their carbon from CO
2
(g) are autotrophs. Organisms
that obtain their carbon from organic compounds are heterotrophs.
Table 2.4 classifies organisms according to their energy and carbon sources.
Photoautotrophs derive their energy from sunlight and their carbon from carbon
dioxide. Photoheterotrophs derive their energy from sunlight and their carbon from
organic material. Lithotrophic autotrophs derive their energy and carbon from
40 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
Table 2.3. Classification of Organisms in Terms of Their Energy
and Carbon Sources
Energy Source
Sunlight Phototroph
Oxidation of inorganic material Lithotroph
Oxidation of organic material Conventional heterotroph
a
Carbon Source
Carbon dioxide Autotroph
Organic material Heterotroph
a
Conventional heterotrophs obtain energy and carbon from organic material.
First shelled invertebrates (0.57)
Primitive fish (0.43–0.5)
Formation of the Earth (4.6)
First land plants (0.395–0.43)
Earliest eukaryotes (1.4)
First prokaryotes (3.5)
Nitrogen fixation (1.5)
Nitrification (1.8)
Billion years ago 4 3 2 1 0
Denitrification (3.2)
Oxygen-producing
photosynthesis
by cyanobacteria (2.3)
Figure 2.8. Timeline of evolution on the Earth.

inorganic material (Fig.
2.9).
Lithotrophic heter
otrophs
derive their ener
gy from
inorganic material and their carbon from organic material. Conventional het-
erotrophs derive their energy and carbon from organic material. Today, all animals,
fungi, protozoa, and most bacteria are conventional heterotrophs.
About 3.5 b.y.a., the first microorganisms appeared on the Earth after a period dur-
ing which the building blocks of life, amino acids, were produced by abiotic
synthesis. Abiotic synthesis is the process by which life is created from chemical reac-
tions and electrical discharges. It was demonstrated in 1953 by American chemist
Stanley Miller (b. 1930), who was working in the laboratory of Harold Urey
(1893–1981). Miller discharged electricity (simulating lightning) through a flask con-
taining H
2
(g), H
2
O(g), CH
4
(g), and NH
3
(g) and liquid water. He let the bubbling
THE SUN, THE EARTH, AND THE EVOLUTION OF THE EARTH’S A
TMOSPHERE
41
Figure 2.9. Hot sulfur springs in Lassen National Park, California. Boiling water of geothermal origin is rich
in hydrogen sulfide. Lithotrophic autotrophic bacteria thrive in the springs and oxidize the hydrogen sulfide
to sulfuric acid, which dissolves the surrounding mineral and converts part of the spring into a “mud pot.”
The steam contains mostly water vapor, but hydrogen sulfide, sulfuric acid, and other gases are also pres-
ent. Courtesy of Alfred Spormann, Stanford University.
Table 2.4. Examples of Organisms Classified According to Their Energ
y and Carbon Sources
Photoautotrophs Green plants, most algae, cyanobacteria, some purple and green bacteria
Photoheterotrophs Some algae, most purple and green bacteria, some cyanobacteria
Lithotrophic autotrophs Hydrogen bacteria, colorless sulfur bacteria, methanogenic bacteria, nitri-
fying bacteria, iron bacteria
Lithotrophic heterotrophs Some colorless sulfur bacteria
Conventional heterotrophs All animals, all fungi, all protozoa, most bacteria
Organism Classification Examples

mixture sit for a week and, after analyzing the results, found that he had produced
complex organic molecules, including amino acids. Later experiments showed that the
same results could be obtained with different gases and with UV radiation as opposed
to with an electrical discharge. In all cases, much of the initial gas had to be highly
reduced (Miller and Orgel, 1974).
In the prebiotic atmosphere, the amino acids required for the production of
deoxyribonucleic acid (DNA) were first developed. About 3.5 b.y.a., the first micro-
scopic cells containing DNA appeared. These cells, termed prokaryotic cells,
contained a single strand of DNA, but had no nucleus. Many early prokaryotes were
conventional heterotrophs because they obtained their energy and carbon from organic
molecules produced during abiotic synthesis. Today, prokaryotic microorganisms
include certain bacteria and blue-green algae.
2.3.3.1. Early Carbon Dioxide
During early biotic evolution, the major energy-producing process, carried out by
prokaryotic conventional heterotrophs, was fermentation, which produced carbon
dioxide gas. One fermentation reaction is
C
6
H
12
O
6
(aq) 2C
2
H
5
OH(aq) 2CO
2
(g)
Glucose Ethanol Carbon (2.3)
dioxide
which is exothermic (energy releasing). The energy source, glucose in this case, is
only partially oxidized to carbon dioxide; thus, the reaction is inefficient.
2.3.3.2. Early Methane
A source of methane gas in the Earth’s early atmosphere (and today) was metabo-
lism by methanogenic bacteria. Such bacteria obtain their carbon from carbon dioxide
and their energy from oxidation of molecular hydrogen; thus, methanogenic bacteria
are lithotrophic autotrophs. Their methane-producing reaction is
4H
2
(g) CO
2
(g) CH
4
(g) 2H
2
O(aq)
Molecular Carbon Methane Liquid (2.4)
hydrogen dioxide water
Methanogenic bacteria use about 90 to 95 percent of carbon dioxide available to them
for this process. The rest is used for synthesis of cell carbon. In Reaction 2.4,
CO
2
(g)
is reduced to CH
4
(g). A reaction such as this, in which cells produce energy by break-
ing down compounds in the absence of molecular oxygen, is called an anaerobic
respiration reaction, where anaerobic means in the absence of oxygen. Anaerobic res-
piration produces energy more efficiently than does fermentation.
2.3.3.3. Early Molecular Nitrogen
In the Earth’s early atmosphere, the most important source of molecular nitrogen
was ammonia photolysis by the two step process,
NH
3
(g) h •N
•
•
(g) 3H
•
(g)
Ammonia Atomic Atomic (2.5)
nitrogen hydrogen
•N
•
•
(g) •N
•
•
(g)
M
N
2
(g)
Atomic nitrogen Molecular (2.6)
nitrogen
42 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION

Once oxygen levels built up in the air sufficiently (about 0.4 b.y.a.), ammonia photolysis
became obsolete because oxygen absorbs the sun’s wavelengths capable of photolyzing
ammonia. About 3.2 b.y.a., some anaerobic conventional heterotrophs developed a new
mechanism of producing molecular nitrogen. In this two step process, called denitrifica-
tion, organic compounds react with the nitrate ion [NO
3
] and the resulting nitrite ion
[NO
2
] by
Organic compound NO
3
CO
2.
(g) NO
2
..
Nitrate Carbon Nitrite (2.7)
ion dioxide ion
Organic compound NO
2
CO
2
(g) N
2
(g) ..
Nitrite Carbon Molecular (2.8)
ion dioxide nitrogen
Denitrification is the source of most molecular nitrogen in the air today
.
2.3.3.4. Anoxygenic Photosynthesis
Most organisms in the Earth’s early atmosphere relied on the conversion of organ-
ic or inorganic material to obtain their energy. During the microbial era,
such
organisms most likely lived underground or in water to avoid exposure to harmful UV
radiation. At some point, certain bacteria, called phototrophs, developed the ability to
obtain their energy from sunlight by a new process, called photosynthesis. An exam-
ple of a photosynthetic reaction by blue, green, and yellow sulfur bacteria, which are
photoautotrophs, is
CO
2
(g) 2H
2
S(g) h CH
2
O(aq) H
2
O(aq) 2S
•
(g)
Carbon Hydrogen Carbohydrate Liquid Atomic (2.9)
dioxide sulfide water sulfur
where CH
2
O(aq) represents a generic carbohydrate dissolved in water. Because
reduced compounds, such as H
2
S(g), were not abundant on the surface of the Earth,
photoautotrophs flourished only in limited environments. Early sulfur-producing
photosynthesis did not result in the production of oxygen; thus, it is referred to as
anoxygenic photosynthesis.
2.3.4. The Oxygen Age
The earth’s atmosphere lacked molecular oxygen and ozone until the onset of
oxygen producing photosynthesis about 2.3 b.y.a, halfway into the present lifetime
of the earth. For the next 1.9 billion years, oxygen-producing photosynthesis was
carried out primarily by c
yanobacteria (Figure 2.10). During this period, oxygen
buildup was slow. About 1.4 b.y.a., oxygen levels were still only 1 percent of those
today. Land plants evolved from blue-green algae, descendents of cyanobacteria,
about 395–430 million years ago (m.y.a.). Like cyanobacteria, plants photosynthe-
sized to produce oxygen. Subsequent to land-plant evolution, oxygen levels began
to increase rapidly. Today, 21 out of every 100 molecules in the air are molecular
oxygen.
Oxygen-producing photosynthesis in plants is similar to that in bacteria. In both
cases, CO
2
(g) and sunlight are required, and reactions occur in chlorophylls.
Chlorophylls reside in photosynthetic membranes. In bacteria, the membranes are cell
membranes; in plants and algae, photosynthetic membranes are found in chloroplasts.
THE SUN, THE EARTH, AND THE EVOLUTION OF THE EARTH’S A
TMOSPHERE
43

Chlorophylls are made of pigments, which are organic molecules that absorb visi-
ble light. Plant and tree leaves generally contain two pigments, chlorophyll a and b,
both of which absorb blue wavelengths (shorter than 500 nm) and red wavelengths
(longer than 600 nm) of visible light. Chlorophyll a absorbs red wavelengths more
efficiently than does chlorophyll b, and chlorophyll b absorbs blue wavelengths more
efficiently than does chlorophyll a. Because neither chlorophyll absorbs between 500
and 600 nm, the green part of the visible spectrum, green wavelengths are reflected by
chlorophyll, giving leaves a green color. Photosynthetic bacteria generally appear pur-
ple, blue, green, or yellow, indicating that their pigments absorb blue, green, and/or
red wavelengths to different degrees.
The oxygen-producing photosynthesis process in green plants is
6CO
2
(g) 6H
2
O(aq) h C
6
H
12
O
6
(aq) 6O
2
(g)
Carbon Liquid Glucose Molecular (2.10)
dioxide water oxygen
where the result, glucose, is dissolved in water in the photosynthetic membrane of the
plant. The source of molecular oxygen during photosynthesis in green plants is not
carbon dioxide, but water. This can be seen by first dividing Reaction 2.10 by 6, then
adding water to each side of the equation. The result is
CO
2
(g) 2H
2
O(aq) h CH
2
O(aq) H
2
O(aq) O
2
(g)
Carbon Liquid Carbohydrate Liquid Molecular (2.11)
dioxide water water oxygen
44 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
Figure 2.10. Hot spring in Yellowstone National Park, Wyoming. The hot, mineral-rich water provides ideal
conditions for colored photosynthetic cyanobacteria to grow at the perimeter of the spring, where the
temperatures drop to about 70 C. The colors identify different photosynthetic bacteria with different tem-
perature optima. Courtesy of Alfred Spormann, Stanford University.

A comparison of Reaction 2.11 with Reaction 2.9 indicates that because the source of
atomic sulfur in Reaction 2.9 is hydrogen sulfide, the analogous source of oxygen in
Reaction 2.11 should be water. This was first hypothesized in 1931 by Cornelius B.
Van Niel, a Dutch microbiologist working at Stanford University, and later proved to
be correct experimentally with the use of isotopically labeled water.
With photosynthesis in cyanobacteria and green plants came the production of molec-
ular oxygen and ozone, which helped to shield the Earth’s surface from UV radiation.
Molecular oxygen absorbs far-UV radiation, and ozone absorbs far-UV, UV-C and a large
portion of UV-B radiation. With the slow production of these gases, the surface of the
Earth became protected from such radiation. The increase in ozone allowed organisms to
migrate to the top of the oceans and land plants to develop 430–395 m.y.a.
2.3.5. Aerobic Respiration and the Oxygen Cycle
The introduction of molecular oxygen and ozone 2.3 b.y.a. also resulted in biological
changes in organisms that shaped our present atmosphere. Most important was the
development of aerobic respiration, which is the process by which molecular oxygen
reacts with organic cell material to produce energy during cellular respiration.
Cellular respiration is the oxidation of organic molecules in living cells.
Whereas aerobic respiration may have developed first in prokaryotes (bacteria and
blue-green algae), its spread coincided with the development of another type of organism,
the eukaryote, about 1.4 b.y.a. A eukaryotic cell contains DNA surrounded by a true
membrane-enclosed nucleus. This differs from a prokaryotic cell, which contains a single
strand of DNA but not a nucleus. Unlike prokaryotes, many eukaryotes became multicel-
lular. Today, the cells of all higher animals, plants, fungi, protozoa, and most algae are
eukaryotic. Prokaryotic cells never evolved past the microbial stage.
Almost all eukaryotic cells respire aerobically. In fact, such cells usually switch
from fermentation to aerobic respiration when oxygen concentrations reach about
1 percent of the present oxygen level (Pollack and Yung, 1980). Thus, eukaryotic cells
probably developed only 1.4 b.y.a., after the oxygen level increased to above 1 percent
of its present level.
The products of aerobic respiration are carbon dioxide and water. Aerobic respira-
tion of glucose, a typical cell component, occurs by
C
6
H
12
O
6
(aq) 6O
2
(g) 6CO
2
(g) 6H
2
O(aq)
Glucose Molecular Carbon Liquid (2.12)
oxygen dioxide water
This process produces energy more efficiently than does fermentation or anaerobic
respiration. Thus, Reaction 2.12 was an evolutionary improvement.
Table 2.5 summarizes the current sources and sinks of O
2
(g). The primary source is
photosynthesis. The major sinks are photolysis in and above the stratosphere and aero-
bic respiration.
2.3.6. The Nitrogen Cycle
With the development of aerobic respiration came the evolution of organisms that
affect the nitrogen cycle. This cycle centers around molecular nitrogen [N
2
(g)], the
most abundant gas in the air today (making up about 78 percent of it by volume).
THE SUN, THE EARTH, AND THE EVOLUTION OF THE EARTH’S A
TMOSPHERE
45
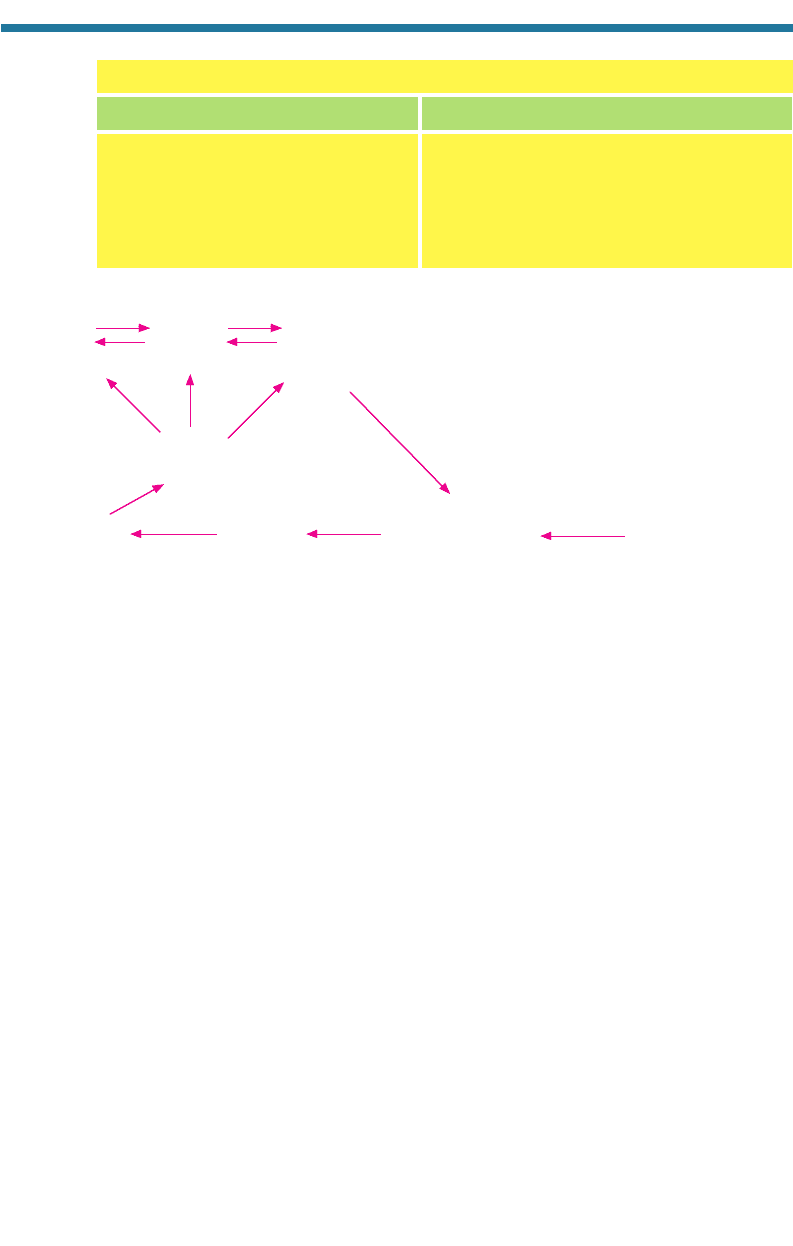
Figure 2.11 summarizes the major steps in the nitrogen cycle. Four of the five steps are
carried out by bacteria in soils. The fifth involves nonbiological chemical reactions
occurring in the air.
The direct source of molecular nitrogen in the air is denitrification, the two step
process carried out by anaerobic bacteria in soils that was described by Reactions 2.7
and 2.8. The second step of denitrification produces nitric oxide [NO(g)], nitrous oxide
[N
2
O(g)], or N
2
(g), with N
2
(g) being the dominant product. N
2
(g) is also produced
chemically from N
2
O(g), which can form from NO(g).
N
2
(g) is slowly removed from the air by nitrogen fixation. During this process,
nitrogen-fixing bacteria, such as Rhizobium, Azotobacter, and Beijerinckia, convert
N
2
(g) to ammonium [NH
4
], some of which evaporates back to the air as ammonia gas
[NH
3
(g)]. Another source of ammonium in soils is ammonification, a process by
which bacteria decompose organic compounds to ammonium. Today, an anthro-
pogenic source of ammonium is fertilizer.
Ammonium is converted to nitrate in soils during a two step process called nitri-
fication. This process occurs only in aerobic environments. In the first step,
nitrosofying (nitrite-forming) bacteria produce nitrite from ammonium. In the second
step, nitrifying (nitrate-forming) bacteria produce nitrate from nitrite. Once nitrate is
formed, the nitrogen cycle continues through the denitrification process.
N
2
(g) has few chemical sinks. Because its chemical loss is slow and because
its removal by nitrogen fixation is slower than is its production by denitrification,
N
2
(g)’s concentration has built up over time. Table 2.6 summarizes the sources and
sinks of N
2
(g).
46 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
Table 2.5. Sources and Sinks of Atmospheric Molecular Oxygen
Photosynthesis by green plants Photolysis and kinetic reaction
Chemical production in the Aerobic respiration
stratosphere and above Dissolution into ocean water
Rusting
Chemical reaction on soil surfaces
Fuel combustion and biomass burning
Sources Sinks
N
2
(g)
Molecular nitrogen
N
2
O(g)
Nitrous oxide
NO(g)
Nitric oxide
NO
2
-
NO
2
-
Nitrite ion
NO
3
-
Nitrate ion
NH
3
(g), NH
4
+
Ammonia, ammonium ion
Nitrogen fixation
(aerobic)
Denitrification
(anaerobic)
Ammonification
Nitrite ion
Nitrification
(aerobic)
Organic compounds
containing
N
Atmospheric reaction
Figure 2.11. Diagram showing bacterial processes affecting the nitrogen cycle.
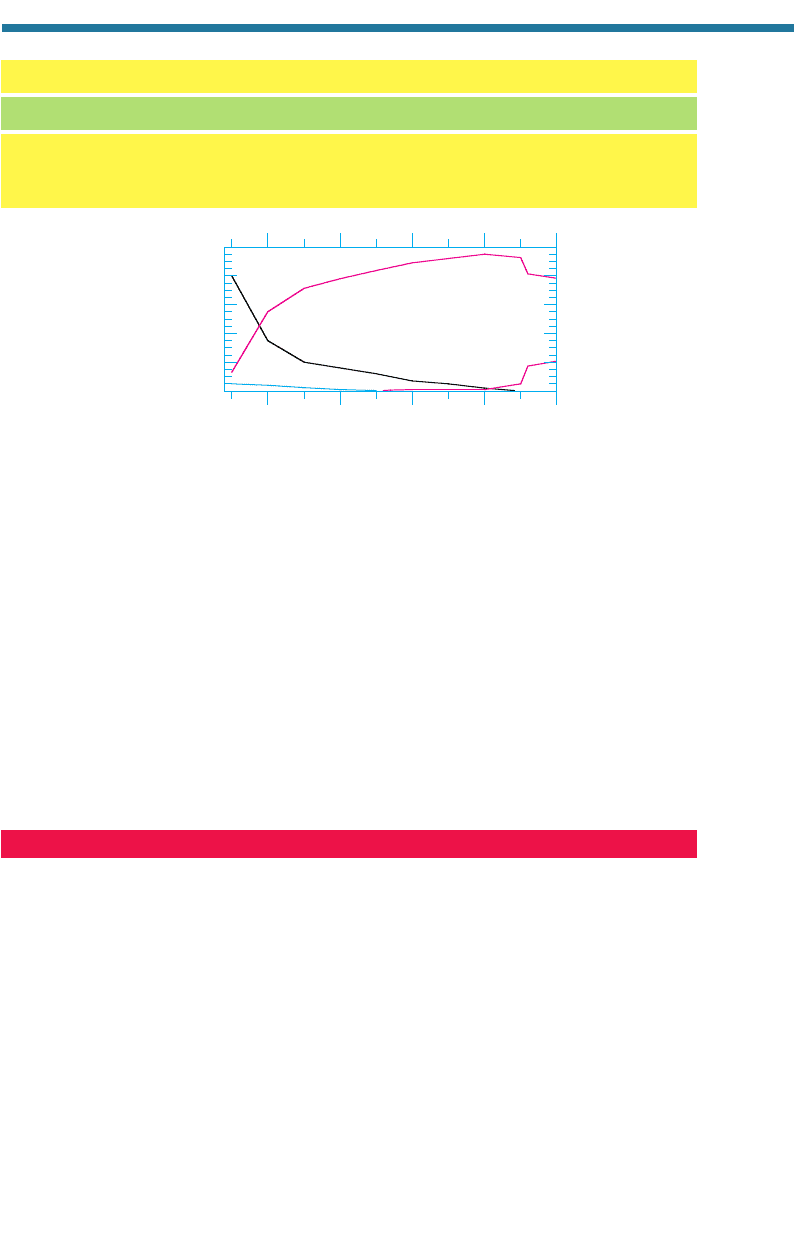
Figure 2.12. Estimated change in composition during the history of the Earth’s second atmos-
phere.
Modified from Cattermole and Moore (1985).
0
20
40
60
80
100
01234
Percentage of total
air by volume
Billions of years ago
N
2
(g)
O
2
(g)
H
2
(g)
CO
2
(g)
2.3.7. Summary of Atmospheric Evolution
Figure 2.12 summarizes the estimated variations in N
2
(g), O
2
(g), CO
2
(g), and H
2
(g)
during the evolution of the Earth’s second atmosphere. The figure shows that the
atmosphere of the early Earth may have been dominated by carbon dioxide. Nitrogen
gradually increased due to denitrification. The oxygen concentration increased follow-
ing the onset of oxygen-producing photosynthesis 2.3 b.y.a. It reached 1 percent of its
present level 1.4 b.y.a., but did not approach its present level until after the evolution of
green plants around 400 m.y.a.
2.4. SUMMARY
The sun formed from the condensation of the solar nebula about 4.6 b.y.a. Solar radia-
tion incident on the Earth originates from the sun’s photosphere. The photosphere emits
radiation with an effective temperature near 6,000 K. The solar spectrum consists of
UV, visible, and near-IR wavelength regimes. The Earth formed from the same nebula
as the sun. Most of the Earth’s growth was due to asteroid and meteorite bombardment.
The composition of the Earth, percentage-wise, is similar to that of stony meteorites.
Dense compounds and compounds with high melting points settled to the center of the
Earth. Light compounds and those with low melting points became concentrated in the
crust. The first atmosphere of the Earth, which consisted of hydrogen and helium, may
have been swept away by an enhanced solar wind during early nuclear explosions in the
sun. The second atmosphere, which resulted from outgassing, initially consisted of car-
bon dioxide, water vapor, and assorted gases. When microbes first evolved, they
converted carbon dioxide, ammonia, hydrogen sulfide, and organic material to methane,
molecular nitrogen, sulfur dioxide, and carbon dioxide, respectively. Oxygen-producing
photosynthesis led to the production of oxygen and ozone. The presence of oxygen
THE SUN, THE EARTH, AND THE EVOLUTION OF THE EARTH’S A
TMOSPHERE
47
Table 2.6. Sources and Sinks of Atmospheric Molecular Nitrogen
Bacterial denitrification Bacterial nitrogen fixation
Atmospheric reaction and photolysis of N
2
O(g) Atmospheric reaction
High-temperature combustion
Sources Sinks
