Jacobson M.Z. Atmospheric Pollution
Подождите немного. Документ загружается.


THE SUN, THE EARTH,
AND THE EVOLUTION OF
THE EARTH’S
ATMOSPHERE
2
30
A
nthropogenic pollution problems result from the enhancement of gas and
aerosol-particle concentrations above background concentrations. In this
chapter, the evolution of the background atmosphere is discussed. The
discussion requires a description of the sun and its origins because sunlight has
affected much of the evolution of the Earth’s atmosphere. The description also
requires a discussion of the Earth’s composition and structure because the inner Earth
affects atmospheric composition through outgassing, and the crust affects atmospher-
ic composition through exchange processes, including soil-dust emission. Earth’s
earliest atmosphere contained mostly hydrogen and helium. Carbon dioxide replaced
these gases during the onset of the Earth’s second atmosphere. Today, nitrogen and
oxygen are the prevalent gases. Processes controlling the changes in atmospheric
composition over time include outgassing from the Earth’s interior, microbial metab-
olism, and atmospheric chemistry. These processes still affect the natural composition
of the air today.
2.1. THE SUN AND ITS ORIGIN
The sun provides the energy to power the Earth. Most of that energy originates from
the sun’s surface, not from its interior. The reason for this is discussed as follows.
About 15 billion years ago (b.y.a.), all mass in the known uni
verse may have been
compressed into a single point, estimated to have a density of 10
9
kg m
3
and a tem-
perature of 10
12
K (kelvin). With the “Big Bang,” this point of mass exploded, ejecting
material in all directions. Aggregates of ejected material collapsed gravitationally
to form the earliest stars. When temperatures in the cores of early stars reached 10
million K, nuclear fusion of hydrogen (H) into helium (He) and higher elements
began, releasing energy that powered the stars. As early stars aged, they ultimately
exploded, ejecting stellar material into space. Table 2.1 gives the abundance of hydro-
gen in the universe today relative to the abundances of other interstellar elements.
About 4.6 b.y.a., some interstellar material aggregated to form a cloudy mass, the
solar nebula. The composition of the solar nebula was the same as that of 95 percent
of the other stars in the universe. Gravitational collapse of the solar nebula resulted in
the formation of the sun.
Table 2.1. Cosmic Abundance of Hydrogen Relative to Those of Other Elements
Hydrogen (H) 1.01 1:1 Silicon (Si)
a
28.1 26,000:1
Helium (He) 4.00 14:1 Iron (Fe)
a
55.8 29,000:1
Oxygen (O) 16.0 1,400:1 Sulfur (S) 32.1 53,000:1
Carbon (C) 12.0 2,300:1 Argon (Ar) 39.9 260,000:1
Neon (Ne) 20.2 10,000:1 Aluminum (Al)
a
27.0 306,000:1
Nitrogen (N) 14.0 11,000:1 Calcium (Ca)
a
40.1 413,000:1
Magnesium (Mg)
a
24.3 24,000:1 Sodium (Na)
a
23.0 433,000:1
Abundance of Abundance of
H Relative H Relative
Element Atomic Mass to Element Element Atomic Mass to Element
a
Rock-forming elements. All other elements vaporize more readily.
Adapted from Goody (1995).
Today, the sun is divided into concentric layers, including interior and atmospheric
layers. About 90 percent of atoms in the sun are hydrogen and 9.9 percent are helium.
The remainder are the other natural elements of the periodic table.
The sun’s interior consists of liquid and gas, and its atmosphere consists predomi-
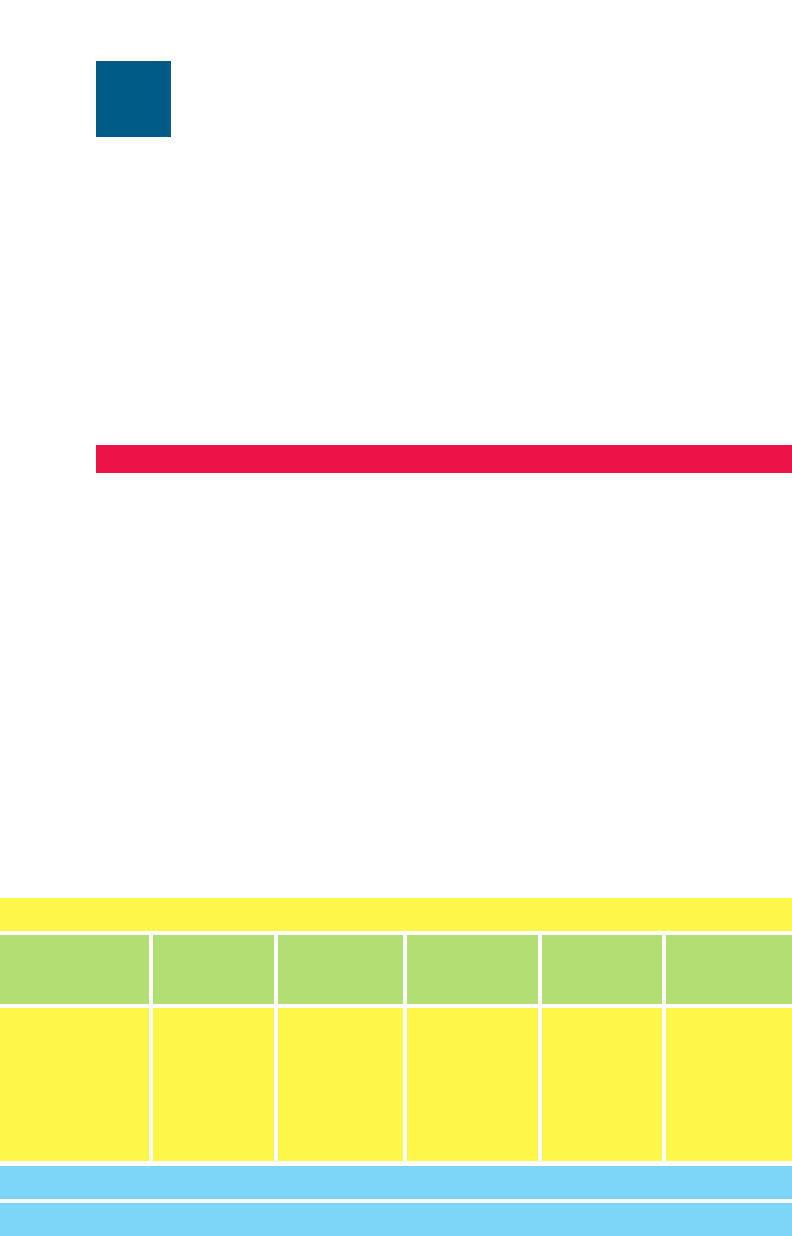
nately of gas. The interior consists of the core, the intermediate interior, and the hydrogen
convection zone (Fig. 2.1). The photosphere, which is primarily gaseous, is a transition
region between the sun’s atmosphere and its interior. Beyond the photosphere, the
sun’s atmosphere consists of the chromosphere, the corona, and solar wind discharge.
The sun has an effective radius (R
p
) of 696,000 km, or 109 times the radius of the
Earth (6378 km). The sun’s effective radius is the distance between the center of the
sun and the top of the photosphere. The mass of the sun is about 1.99 10
30
kg, or
333,000 times the mass of the Earth (5.98 10
24
kg). The sun’s surface gravity at the
top of the photosphere is about 274 m s
2
, or 28 times that of the Earth (9.8 m s
2
).
The sun’s core lies between its center and about 0.25 R
p
. Temperatures in the
core reach 15 million K. At these temperatures, electrons are stripped from hydrogen
atoms. The remaining nuclei (single protons) collide in intense thermonuclear fusion
reactions, producing helium and releasing energy in the form of photons of radiation
that power the sun. Energy from the core radiates to the intermediate interior, which
has a thickness of about 0.61 R
p
and a temperature ranging from 8 million K at its base
to about 5 million K at its top. Energy transfer through the intermediate interior is also
radiative. The next layer is the hydrogen convection zone (HCZ), a region in which
convection of hydrogen atoms due to buoyancy takes over from radiation as the pre-
dominant mechanism of transferring energy toward the sun’s surface. Temperatures at
the base of the HCZ are around 5 million K; temperatures at its top are near 6,400 K.
In the HCZ, a strong temperature gradient exists and the mean free path (average dis-
tance between collisions) of photons with hydrogen and helium atoms decreases with
increasing distance from the core. The HCZ is a region that prevents much of the sun’s
internal radiation from escaping to its surface. In fact, a photon of radiation emitted
THE SUN, THE EARTH, AND THE EVOLUTION OF THE EARTH’S A
TMOSPHERE
31
Figure 2.1. Structure of the sun.
Core
Intermediate
interior
Hydrogen
convection
zone
Photosphere
500-km thick
Chromosphere
2500-km thick
Corona
1.0R
p
0.86R
p
0.25R
p
Solar wind
Solar radiation

from the core of the sun takes about 10 million years to reach the top of the HCZ. The
HCZ thickness is difficult to determine and may range from about 0.14 to 0.3 R
p
.
Above the HCZ lies the photosphere (“light sphere”), which is a relatively thin
(500 km thick) transition region between the sun’s interior and its atmosphere.
Temperatures in the photosphere range from 6,400 K at its base to 4,000 K at its top
and average 5,785 K. The photosphere is the source of most solar energy that reaches
the planets, including the Earth. Although the sun’s interior is much hotter than is its
photosphere, most energy produced in its interior is confined by the HCZ.
Above the photosphere lies the chromosphere (“color sphere”), which is a 2,500
km thick region of hot gases. Temperatures at the base of the chromosphere are around
4,000 K. Those at the top are up to 1 million K. The name chromosphere arises
because at the high temperatures found in this region, hydrogen is energized and
decays back to its ground state, emitting wavelengths of radiation in the visible part of
the solar spectrum. For example, hydrogen decay results in radiation emission at
0.6563 m, which is in the red part of the spectrum, giving the chromosphere a char-
acteristic red coloration observed during solar eclipses.
The corona is the outer shell of the solar atmosphere and has an average tempera-
ture of about 1 to 2 million K. Because of the high temperatures, all gases in the
corona, particularly hydrogen and helium, are ionized. A low-concentration, steady
stream of these ions escapes the corona and the sun’s gravitational field and propa-
gates through space, intercepting the planets with speeds ranging from 300 to 1000
km s
1
. This stream is called the solar wind. The solar wind is the outer boundary of
the corona and extends from the chromosphere to the outermost reaches of the solar
system.
The Earth–sun distance (R
es
) is about 150 million km. At the Earth, the solar
wind temperature is about 200,000 K, and the number concentration of solar-wind
32 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
Figure 2.2. The Aurora Australis as seen from Kangaroo Island, southern Australia. Photo by
David Miller, National Geophysical Data Center, available from NOAA Central Library.

ions is a few to tens per cubic centimeter of space. As the solar wind approaches the
Earth, the Earth’s magnetic fields bend the path of the wind toward the North and
South Poles. In the atmosphere above these regions the ionized gases collide with air
molecules, creating luminous bands of streaming, colored lights. In the Northern
Hemisphere, these lights are called the Northern Lights or Aurora Borealis (“north-
ern dawn” in Latin), and in the Southern Hemisphere, they are called the Southern
Lights or Aurora Australis (“southern dawn”). These lights, one of the seven natural
wonders of the Earth, can be seen at high latitudes, such as in northern Scotland,
Scandinavia, and parts of Canada in the Northern Hemisphere and in southern
Australia and Argentina in the Southern Hemisphere. Figure 2.2 shows a photograph
of the Aurora Australis.
2.2. SPECTRA OF THE RADIATION OF THE SUN AND THE EARTH
Life on Earth would not have evolved to its present state without heating by solar
radiation. Next, the sun’s radiation spectrum is described.
Radiation is the emission or propagation of energy in the form of a photon or an
electromagnetic wave. Whether radiation is considered a photon or a wave is still debat-
ed. A photon is a particle or quantum of energy that has no mass, no electric charge,
and an indefinite lifetime. An electromagnetic wave is a disturbance traveling through
a medium, such as air or space, that transfers energy from one object to another without
permanently displacing the medium itself.
Because radiative energy can be transferred even in a vacuum, it is not necessary
for gas molecules to be present for radiative energy transfer to occur. Thus, such trans-
fer can occur through space, where few gas molecules exist, or through the Earth’s
atmosphere, where many molecules exist.
Radiation is emitted by all bodies in the universe that have a temperature above
absolute zero (0 K). During emission, a body releases electromagnetic energy at
different wavelengths, where a wavelength is the difference in distance between
peaks or troughs in a wave. The intensity of emission from a body varies with wave-
length, temperature, and efficiency of emission. Bodies that emit radiation with
perfect efficiency are termed blackbodies. A blackbody is a body that absorbs all
radiation incident on it. No incident radiation is reflected by a blackbody. No bodies
are true blackbodies, although the Earth and the sun are close, as are black carbon,
platinum black, and black gold. The term blackbody was coined because good
absorbers of visible radiation generally appear black. However, good absorbers of
infrared radiation are not necessarily black. For example, one such absorber is white
oil-based paint.
Bodies that absorb radiation incident upon them with perfect efficiency also emit
radiation with perfect efficiency. The wavelength of peak intensity of emission of a
blackbody is inversely proportional to the absolute temperature of the body. This law,
called Wien’s displacement law, was derived in 1893 by German physicist Wilhelm
Wien (1864−1928). Wien’s law states
(2.1)
where
p
is the wavelength (in micrometers, m) of peak blackbody emission, and T is
the temperature (K) of the body. Wien won a Nobel prize in 1911 for his discovery.
p
(m)
2,897
T(K)
THE SUN, THE EARTH, AND THE EVOLUTION OF THE EARTH’S A
TMOSPHERE
33
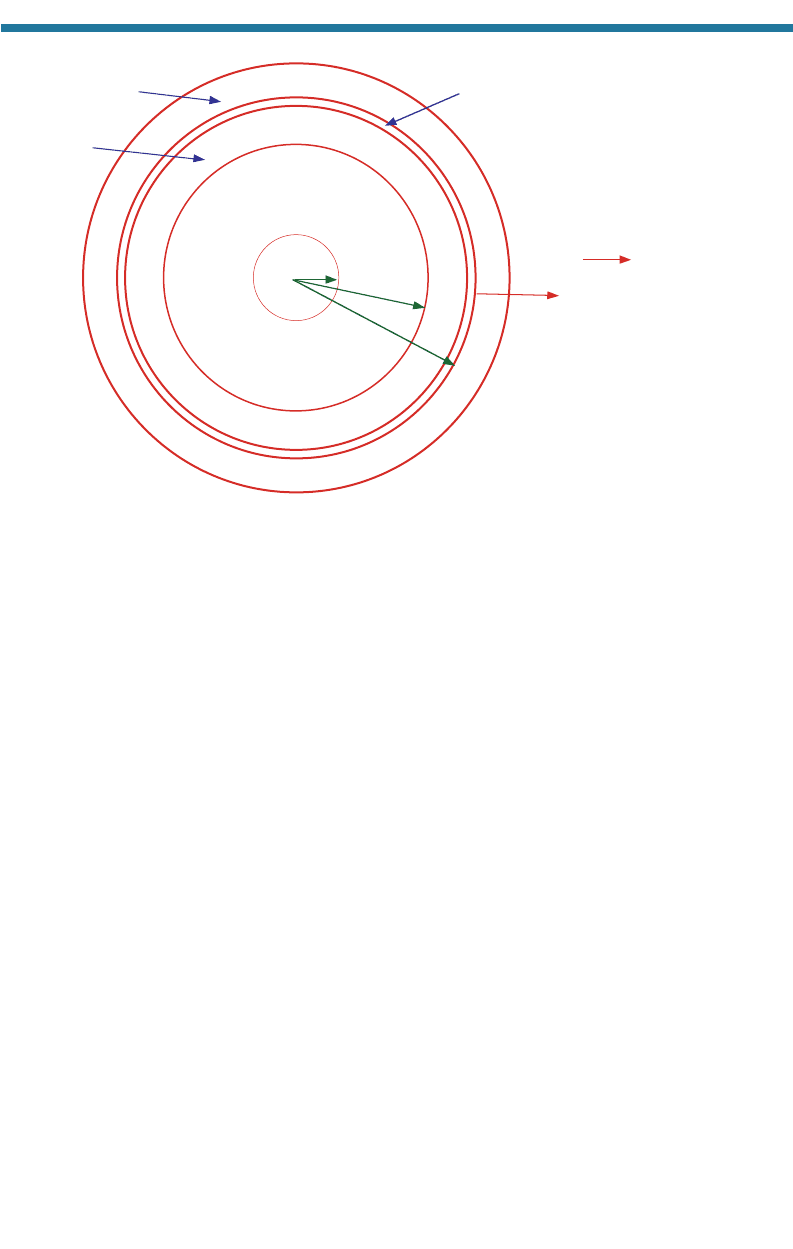
At any wavelength, the intensity of radiative emission from an object increases
with increasing temperature. Thus, hotter bodies (such as the sun) emit radiation more
intensely than do colder bodies (such as the Earth). Figure 2.3 shows the radiation
intensity versus wavelength for blackbodies at four temperatures. The figure shows
that at 15 million K, a temperature at which nuclear fusion reactions occur in the sun’s
center, gamma radiation wavelengths (10
8
to 10
4
m) and X radiation wave-
lengths (10
4
to 0.01 m) are the most intensely emitted wavelengths. At 6,000 K,
visible wavelengths (0.38–0.75 m) are the most intensely emitted wavelengths,
although shorter ultraviolet (UV) wavelengths (0.01–0.38 m), and longer infrared
(IR) wavelengths (0.75–100 m) are also emitted. At 300 K, infrared wavelengths are
the most intensely emitted wavelengths.
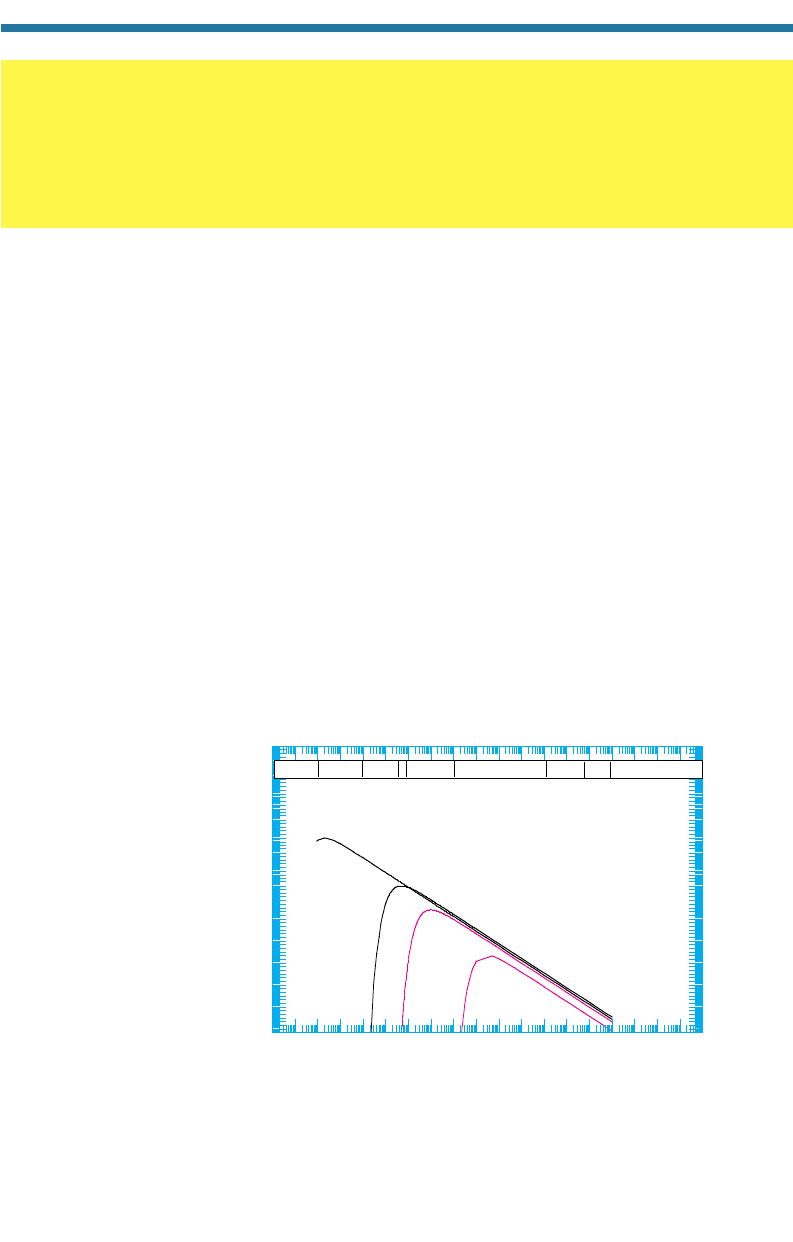
Figure 2.4 focuses on the 6,000 and the 300 K spectra in Fig. 2.3. These are the
radiation spectra for the sun’s photosphere and the Earth, respectively. The solar spec-
trum is divided into the UV, visible, and IR spectra. The UV spectrum is divided into
the far UV (0.01–0.25 m) and near UV (0.25–0.38 m) spectra, as shown in
Fig. 2.5. The near UV spectrum is further di
vided into
UV
-A
(0.32–0.38 m), UV
-B
(0.29–0.32 m), and UV-C (0.25–0.29 m) wavelengths. The visible spectrum
34 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
10
-32
10
-24
10
-16
10
-8
10
0
10
8
10
16
10
24
10
32
10
40
10
-6
10
-4
10
-2
10
0
10
2
10
4
10
6
10
8
10
10
10
12
10
-6
10
-4
10
-2
10
0
10
2
10
4
10
6
10
8
10
10
10
12
Radiation intensity (W m
-2
μm
-1
)
Wavelength (μm)
6,000 K
15 million K
Gamma
X
UV
Visible
IR
Short radio
AM radio
Long radio
Television and FM radio
300 K
1 K
Figure 2.3. Blackbody radiation emission versus wavelength at four temperatures. Units are
watts (joules of energy per second) per square meter of area per micrometer wavelength. The
15 million K spectrum represents emission from the center of the sun (most of which does
not penetrate to the sun’s exterior) The 6,000 K spectrum represents emission from the
sun’s surface and received at the top of the Earth’s atmosphere (not at its surface). The 300
K spectrum represents emission from the Earth’s surface. The 1 K spectrum is close to the
coldest temperature possible (0 K).
EXAMPLE 2.1.
Calculate the peak wavelength of radiative emission for each the sun and the Earth.
Solution
The effective temperature of the sun’s photosphere is 5,785 K, giving the sun a blackbody emission
peak wavelength of 0.5 m from Equation 2.1. The average sur face temperature of the Earth is 288
K, giving the Earth a blackbody emission peak wavelength of 10 m.
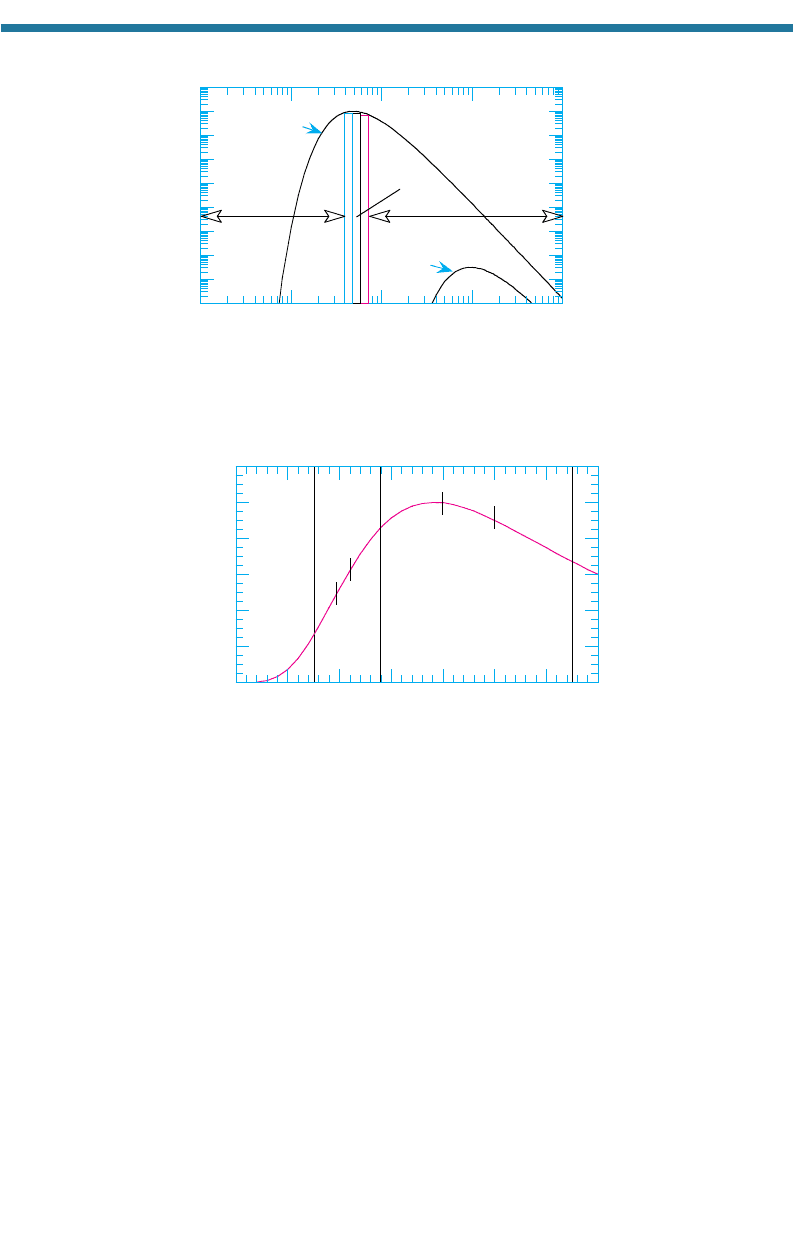
contains the colors of the rainbow. For convenience, visible light is divided into blue
(0.38–0.5 m), green (0.5–0.6 m), and red (0.6–0.75 m) wavelengths. Infrared
wavelengths are divided into solar (near)-infrared (0.75–4 m) and thermal (far)-
infrared (4–100 m) wavelengths. The intensity of the sun’s emission is strongest in
the visible spectrum. That of the Earth is strongest in the thermal-IR spectrum.
Figures 2.3 to 2.5 show wavelength dependencies of the intensity of radiation
emission at a given temperature. Integrating the intensity ov
er all wavelengths (sum-
ming the area under any of the curves) gives the total intensity of emission of a body at
a given temperature. This intensity is proportional to the fourth power of the object’s
kelvin temperature (T) and is given by the Stefan–Boltzmann law, derived empirical-
ly in 1879 by Austrian physicist Josef Stefan (1835–1893) and theoretically in 1889
by Austrian physicist Ludwig Boltzmann (1844–1906). The law states
F
b
B
T
4
(2.2)
where F
b
is the radiation intensity (W m
2
), summed over all wavelengths, emitted by
a body at a given temperature, is the emissivity of the body, and
B
5.67 10
8
W m
2
K
4
is the Stefan–Boltzmann constant. The emissivity, which ranges from 0 to
1, is the efficiency at which a body emits radiation in comparison with the emissivity
THE SUN, THE EARTH, AND THE EVOLUTION OF THE EARTH’S A
TMOSPHERE
35
10
-4
10
-2
10
0
10
2
10
4
0.01 0.1 1 10 100
Radiation intensity (W m
-2
μm
-1
)
Wavelength (μm)
Sun
Earth
UV
Visible
IR
Figure 2.4. Radiation spectrum as a function of wavelength for the sun’s photosphere and the
Earth when both are considered blackbodies. The sun’s spectrum is received at the top of
the Earth’s atmosphere.
2 × 10
3
4 × 10
3
6 × 10
3
8 × 10
3
1 × 10
3
1.2 × 10
4
0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8
Radiation intensity (W m
-2
μm
-1
)
Wavelength (μm)
UV-C
UV-B
UV-A
Visible
Far
UV
Near
UV
Red
Green
Blue
Figure 2.5. UV and visible por
tions of the solar spectr
um. This spectrum is received at the
top of the Earth’s atmosphere.
of a blackbody, which is unity. Soil has an emissivity of 0.9 to 0.98, and water has an
emissivity of 0.92 to 0.97. All the curves in Figs. 2.3 to 2.5 show emission spectra for
blackbodies (1).
36 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
EXAMPLE 2.2.
How does doubling the kelvin temperature of a blackbody change the intensity of radiative emission of
the body? What is the ratio of intensity of the sun’s radiation compared with that of the Earth’s?
Solution
From Equation 2.2, the doubling of the kelvin temperature of a body increases its intensity of radiative
emission by a factor of 16. The temperature of the sun’s photosphere (5,785 K) is about 20 times that
of the Earth (288 K). Assuming both are blackbodies (1), the intensity of the sun’s radiation (63.5
million W m
2
) is 163,000 times that of the Earth’s (390 W m
2
).
2.3. PRIMORDIAL EVOLUTION OF THE EARTH
AND ITS ATMOSPHERE
Earth formed when rock-forming elements (identified in Table 2.1), present as gases at
high temperatures in the solar nebula, condensed into small solid grains as the nebula
cooled. The grains grew by collision to centimeter-sized particles. Additional grains
accreted onto the particles, resulting in planetesimals, which are small-body precur-
sors to planet formation. Accretion of grains and particles onto planetesimals resulted
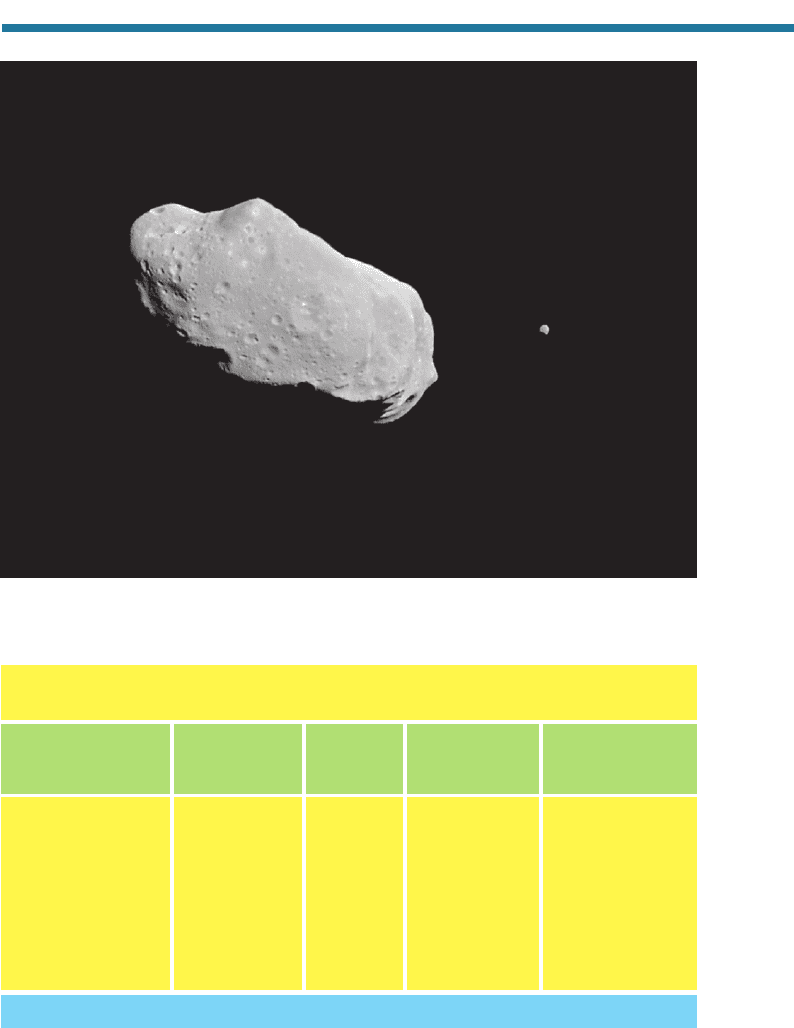
in the formation of asteroids (Fig. 2.6), which are rocky bodies 1 to 1,000 km in size
that orbit the sun. Asteroids collided to form the planets. The growth of planets was
aided by the bombardment of meteorites, which are solid minerals or rocks that reach
the planet’s surface without vaporizing. Meteorite bombardment was intense for about
500 million years. Although the solar nebula has since cooled and most of it has been
converted to solar or planetary material or has been swept away from the solar system,
some planetary growth still continues today, as leftover asteroids and meteorites
occasionally strike the planets. Table 2.2 shows the average composition of stony
meteorites, the total Earth, and the Earth’s continental and oceanic crusts. The table
indicates that meteorite composition is relatively similar to that of the total Earth, sup-
porting the theory that meteorites played a role in the Earth’s formation.
Meteorites and asteroids consist of rock-forming elements (e.g., Mg, Si, Fe, Al,
Ca, Na, Ni, Cr, Mn) that condensed from the gas phase in the cooling solar nebula, and
noncondensable elements (e.g., H, He, O, C, Ne, N, S, Ar,
P). How did noncondens-
able elements enter meteorites and asteroids, particularly as they were too light to
attract to these bodies gravitationally? One theory is that noncondensable elements may
have chemically reacted as gases in the solar nebula to form high molecular weight
compounds that were condensable, although less condensable (more volatile) than were
rock-forming elements. When meteorites and asteroids collided with the Earth, they
brought with them volatile compounds and rock-forming elements. Whereas some of
the volatiles vaporized on impact, others have taken longer to vaporize and have been
outgassed ever since through volcanos, fumaroles, steam wells, and geysers.
Earth’s first atmosphere likely contained hydrogen (H) and helium (He), the most
abundant elements in the solar nebula. During the formation of the Earth, the sun was
also forming. Early stars are known to blast off a large amount of gas into space. This
outgassed solar material, the solar wind, was previously introduced as an extension of
THE SUN, THE EARTH, AND THE EVOLUTION OF THE EARTH’S A
TMOSPHERE
37
Figure 2.6. The asteroid Ida and its moon, Dactyl, taken by the Galileo spacecraft as it
passed within 10,878 km of the asteroid on August 28, 1993. Available from National Space
Science Data Center.
Table 2.2. Mass Percentages of Major Elements in Stony Meteorites, the Total
Earth, and the Earth’s Continental and Oceanic Crusts
Oxygen (O) 33.24 29.50 46.6 45.4
Iron (Fe) 27.24 34.60 5.0 6.4
Silicon (Si) 17.10 15.20 27.2 22.8
Magnesium (Mg) 14.29 12.70 2.1 4.1
Sulfur (S) 1.93 1.93 0.026 0.026
Nickel (Ni) 1.64 2.39 0.075 0.075
Calcium (Ca) 1.27 1.13 3.6 8.8
Aluminum (Al) 1.22 1.09 8.1 8.7
Sodium (Na) 0.64 0.57 2.8 1.9
Earth’s
Stony Total Continental Earth’s
Element Meteorites Earth Crust Oceanic Crust
Adapted from Cattermole and Moore (1985).
the sun’s corona. During the birth of the sun, nuclear reactions in the sun were
enhanced, and solar wind speeds and densities were much larger than they are today.
This early stage of the sun is called the T-Tauri stage after the first star observed
at this point in its evolution. The enhanced solar wind is thought to have stripped away
the first atmosphere of not only the Earth, but also all other planets in the solar system.
Additional H and He were lost from the Earth’s first atmosphere after escaping the
