Jacobson M.Z. Atmospheric Pollution
Подождите немного. Документ загружается.

Ozone absorbs wavelengths shorter than 0.35
m (strongly below 0.31 m and
weakly at 0.31 to 0.35 m). Ozone also absorbs weakly at 0.45 to 0.75 m.
Stratospheric ozone allows little UV-C, some UV-B, and most UV-A radiation to reach
the troposphere. Ozone in the background troposphere absorbs some of the UV-B and
UV-A not absorbed in the stratosphere. In polluted air, additional absorbers of UV-B
radiation include nitrated aromatic gases and aerosol particle components,
such as
black carbon (BC), nitrated aromatics, polycyclic aromatic hydrocarbons (PAHs), and
soil-dust components (Section 7.1.3.1).
The major UV-A-absorbing gas is nitrogen dioxide [NO
2
(g)]. Its mixing ratio in
clean air is too small to affect UV-A. In polluted air
, its mixing ratio is high only in the
morning, when UV-A intensity is low. Other UV-A absorbers in polluted air include
the same aerosol particle components that absorb UV-B radiation.
Gas- and aerosol particle absorption is not the only mechanism that reduces inci-
dent downward UV radiation. Gas and aerosol particle backscattering, ground reflection,
and cloud reflection return some incident UV radiation to space as well.
11.3. CHEMISTRY OF THE NATURAL OZONE LAYER
The chemistry of the natural ozone layer involves primarily oxygen-containing com-
pounds, but the shape of the stratospheric ozone vertical profile is affected by nitrogen-
and hydrogen-containing compounds as well. Next, the chemistry of the natural ozone
layer is discussed.
11.3.1. The Chapman Cycle
The photochemistry of the natural stratosphere is similar to that of the free tro-
posphere, except that stratospheric ozone is produced after photolysis of molecular
oxygen, whereas tropospheric ozone is produced after photolysis of nitrogen dioxide.
Next, reactions naturally producing and destroying stratospheric ozone are described.
In the stratosphere, far-UV wavelengths (shorter than 0.245 m) break down
molecular oxygen by
O
2
(g) h •O
•
(
1
D)(g) •O
•
(g) 175 nm
Molecular Excited Ground-
oxygen atomic state atomic
(11.1)
oxygen oxygen
278 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
Table 11.1. Summary of Major Absorbers of UV Radiation
Far-UV 0.01–0.25 N
2
(g) Thermosphere, mesosphere
O
2
(g) Thermosphere, mesosphere,
stratosphere
Near-UV
UV-C 0.25–0.29 O
3
(g) Stratosphere
UV-B 0.29–0.32 O
3
(g) Stratosphere, troposphere
Particulate components Polluted troposphere
UV-A 0.32–0.38 NO
2
(g) Polluted troposphere
Particulate components Polluted troposphere
Name of Spectrum Wavelengths (m) Dominant Absorbers Location of Absorption
O
2
(g) h •O
•
(g) •O
•
(g) 175 < 245 nm
Molecular Ground-
oxygen state atomic
(11.2)
oxygen
The first reaction is important only at the top of the stratosphere because wavelengths
shorter than 0.175 m do not penetrate lower. Neither reaction is important in the tropo-
sphere. Excited atomic oxygen from Reaction 11.1 rapidly converts to the ground state by
•O
•
(
1
D)(g)
M
•O
•
(g)
Excited Ground-
atomic state atomic
(11.3)
oxygen oxygen
Ozone then forms by
This reaction also occurs in the troposphere, where the O(g) in that case originates
from NO
2
(g) photolysis, not from O
2
(g) photolysis. Ozone is destroyed naturally in the
stratosphere and troposphere by
O
3
(g) h O
2
(g) •O
•
(
1
D)(g) 310 nm
Ozone Molecular Excited
oxygen atomic
(11.5)
oxygen
O
3
(g) h O
2
(g) •O
•
(g) 310 nm
Ozone Molecular Ground-
oxygen state atomic
(11.6)
oxygen
Stratospheric ozone is also destroyed by
•O
•
(g) O
3
(g) 2O
2
(g)
Ground- Ozone Molecular (11.7)
state atomic oxygen
oxygen
In 1930, English physicist Sidney Chapman (1888–1970; Fig. 11.6) suggested
that ozone in the stratosphere must be produced from UV photolysis of molecular
oxygen. He further postulated that Reactions 11.2, 11.4, 11.6, 11.7, and the reaction
•O
•
(g) •O
•
(g)
M
O
2
(g)
Ground- Molecular
state atomic oxygen
(11.8)
oxygen
GLOBAL STRATOSPHERIC OZONE REDUCTION 279
•O
•
(g) O
2
(g)
M
O
3
(g)
Ground- Molecular Ozone
state atomic oxygen
(11.4)
oxygen

describe the natural formation and destruction of ozone in the stratosphere (Chapman,
1930). These reactions make up the Chapman cycle, and they simulate the process
fairly well. Some Chapman reactions are more important than are others. Reactions
11.2, 11.4, and 11.6 affect ozone the most. The non-
Chapman reaction, 11.5, is also important.
Some of the Chapman cycle reactions can be used
to explain why the altitudes of peak ozone concentra-
tion and mixing ratio occur where they do in Fig. 11.4.
Oxygen density, like air density, decreases exponen-
tially with increasing altitude. UV intensity decreases
with decreasing altitude. Peak ozone densities occur
where sufficient radiation encounters sufficient oxygen
density, which is near 25 to 32 km (Fig. 11.4). At high-
er altitudes, the oxygen density is too low for its
photolysis by Reactions 11.1 and 11.2 to produce peak
ozone densities; at lower altitudes, the radiation is not
intense enough for oxygen photolysis to produce peak
ozone densities.
11.3.2. Effects of Nitrogen on the Natural
Ozone Layer
Oxides of nitrogen [NO(g) and NO
2
(g)] naturally
destroy ozone, primarily in the upper stratosphere,
helping shape the vertical profile of the ozone layer. In the troposphere, the major
sources of nitric oxide are surface emissions and lightning. The major source of NO(g)
in the stratosphere is transport from the troposphere and the breakdown of nitrous
oxide [N
2
O(g)] (laughing gas), a colorless gas emitted during denitrification by anaer-
obic bacteria in soils (Section 2.3.5). It is also emitted by bacteria in fertilizers,
sewage, and the oceans and during biomass burning, automobile combustion, aircraft
combustion, nylon manufacturing, and the use of spray cans. In the troposphere,
N
2
O(g) is lost by transport to the stratosphere, deposition to the surface, and chemical
reaction. Because its loss rate from the troposphere is slow, nitrous oxide is long lived
and well diluted in the troposphere, with an average mixing ratio of about 0.31 ppmv.
The mixing ratio of N
2
O(g) is relatively constant up to about 15 to 20 km, but decreases
above that as a result of photolysis. Throughout the atmosphere, N
2
O(g) produces
nitric oxide by
N
2
O(g) •O
•
(
1
D)(g) N
•
O(g) N
•
O(g)
Nitrous Excited Nitric oxide
oxide atomic
(11.9)
oxygen
Nitric oxide naturally reduces ozone in the upper stratosphere by
280 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
Figure 11.6. Sidney Chapman (1888–1970).
N
•
O(g) O
3
(g) N
•
O
2
(g) O
2
(g)
Nitric Ozone Nitrogen Molecular
oxide dioxide oxygen
(11.10)
The result of this sequence is that one molecule of ozone is destro
yed, but neither
NO(g) nor NO
2
(g) is lost. This sequence is called a catalytic ozone destruction cycle
because the species causing the O
3
(g) loss, NO(g), is recycled. This particular cycle is
the NO
x
(g) catalytic ozone destruction cycle, where NO
x
(g) NO(g) NO
2
(g), and
NO(g) is the catalyst. The number of times the cycle is executed before NO
x
(g) is
removed from the cycle by reaction with another gas is the chain length. In the upper
stratosphere, the chain length of this cycle is about 10
5
(Lary, 1997). Thus, 10
5
mole-
cules of O
3
(g) are destroyed before one NO
x
(g) molecule is removed from the cycle. In
the lower stratosphere, the chain length decreases to near 10. When NO
x
(g) is removed
from this cycle, its major loss processes are the formation of nitric acid and peroxyni-
tric acid by the reactions
N
•
O
2
(g) O
•
H(g)
M
HNO
3
(g)
Nitrogen Hydroxyl Nitric (11.13)
dioxide radical acid
HO
•
2
(g) N
•
O
2
(g)
M
HO
2
NO
2
(g)
Hydroperoxy Nitrogen Peroxynitric (11.14)
radical dioxide acid
Nitric acid and peroxynitric acid photolyze back to the reactants that formed them,
but such processes are slow. Peroxynitric acid also decomposes thermally, but
thermal decomposition is slow in the stratosphere because temperatures are low
there.
The natural NO
x
(g) catalytic cycle erodes the ozone layer above ozone’s peak alti-
tude shown in Fig. 11.4. Although the NO
x
(g) catalytic cycle is largely natural, an
unnatural source of stratospheric NO
x
(g) and, therefore, ozone destruction, is stratos-
pheric aircraft emission of NO(g) and N
2
O(g). In the 1970s and 1980s, scientists were
concerned that the introduction of a fleet of supersonic transport (SST) jets into the
stratosphere would enhance N
2
O(g) emissions sufficiently to damage the ozone layer
by Reactions 11.9 through 11.12. This concern disappeared because the plan to intro-
duce a fleet of stratospheric jets never materialized.
11.3.3. Effects of Hydrogen on the Natural Ozone Layer
Hydrogen-containing compounds, particularly the hydroxyl radical [OH(g)] and the
hydroperoxy radical [HO
2
(g)], are responsible for shaping the ozone profile in the
lower stratosphere. The hydroxyl radical is produced in the stratosphere by one of sev-
eral reactions
GLOBAL STRATOSPHERIC OZONE REDUCTION 281
N
•
O
2
(g) •O
•
(g) N
•
O(g) O
2
(g)
Nitrogen Ground- Nitric Molecular
dioxide state atomic oxide oxygen
oxygen
(11.11)
•O
•
(g) O
3
(g) 2O
2
(g)
Ground- Ozone Molecular
state atomic oxygen
oxygen
(net process) (11.12)
(11.15)
The hydroxyl radical participates in an HO
x
(g) catalytic ozone destruction cycle,
where HO
x
(g) OH(g) HO
2
(g). HO
x
(g) catalytic cycles are important in the lower
stratosphere. The most effective HO
x
(g) cycle, which has a chain length in the lower
stratosphere of 1 to 40 (Lary, 1997), is
OH(g)
OH(g) +
CH
3
(g)
H
Hydroxyl
radical
Excited
atomic
oxygen
O(
1
D)(g) +
H
2
O(g)
CH
4
(g)
H
2
(g)
Methane
Molecular
hydrogen
Water
vapor
Hydroxyl
radical
Methyl
radical
Atomic
hydrogen
HO
x
(g) species can be removed temporarily from catalytic cycles by Reactions 11.13
and 11.14 and by the reaction
HO
•
2
(g) O
•
H(g) H
2
O(g) O
2
(g)
Hydroperoxy Hydroxyl Water Molecular (11.19)
radical radical vapor oxygen
This mechanism is particularly efficient at removing HO
x
(g) from catalytic cycles
because it removes two HO
x
(g) molecules at a time.
11.3.4. Effects of Carbon on the Natural Ozone Layer
Carbon monoxide and methane produce ozone by the reaction mechanisms shown in
Sections 4.2.4 and 4.2.5, respectively. The contributions of CO(g) and CH
4
(g) to ozone
production are small in the stratosphere. A by-product of methane oxidation in the
stratosphere is water vapor, produced by
CH
4
(g) O
•
H(g) C
•
H
3
(g) H
2
O(g)
Methane Hydroxyl Methyl Water (11.20)
radical radical vapor
282 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
O
•
H(g) O
3
(g) HO
•
2
(g) O
2
(g)
Hydroxyl Ozone Hydroperoxy Molecular
radical radical oxygen
(11.16)
HO
•
2
(g) O
3
(g) O
•
H(g) 2O
2
(g)
Hydroperoxy Ozone Hydroxyl Molecular
radical radical oxygen
(11.17)
2O
3
(g) 3O
2
(g)
Ozone Molecular
oxygen
(net process) (11.18)
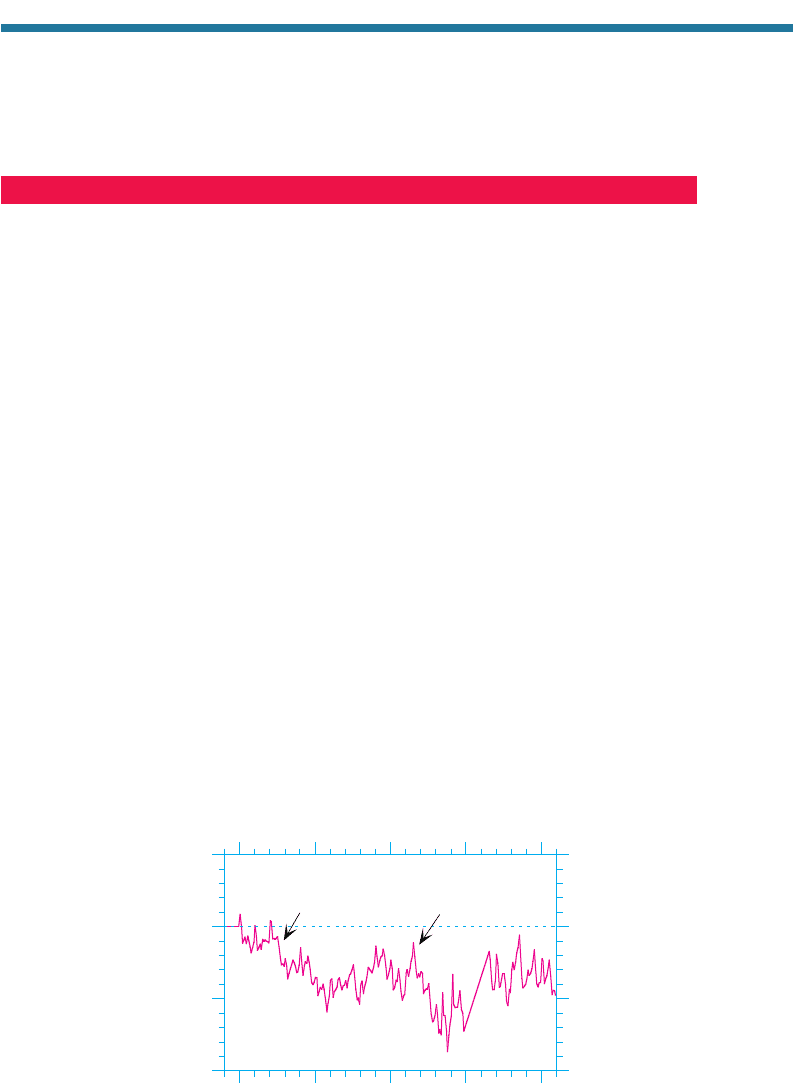
Because water vapor mixing ratios in the stratosphere are low and transport of water
vapor from the troposphere to stratosphere is slow, this reaction is a relatively impor-
tant source of water vapor in the stratosphere.
11.4. RECENT CHANGES TO THE OZONE LAYER
Changes in stratospheric ozone since the early 1970s can be divided into global stratos-
pheric changes, Antarctic stratospheric changes, and Arctic stratospheric changes.
11.4.1. Changes on a Global Scale
Figure 11.7 shows that between 1979 and 2000, the global stratospheric ozone column
abundance decreased by approximately 3.5 percent (from 304.0 to 293.4 DU). Unusual
decreases in global ozone occurred following the El Chichón (Mexico) volcanic erup-
tion in April 1982, and the Mount Pinatubo (Philippines) eruption in June 1991 (Fig.
11.8). These eruption injected particles into the stratosphere. On the surfaces of these
particles, chemical reactions involving chlorine took place that contributed to ozone
loss. Over time, however, the concentration of these particles decreased, and the global
ozone layer partially recovered. Because volcanic particles were responsible for only
temporarily ozone losses, the net loss of ozone over the globe from 1979 to 2000 was
still about 3.5 percent. The decrease between 60S and 60N was 2.5 percent (298.08 to
290.68 DU), that between 60°N and 90N was 7.0 percent (370.35 to 344.29 DU),
and
that between 60°S and 90°S was 14.3 percent (335.20 to 287.23 DU).
11.4.2. Antarctic Stratospheric Changes
Between 1950 and 1980, no measurements from three ground-based stations in the
Antarctic showed ozone levels less than 220 DU, a threshold for defining Antarctic
ozone depletion. Every Southern Hemisphere spring (September–November) since 1980,
GLOBAL STRATOSPHERIC OZONE REDUCTION 283
-10
-5
0
5
1980 1985 1990 1995 2000
Percentage difference in global ozone
from 1979 monthly average
Year
Mount Pinatubo
(June 1991)
El Chichón
(April 1982)
Figure 11.7. Percentage change in the monthly averaged global (90S to 90N) ozone column
abundance between a given month and the same month in 1979. Data were obtained from
the satellite-based Total Ozone Mapping Spectrometer (TOMS) and made available by NASA
Goddard Space Flight Center, Greenbelt, Maryland. No data were available from December
1994 to July 1996.

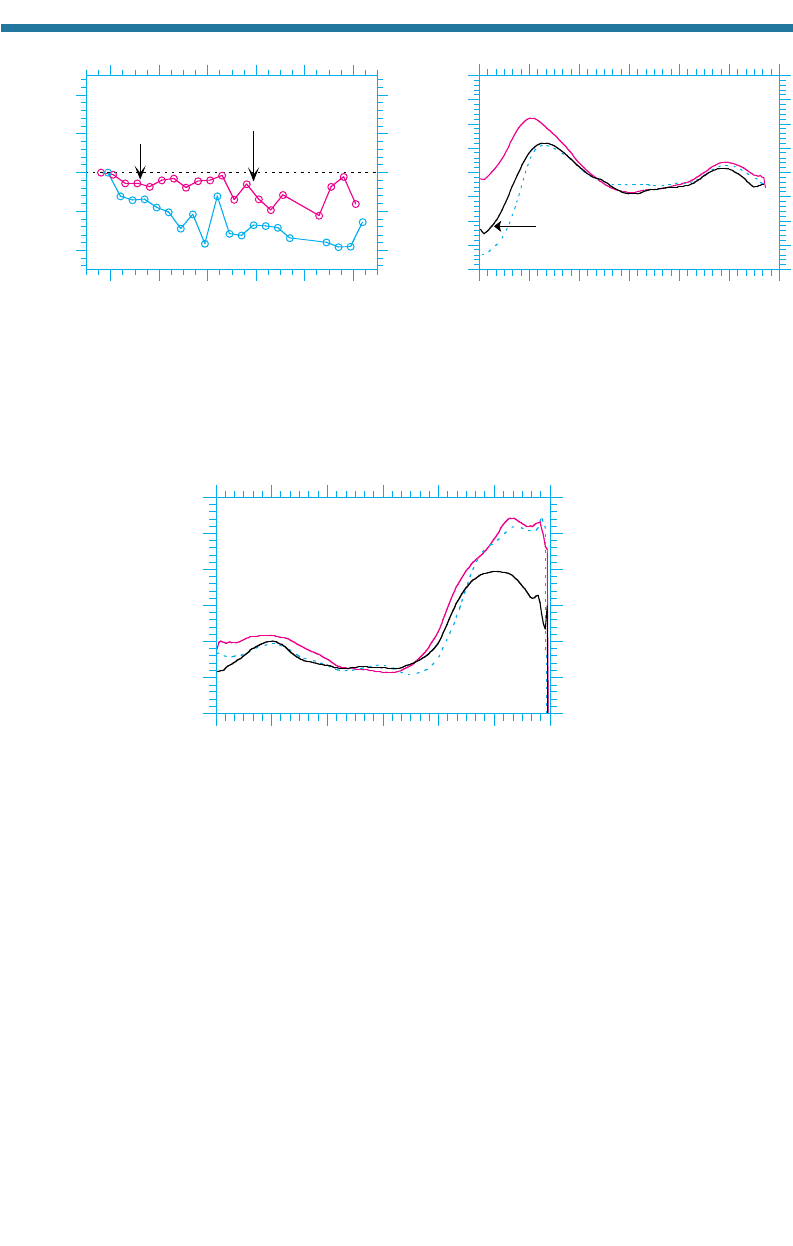
measurements of stratospheric ozone have shown a depletion. Farman et al. (1985) first
reported depletions of more than 30 percent relative to pre-1980 measurements. Since
then, measurements over the South Pole have indicated depletions of up to 70 percent of
the column ozone for a period of a week in early October. The largest average depletion
for the month of September from 60 to 90°S since 1979 was 32.8 percent and occurred
in 2000. The largest depletion for the month of October from 60 to 90°S since 1979 was
38.3 percent and occurred in 1998 [Fig. 11.9(a)]. Most ozone depletion has occurred
between altitudes of 12 and 20 km. The large reduction of stratospheric ozone over the
Antarctic in the Southern Hemisphere spring each year is the Antarctic ozone hole. The
areal extent of the ozone hole is no
w greater than the size of North America.
Figure 11.9(b) shows the zonally and October-averaged ozone column abundance
versus latitude for 1979, 1999, and 2000. The figure shows that in 1999, the October
average over the South Pole was 131 DU, which compares with 286 DU in 1979. The
October average was slightly higher in 2000 than in 1999, but the September average
(not shown) was lower in 2000 than in 1999. October ozone levels over 45S (the lati-
tude of southern New Zealand, Chile, and Argentina) were 5 to 6 percent lower in
284 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
Figure 11.8. Mount Pinatubo eruption, June 12, 1991. Three days later, a larger eruption, the
second largest in the twentieth century, occurred. Photo by Dave Harlow, USGS.
1999 and 2000 than in 1979. Ozone levels over 30S (the latitude of Australia, South
Africa, Chile, Argentina, and southern Brazil) were about 3 percent lo
wer in 1999 and
2000 than in 1979. Temporary losses of ozone over these countries have caused con-
cern due the effects of the resulting enhanced UV-B radiation on health (Section 11.9).
11.4.3. Arctic Stratospheric Changes
Since 1979, the stratospheric ozone layer over the North Pole has declined during
the Northern Hemisphere late winter and spring (March–May). This reduction is
the Arctic ozone dent. Figure 11.9(a) indicates that the Arctic ozone dent in March
has consistently been less severe than has the corresponding springtime Antarctic
ozone hole in October. Figure 11.10, however, shows that ozone levels over the
Arctic during March 2000 were nearly 16 percent lower than were those during
GLOBAL STRATOSPHERIC OZONE REDUCTION 285
-40
-20
0
20
40
1980 1984 1988 1992 1996 2000
Percentage difference in ozone
from 1979 monthly average
Year
Mount Pinatubo
(June 1991)
60–90°N
March
El Chich´on
(April 1982)
60–90°S
October
100
150
200
250
300
350
400
450
500
-90 -60 -30 0 30 60 90
Ozone (DU)
Latitude (degrees)
October zonal average
1999
1979
2000
(a) (b)
Figure 11.9. (a) Percentage difference in March-averaged 60 to 90N and October-averaged 60 to 90S
ozone column abundances between the given year and 1979. (b) Variation with latitude of October month-
ly and zonally averaged column abundances of ozone in 1979, 1999, and 2000. Data were obtained from
the satellite-based Total Ozone Mapping Spectrometer (TOMS) and made available by NASA Goddard
Space Flight Center, Greenbelt, Maryland. No data were available from December 1994 to July 1996.
200
250
300
350
400
450
500
-90 -60 -30 0 30 60 90
Ozone (DU)
Latitude (degrees)
March zonal average
2000
1979
1999
Figure 11.10. Variation with latitude of March monthly and zonally averaged column abun-
dance of ozone in 1979, 1999, and 2000. Data were obtained from the satellite-based Total
Ozone Mapping Spectrometer (TOMS) and made available by NASA Goddard Space Flight
Center, Greenbelt, Maryland.
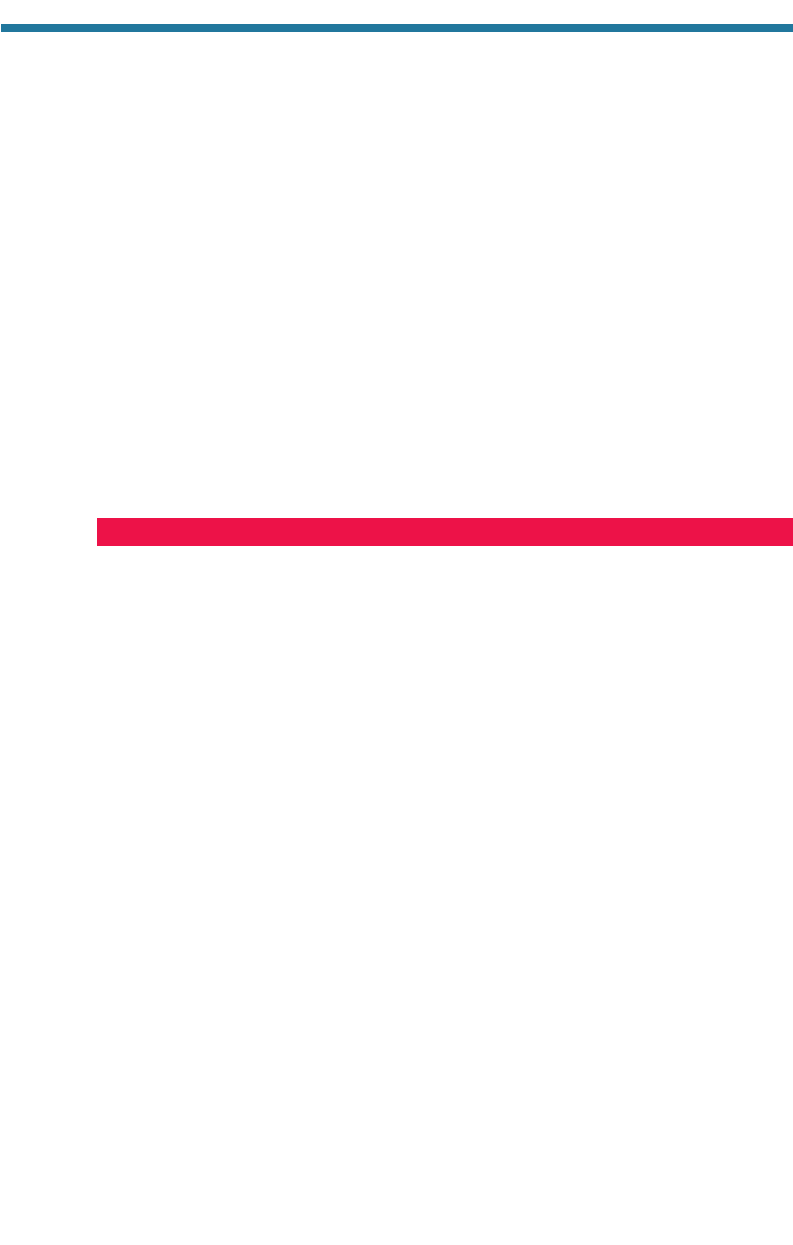
March 1979. Ozone levels over the Arctic in March 1999 were nearly the same as
those in March 1979.
11.4.4. Summary of Effects of Ozone and Air Pollution Changes
on UV Radiation
The yearly global reduction and seasonal regional destruction of stratospheric ozone
affect the amount of UV radiation reaching the surface. In comparison with their levels
in the 1970s, levels of ground UV-B radiation in 1998 were about 7 percent higher in
Northern Hemisphere midlatitudes in winter and spring, 4 percent higher in Northern
Hemisphere midlatitudes in summer and f
all, 6 percent higher in Southern Hemisphere
midlatitudes during the entire year, 130 percent higher in the Antarctic in the Southern
Hemisphere spring, and 22 percent higher in the Arctic in the Northern Hemisphere
spring (Madronich et al., 1998). Localized measurements of UV at Lauder, New Zealand
(45S), for example, show that surface UV-B radiation doses during the summer of
1998–1999 were 12 percent higher than they were in the first years of the 1990s
(McKenzie et al., 1999).
11.5. EFFECTS OF CHLORINE ON GLOBAL OZONE REDUCTION
Ozone reductions since the late 1970s correlate with increases in chlorine and bromine
in the stratosphere. Molina and Rowland (1974) first recognized that anthropogenic
chlorine compounds could destroy stratospheric ozone. Since then, scientists have
strengthened the links among global ozone reduction, Antarctic ozone depletion, and
the presence of chlorine- and bromine-containing compounds in the stratosphere.
11.5.1. CFCs and Related Compounds
The compounds that play the most important role in reducing stratospheric ozone are
chlorofluorocarbons (CFCs). Important CFCs are identified in Table 11.2. CFCs are
gases formed synthetically by replacing all hydrogen atoms in methane [CH
4
(g)] or
ethane [C
2
H
6
(g)] with chlorine and/or fluorine atoms. For example, CFC-12
[CF
2
Cl
2
(g), dichlordifluoromethane] is formed by replacing the four hydrogen atoms
in methane with two chlorine and two fluorine atoms.
11.5.1.1. Invention of CFCs
CFCs were invented on a Saturday afternoon in 1928 by Thomas Midgley and his
assistants, Albert L. Henne (1901–1967) and Robert R. McNary (1903–1988), at the
Thomas and Hochwalt Laboratory, 127 North Ludlow Street, Dayton, Ohio. Midgley
is the same scientist who invented tetraethyl lead (Ethyl) gasoline (Section 3.6.9).
Some argue that Midgley’s inventions led to the two greatest environmental disasters
of the twentieth century.
Midgley and his assistants developed CFC-12 effectively on the same day that a
representative of General Motors’ Frigidaire division asked Midgley to find a nontoxic,
nonflammable substitute for an existing refrigerant, ammonia, a flammable and toxic
gas. CFC-12 and subsequent CFCs were inexpensive, nontoxic, nonflammable, nonex-
plosive, insoluble, and chemically unreactive under tropospheric conditions; thus, they
became popular. Midgley demonstrated the nontoxic and nonflammable properties of
286 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
GLOBAL STRATOSPHERIC OZONE REDUCTION 287
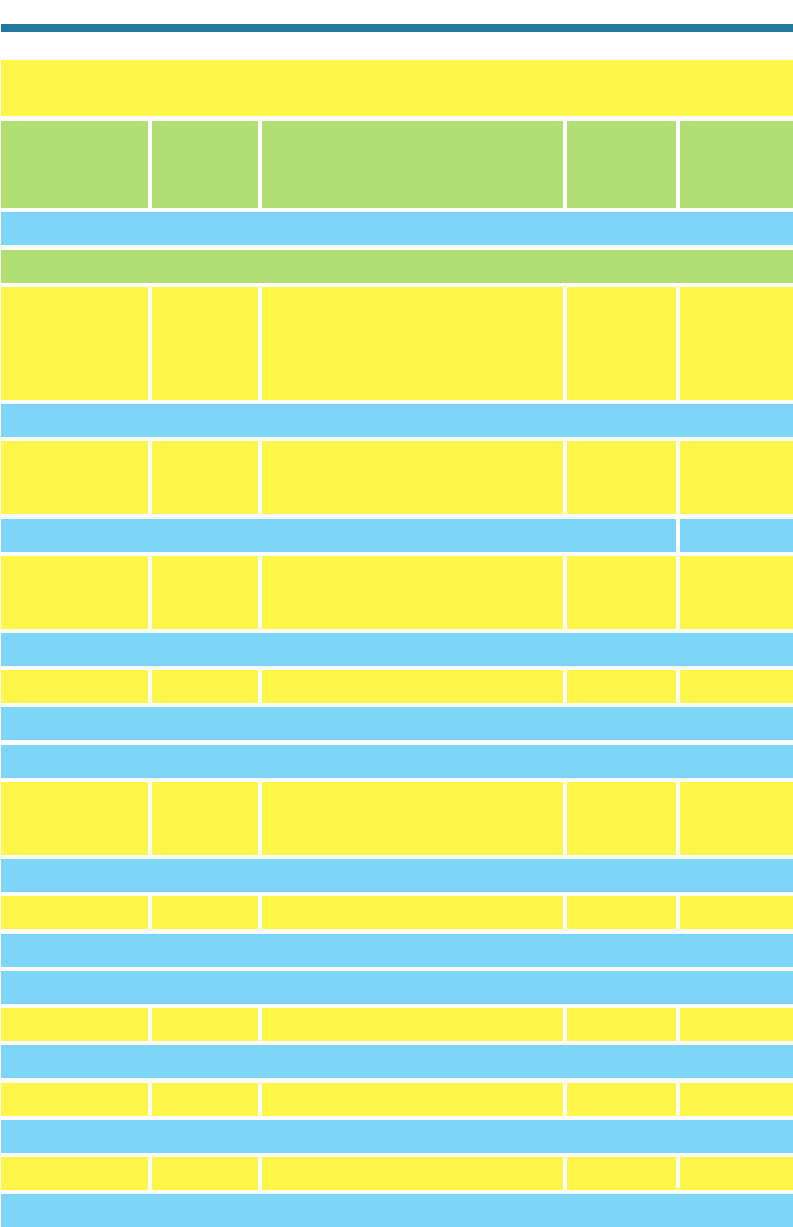
Table 11.2. Mixing Ratios and Lifetimes of Selected Chlorocarbons,
Bromocarbons, and Fluorocarbons
CFCl
3
(g) CFC-11 Trichlorofluoromethane 270 45
CF
2
Cl
2
(g) CFC-12 Dichlorodifluoromethane 550 100
CFCl
2
CF
2
Cl(g) CFC-113 1-Fluorodichloro, 2-difluorochloroethane 70 85
CF
2
ClCF
2
Cl(g) CFC-114 15 220
CF
2
ClCF
3
(g) CFC-115 5 550
CF
2
ClH(g) HCFC-22 Chlorodifluoromethane 130 11.8
CH
3
CFCl
2
(g) HCFC-141b 6 9.2
CH
3
CF
2
Cl(g) HCFC-142b 2-Difluorochloroethane 8 18.5
CCl
4
(g) Carbon tetrachloride 100 35
CH
3
CCl
3
(g) Methyl chloroform 90 4.8
CH
3
Cl(g) Methyl chloride 610 1.3
HCl(g) Hydrochloric acid 10–1,000 1
Estimated
Tropospheric Overall
Mixing Ratio Atmospheric
Chemical Formula Trade Name Chemical Name (pptv) Lifetime (yrs)
CF
3
Br(g) H-1301 Trifluorobromomethane 2 65
CF
2
ClBr(g) H-1211 Difluorochlorobromomethane 2 11
CF
2
BrCF
2
Br H-2402 1-Difluorobromo, 2-difluorobromoethane 1.5 22–30
CH
3
Br(g) Methyl bromide 12 0.7
CH
2
FCF
3
(g) HFC-134a 1-Fluoro, 2-trifluoroethane 4 13.6
C
2
F
6
(g) Perfluoroethane 4 10,000
SF
6
(g) Sulfur hexafluoride 3.7 3,200
Sources: Shen et al. (1995); Singh (1995); WMO (1998); Mauna Loa Data Center (2001).
Other Chlorocarbons
Other Chlorinated Compounds
BROMOCARBONS
Halons
Other Bromocarbons
FLUOROCARBONS AND FLUORINE COMPOUNDS
Hydrofluorocarbons (HFCs)
Perfluorocarbons (PFCs)
Other Fluorinated Compounds
Hydrochlorofluorocarbons (HCFCs)
CHLOROCARBONS AND CHLORINE COMPOUNDS
Chlorofluorocarbons (CFCs)
