Jacobson M.Z. Atmospheric Pollution
Подождите немного. Документ загружается.


people most likely to be exposed to asbestos in concentrations high enough to cause
health problems. In the United States, asbestos is defined as a hazardous air pollutant
under CAAA90.
Sources and Sinks
Once insulation containing asbestos has been installed, the asbestos is not expected
to cause damage to humans unless the insulation is disturbed. At that time, fibers can
be scattered into the air, where they can remain for minutes to days until they deposit
to the ground or are inhaled. Thus, the only source of asbestos in indoor air is turbulent
uplift, and the only sink is deposition.
Concentrations
Indoor concentrations of asbestos vary from building to building. Lee et al. (1992)
found an average of 0.02 structures per cubic centimeter in 315 public, commercial,
residential, school, and university buildings in the United States. The concentration of
fibers longer than 5 m was only 0.00013 structures per cubic centimeter
.
Health Effects
The primary health effects of asbestos exposure are lung cancer, mesothelioma,
and asbestosis. Mesothelioma is a cancer of the mesothelial membrane lining the
lungs, and asbestosis is a slow, debilitating disease of the lungs.
The most dangerous
asbestos fibers are those longer than 5 to 10 m but with a diameter less than 1 m.
Exposure to 0.0004 structures per cubic centimeter is expected to cause a lifetime
cancer risk of 160 cases of mesothelioma per 1 million people (Turco, 1997). The
time between first exposure to asbestos and the appearance of tumors is estimated to
be 20 to 50 years (Jones, 1999). Cigarette smoking and e
xposure to asbestos are
believed to amplify the rates of lung cancer in comparison with the rates of cancer
associated with just smoking or just exposure to asbestos. Short-term acute exposure
to asbestos can lead to skin irritation (Spengler and Sexton, 1983).
9.1.11. Fungal Spores, Bacteria, and Viruses
Fungal spores, bacteria, and viruses are common indoor air contaminants. Sources
of fungal spores are soils, plants, foodstuff, internal surfaces, and outdoor air. Sources
of bacteria and viruses are humans, animals, plants, air conditioners,
and outdoor air.
Fungi grow well on damp surfaces. Common fungi in buildings include Penicillium,
Cladosporium, and Aspergillus. Common bacteria include Bacillus, Staphylococcus,
and Micrococcus (Jones, 1999). Some diseases associated with fungi, bacteria, and
viruses include rhinitis (a respiratory illness), asthma, humidifier fever, extrinsic aller-
gic alveolitis, and atopic dermatitis. Most human illnesses due to viral and bacterial
infection are due to human-to-human transmission of microorganisms rather than to
building-to-human transmission (Ayars, 1997).
9.1.12. Environmental Tobacco Smoke
When a cigarette is smoked, some of the smoke is inhaled and swallowed, some is
inhaled and exhaled (mainstream smoke), and the rest is emitted from the cigarette
(sidestream smoke). The mainstream plus sidestream smoke is called environmental
tobacco smoke (ETS), a mixture of more than 4,000 aerosol particle components and
248 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION

gases, at least 50 of which are known carcinogens. ETS, itself, has been classified by
the U.S. EPA as a carcinogen. Also called second-hand smoke, ETS, builds up in
enclosed spaces, increasing danger to others in the vicinity. Even in well-ventilated
indoor areas, particle and gas concentrations associated with ETS increase. Although
the cumulative effect of ETS on outdoor air pollution is relatively small compared
with the effects of other sources of pollution, such as automobiles, ETS concentrations
can build up outdoors in the vicinity of smokers.
Outdoor ETS emission rates are not regulated in the United States, although
regulations prohibiting smoking in many public and private indoor facilities cur-
rently exist at the local, state, and federal levels. Many chemical constituents of
ETS are classified as hazardous air pollutants under CAAA90,
but the Act controls
only sources emitting more than 10 tons of a hazardous substance per year, and
individual cigarettes emit less than 1 g of all pollutants combined. The product of
the number of cigarettes smoked per year multiplied by the emission rate per ciga-
rette is much larger than 10 tons per year, but this statistic is not recognized
by CAAA90.
Sources
In 1990, approximately 26 percent of the U.S. population, or 50 million people,
were estimated to smoke cigarettes regularly (Rando et al., 1997). In 1986, 70 percent
of all children in the United States li
ved in households in which at least one parent
smoked (Weiss, 1986). Table 9.2 shows mainstream and sidestream emissions for
some components emitted from cigarettes. The actual emission rate of a cigarette
depends on the type of tobacco, the density of its packing, the type of wrapping paper,
and the puffing rate of the smoker (Hines et al., 1993).
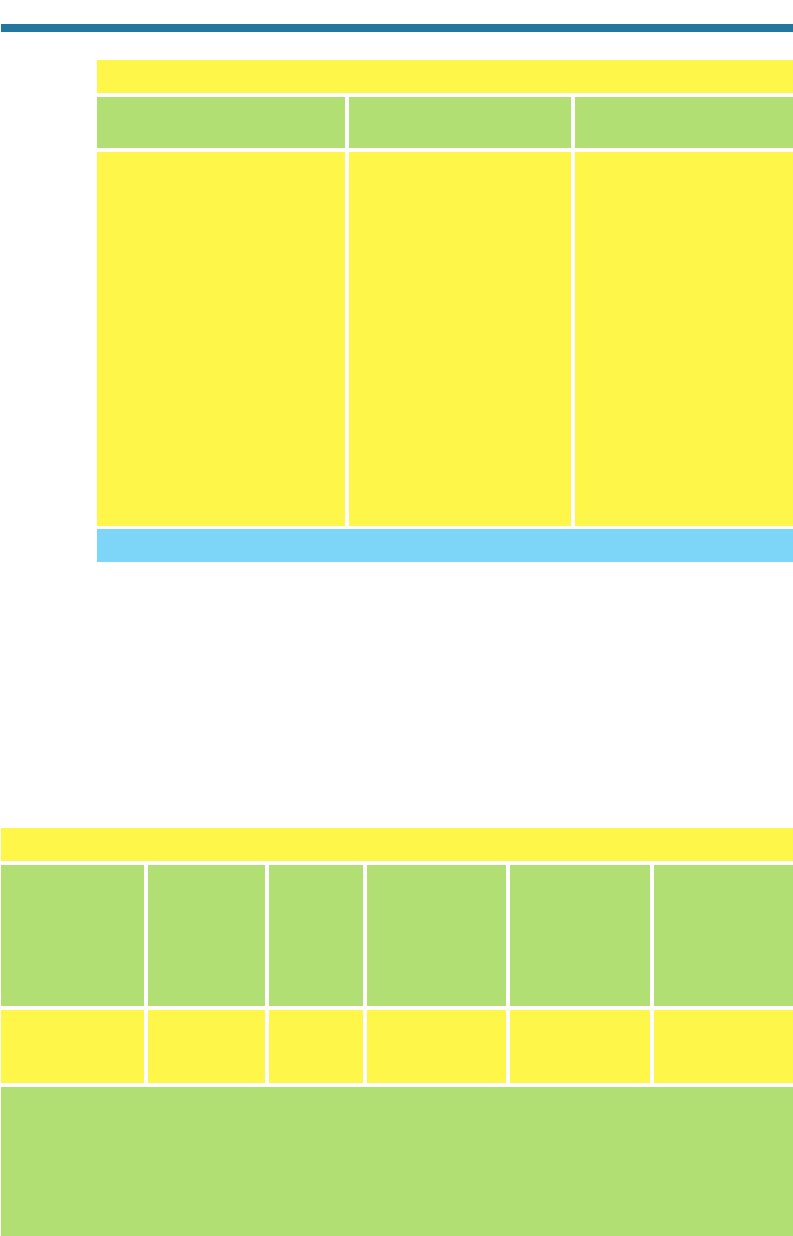
Table 9.2 indicates that emission rates of sidestream smok
e are greater than are
those of mainstream smoke for many pollutants. Thus, in many cases, a person stand-
ing a short distance from a cigarette is exposed to more pollution than is the smoker
(Schlitt and Knöppel, 1989).
Table 9.3 compares emissions from cigarettes with those from vehicles. The table
indicates that driving a vehicle that meets current U.S. EPA emission standards 1 mile
results in particle emissions equivalent to emissions from about 1.4 cigarettes.
Emissions of CO(g) and NO
x
(g) from driving 1 mile are equivalent to those from
smoking 73 and 190 cigarettes, respectively. The total emissions per day of these sub-
stances from cigarettes is much less than that from mobile sources,
but ETS is often
emitted in enclosed spaces, where its concentrations build up.
Concentrations
ETS contributes to the buildup of gas and particle concentrations indoors. Spengler
et al. (1981) found that one pack of cigarettes per day contributes about 20 g m
3
of
particles over a 24-hour period. During the time a cigarette is actually smoked, particle
concentrations increase to 500 to 1,000 g m
3
. Leaderer et al. (1990) found that par-
ticle concentrations in homes with a cigarette smoker were up to three times those in
homes without a smoker.
Health Effects
Short-term exposure to ETS results in eye, nose, and throat irritation for most
individuals, and allergic skin reactions for some (Maroni et al., 1995). ETS also
elevates symptoms for people who have asthma, and may induce asthma in some
INDOOR AIR POLLUTION 249
children (Jones, 1999). A link between exposure to ETS and lower respiratory tract
illness has been found (Somerville et al., 1988). Children exposed to ETS are hos-
pitalized more often than are children not e
xposed (Harlap and Davies, 1974).
Several studies have linked long-term ETS exposure to lung cancer. Janerich et al.
(1990) found that ETS may cause up to 17 percent of lung cancers among non-
smokers. Rando et al. (1997) found a 30 percent increase in the cancer risk to
women whose husbands smoked. Ryan et al. (1992) linked brain cancer tumors
to ETS.
250 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
Table 9.2. Mainstream and Sidestream Emission Rates per Cigarette
Carbon dioxide 10,000–80,000 81,000–640,000
Carbon monoxide 500–26,000 1,200–65,000
Nitrogen oxides 16–600 80–3,500
Ammonia 10–130 400–9,500
Hydrogen cyanide 280–550 48–203
Formaldehyde 20–90 1,000–4,600
Acrolein 10–140 100–1,700
N-nitrosodimethylamine 0.004–0.18 0.04–149
Nicotine 60–2,300 160–7,600
Total par ticulate 100–40,000 130–76,000
Phenol 20–150 52–390
Catechol 40–280 28–196
Naphthalene 2.8 45
Benzo(a)pyrene 0.008–0.04 0.02–0.14
Aniline 0.10–1.20 3–36
2-Naphthylamine 0.004–0.027 0.02–1.1
4-Aminobiphenyl 0.002–0.005 0.06–0.16
N-nitrosonornicotine 0.2–3.7 0.02–18
Mainstream Smoke Sidestream Smoke
Substance (g per Cigarette) (g per Cigarette)
Source: Rando et al., 1997; Jones, 1999.
Table 9.3. Comparison of Emissions from Cigarettes and Mobile Sources
Carbon monoxide 0.0464 3.4 73.3 60 189,000
Nitrogen oxides 0.0021 0.4 190.5 2.7 32,000
Particulates 0.058 0.08 1.4 75 9300
(c) (e)
Number of (d) Estimated
(a) (b) Cigarettes Estimated Mobile-Source
Average Average Resulting in Cigarette Direct
Cigarette Vehicle Same Emissions Emissions in the Emissions in the
Emissions Emissions as 1 Mile of United States United States
Substance (gCigarette)
a
(gmi)
b
Driving
c
(tonsday)
d
(tonsday)
e
a
Sum of average mainstream and sidestream emissions in Table 9.2.
b
1994 federal light-duty vehicle emission standards.
c
Column (b) divided by column (a).
d
Assumes the current population of the United States is 250,000,000 and 26 percent of the population
smokes 20 cigarettes per day.
e
Estimated 1997 emissions from U.S. EPA (1997).

9.2. SICK BUILDING SYNDROME
In some workplaces, employees experience an unusually high rate of headaches, nau-
sea, nasal congestion, chest congestion, eye problems, throat problems, fatigue, fever,
muscle pain, dizziness, and dry skin. These symptoms, present during working hours,
often improve after a person leaves work. The situation described is called sick build-
ing syndrome (SBS), the cause of which is unknown, although it may be due to
certain VOCs, building ventilation systems, or the exposure to many pollutants in low
doses simultaneously. SBS may also be caused by enhanced stress levels and heavy
workloads or a combination of psychological and chemical factors (Jones, 1999).
9.3. REGULATION OF INDOOR AIR POLLUTION
In the United States, National Ambient Air Quality Standards (NAAQS), initiated under
the Clean Air Act Amendments, control outdoor air pollutants only. No regulations control
air pollution concentrations in indoor residences. Standards for pollutant concentrations in
indoor workplaces are set by a government agency, the Occupational Safety and Health
Administration (OSHA), which obtains recommendations for standards from another go
v-
ernment agency, the National Institute for Occupational Safety and Health (NIOSH), and
an independent professional society, the American Conference of Governmental Industrial
Hygienists, Inc. (ACGIH). OSHA and NIOSH were created by the 1970 U.S.
Occupational Safety and Health Act. OSHA is in the U.S. Department of Labor and is
responsible for setting and enforcing workplace standards, whereas NIOSH is in the U.S.
Department of Health and Human Services and is responsible for researching workplace
health issues. ACGIH’s primary mission is to promulgate workplace safety standards.
NIOSH recommends permissible exposure limits (PELs), short-time exposure
limits (STELs), and ceiling concentrations. A PEL is the maximum allowable con-
centration of a pollutant in an indoor workplace over an 8-hour period during a day.
An STEL is the maximum allowable concentration of a pollutant over a 15-minute
period. A ceiling concentration is a concentration that may never be exceeded. ACGIH
sets 8-hour time-weighted average threshold limit values (TWA-TLVs), which are
similar to PELs. For most pollutants, PELs and TWA-TLVs are the same. When differ-
ences occur, they are small.
Indoor standards exist for more than 150 compounds. Table 9.4 compares indoor
with outdoor standards for a few compounds. For ozone and carbon monoxide, the
INDOOR AIR POLLUTION 251
Table 9.4. Comparison of Indoor Workplace Standards with Outdoor Federal and California
State Standards for Selected Gases
Carbon monoxide 35 — 200 9.5 (8 hour) 9 (8 hour)
Nitrogen dioxide — 1 — 0.053 (annual) 0.25 (1 hour)
Ozone 0.1 0.3 — 0.08 (8 hour) 0.09 (1 hour)
Sulfur dioxide 2 5 — 0.14 (24 hour) 0.05 (24 hour)
Indoor 8-hour Indoor Outdoor
PEL and TWA-TLV 15-min STEL Indoor Ceiling Outdoor NAAQS California
Gas (ppmv)
a
(ppmv)
a
(ppmv)
a
(ppmv) Standard (ppmv)
a
National Institute for Occupational Safety and Health (2000).

8-hour PEL/TWA-TLV standards are less stringent than are the outdoor standards
because outdoor standards are designed to protect the entire population, particularly
infants and people afflicted with disease or illness. Indoor standards are designed to
protect workers, who are assumed to be healthier than is the average person. The table
also indicates that the 15-minute STEL for nitrogen dioxide is four times higher (less
stringent) than is the 1-hour outdoor California standard. Stringent outdoor standards
for nitrogen dioxide are set because it is a precursor to photochemical smog. Because
UV sunlight does not penetrate indoors, nitrogen dioxide does not produce ozone
indoors, and indoor regulations of nitrogen dioxide as a smog precursor are not neces-
sary. Indoor standards for nitrogen dioxide are based solely on health concerns.
9.4. SUMMARY
People spend most of their time indoors; thus, most of their exposure to pollution occurs
indoors. Indoor air contains many of the same pollutants as does outdoor air, but pollu-
tion concentrations in indoor and outdoor air usually differ. Indoor mixing ratios of
ozone and sulfur dioxide are usually less than are those outdoors, but indoor mixing
ratios of formaldehyde are usually greater than are those outdoors. Indoor mixing ratios
of carbon monoxide and nitrogen dioxide are generally the same as or less than are those
outdoors, unless appliances or other indoor combustion sources are turned on. Indoor
concentrations of radon and environmental tobacco smoke, when present, are usually
greater than are those outdoors, giving rise to potentially serious health problems to peo-
ple exposed to these pollutants indoors. Indoor air also contains v
olatile organic
compounds, allergens, fungi, bacteria, and viruses. In the United States, indoor air is reg-
ulated only in the workplace and in public buildings; residential air is not regulated.
9.5. PROBLEMS
9.1. Why are ozone mixing ratios almost always lower indoors than outdoors?
9.2. Why are workplace standards for pollutant concentrations generally less strin-
gent than standards for outdoor air?
9.3. What would be the volume mixing ratio (ppmv) of carbon monoxide and the
mass concentration (g m
3
) of particulates if ten cigarettes were smoked in a
5 m 10 m 3 m room. How do these values compare with the California
state 1-hour standard for CO(g) and the 24-hour average standard for PM
10
?
Based on the results, which pollutant do you think is more of a cause for concern
with respect to indoor air quality? Assume the dry air partial pressure is 1013
mb, the temperature is 298 K, and use cigarette emission rates from Table 9.3.
9.4. Why is radium less of a concern than radon?
9.5. Why is removing asbestos from buildings often more dangerous than leaving it
alone?
9.6. Why does the gas-phase chemical decay of organic compounds generally take
longer indoors than outdoors?
252 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION

ACID DEPOSITION
10

254
A
cid deposition occurs when sulfuric acid, nitric acid, or hydrochloric acid,
emitted into or produced in the air as a gas or liquid, deposits to soils,
lakes, farmland, forests, or buildings. Deposition of acid gases is dry acid
deposition, and deposition of acid liquids is wet acid deposition. Wet acid deposition
can be through rain (acid rain), fog (acid fog), or aerosol particles (acid haze). On the
Earth’s surface, acids have a variety of environmental impacts, including damage to
microorganisms, fish, forests, agriculture, and structures. In the air, acids in high con-
centrations are harmful to humans. Acid deposition problems have existed since coal
was first combusted, but were exacerbated during the Industrial Revolution in the eigh-
teenth century. The problem became more severe with the growth of the alkali industry
in 19th-century France and the United Kingdom. In this chapter, the history, science,
and regulation of acid deposition problems are discussed.
10.1. HISTORICAL ASPECTS OF ACID DEPOSITION
Acid deposition is caused by the emission or atmospheric formation of gas- or
aqueous-phase sulfuric acid (H
2
SO
4
), nitric acid (HNO
3
), or hydrochloric acid
(HCl). Historically, coal was the first and largest source of anthropogenically
produced atmospheric acids. Coal combustion produces sulfur dioxide gas [SO
2
(g)]
and hydrochloric acid gas [HCl(g)]. SO
2
(g) produces gas-and aqueous-phase
sulfuric acid. Humans have combusted coal for thousands of years. In the 1200s,
sea coal was brought to London and used in lime
kilns and forges (Section 4.1). It was later burned
in furnaces to produce glass and bricks, in brew-
eries to produce beer and ale, and in homes to
provide heating. During the Industrial
Revolution, which started in the eighteenth centu-
ry, coal was used to provide energy for the steam
engine.
In the late eighteenth century, a second major
source of atmospheric acids emerged. Around 1780,
the demand for sodium carbonate [Na
2
CO
3
(s)]
(also known as soda ash, washing soda, and salt
cake), used in the production of soaps, detergents,
cleansers, glass, paper, bleaches, and dyes, increa-
sed. Although this chemical could be extracted from
barilla plants, which grew near the Mediterranean
Sea, and from sea kelp, a more efficient method of
producing it was desired. In 1781, the French
Academy of Sciences offered a prize to the person
who could develop the most efficient and economic
method of producing soda ash. The prize was never
awarded because of the French Revolution, but
Nicolas Leblanc (1742–1806; Fig. 10.1), encour-
aged by the competition, began experimenting in 1784 to find a new process. In 1789,
he came up with a two-step set of reactions to produce soda ash. In the first step, he
Figure 10.1. Nicolas Leblanc (1742–1806).

dissolved common salt into a sulfuric acid solution at a high temperature in an iron
pan to produce dissolved sodium sulfate (Glauber’s salt) and hydrochloric acid
gas by
High
temperature
2NaCl(s) H
2
SO
4
(aq) Na
2
SO
4
(aq) 2HCl(g)
Sodium Sulfuric Sodium Hydrochloric (10.1)
chloride (salt) acid sulfate acid
In the second step, he heated sodium sulfate together with charcoal and chalk in a kiln
to form sodium carbonate by
High
temperature
Na
2
SO
4
(aq) 2C CaCO
3
(s) Na
2
CO
3
(aq) CaS(s) 2CO
2
(g)
Sodium Carbon Calcium Sodium Calcium Carbon (10.2)
sulfate from carbonate carbonate sulfide dioxide
charcoal from chalk
By-products of the second reaction, when complete, included calcium sulfide
[CaS(s)], an odorous, yellow to light-gray powder, and carbon dioxide gas. In practice,
the reaction was incomplete and produced gas-phase sulfuric acid and soot as well.
The sodium carbonate residue was separated from calcium sulfide by adding water,
which preferentially dissolved the sodium carbonate. The resulting solution was then
dried, producing sodium carbonate crystals. Sodium carbonate w
as then combined
with animal fat to produce soap.
A necessary ingredient for the production of sodium carbonate was aqueous sulfu-
ric acid (Reaction 10.1). This was obtained by burning elemental sulfur (S) powder
with saltpeter [KNO
3
(s)], then dissolving the resulting H
2
SO
4
(g) in water, as was done
by Libavius in 1585. This process was inefficient, releasing volumes of gas-phase sul-
furic acid and nitric oxide [NO(g)] (which converts to nitrogen dioxide, then to nitric
acid in air).
In sum, although the production of sodium carbonate from common salt and sul-
furic acid allowed the alkali industry to become self-sufficient and escape reliance on
the import of natural sodium carbonate, it resulted in the release of HCl(g), H
2
SO
4
(g),
HNO
3
(g), and soot, causing widespread acid deposition and air pollution in France
and the United Kingdom. The Leblanc process also produced large amounts of
impure solid “alkali waste” containing calcium sulfide. The waste was piled near each
factory. When rainwater fell on the waste, some of the calcium sulfide dissolved, pro-
ducing dissolved hydrogen sulfide H
2
S(aq), which evaporated as a harmful, odorous,
colorless gas. Much of the rest of the calcium sulfide oxidized over time to form cal-
cium sulfate [CaSO
4
-2H
2
O(s), gypsum]. Piles of gypsum from soda ash factories can
still be seen today.
Although Leblanc patented the soda ash technique and started a soda ash factory in
1791, he lost his patent and factory to the state, which nationalized patents and facto-
ries in 1793. Leblanc ultimately committed suicide in 1806 because of his inability to
recover from his business losses.
In 1863, an estimated 1.76 million tons of raw material were burned to form soda ash,
producing only 0.28 million tons of useful products. Most of the remaining 1.48 million
ACID DEPOSITION 255

tons was emitted as HCl(g) or other gases and produced as solid waste (Brock, 1992).
Environmental damage due to the alkali industry was severe. HCl(g) and other pollutants
rained down onto agricultural property and cities in France and the U.K., causing proper-
ty and health damage. In the United Kingdom, St. Helens, Newcastle, and Glasgow
countrysides were decimated (Brimblecombe, 1987).
Early complaints against alkali factories were in the form of civil litigation. For
example, in 1838, a Liverpool landowner filed a complaint against an alkali factory,
charging that it destroyed his crops and interfered with his hunting. Ultimately, HCl(g)
from alkali manufacturers was regulated in France and the United Kingdom. In
France, regulation took the form of planning laws that controlled the location of alkali
factories. In the United Kingdom, the 1863 Alkali Act required alkali manufacturers to
reduce 95 percent of their HCl(g) emissions. The act also called for the appointment of
an alkali inspector to watch over the industry.
Impetus for the 1863 Alkali Act came in the early 1860s, when William Gossage
invented a technique to wash HCl(g) from waste gases before it was released from
chimneys. Gossage built his own soda ash factory in Wo
rcestershire in 1830. Spurred
by his neighbors’ complaints about the hydrochloric acid emitted from his factory, he
worked to mitigate the problem. His solution was to convert a windmill into a tower,
fill the tower with brushwood, and spray water down the top of the tower as smoke
rose from the bottom. The water dissolved most of the hydrochloric acid (just as rain
does) and drained it into a nearby waterway (which he was not too concerned about).
Because the technique was so simple and inexpensive and because the consequences
of not implementing the technique were so severe, the United Kingdom easily passed
the 1863 Alkali Act shortly after Gossage’s invention. Subsequent to the Act, Walter
Weldon (in 1866) and Hugh Deacon (in 1868) developed processes for converting
HCl(g) to chlorine that could be used for bleaching powder. These inventions allowed
chlorine, which otherwise would have been wasted, to be recycled.
Despite the success of the Alkali Act at reducing HCl(g), the alkali industry contin-
ued to emit sulfuric acid, nitric acid, soot, and other pollutants in abundance. Because
the act did not control emissions from factories other than those producing soda ash,
pollution problems in the U.K. worsened. Although the Alkali Act was modified in
1881 to regulate other sections of the chemical industry, the number of factories and
the volume of emissions from them had grown so much that the new law had little
effect. In 1899, one writer described St. Helens as
a sordid ugly town. The sky is a low-hanging roof of smeary smoke. The
atmosphere is a blend of railway tunnel, hospital ward, gas works and open
sewer. The features of the place are chimneys, furnaces, steam jets, smoke
clouds and coal mines. (Blatchford, 1899)
The first Alkali Act inspector in the United Kingdom was Scottish chemist Robert
Angus Smith (1817–1884; Fig. 10.2). He was charged with ensuring industry reduced
HCl(g) emissions by 95 percent. Smith was also a field experimentalist. In 1872, he
published Air and Rain: The Beginnings of a Chemical Climatology, in which he dis-
cussed results of the first monitoring network for air pollution in Great Britain. As part
of the analysis, he recorded the gas-phase mixing ratios of molecular oxygen and car-
bon dioxide and measured the composition of chloride, sulfate, nitrate, and ammonium
in rainwater in the British Isles. In his book, Smith introduced the term acid rain to
describe the high sulfate concentrations in rain near coal-burning facilities.
256 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION

Leblanc’s soda ash process dominated the alkali industry until the 1880s. In 1861,
Belgian chemist Ernst Solvay (1838–1922) developed a more efficient process of pro-
ducing soda ash by raining a salt produced from
sodium chloride and ammonia gas down a tower
(Solvay tower) over an upcurrent of carbon dioxide
gas. The technique required no sulfuric acid or
potassium nitrate (saltpeter). The disadvantages were
the cost of the tower and the fact that the final chlo-
ride was tied up in a form that could not easily be
reused. Only by the 1880s did the Solvay process
overtake the LeBlanc process in economic efficiency
and popularity.
Throughout the twentieth century, acid deposi-
tion problems continued to plague cities in the
United Kingdom and municipalities in other coun-
tries where coal and chemical b
urning occurred. The
fatal pollution episodes in London, discussed in
Chapter 4, included contributions from acidic com-
pounds in smoke.
Acid deposition became an issue of international
interest in the 1950s and 1960s, when a relationship
was found between sulfur emissions in continental
Europe and acidification of Scandinavian lakes.
These studies led to a 1972 United Nations
Conference on Human Environment in Stockholm
that called for an international ef
fort to reduce acidi-
fication. Since then, numerous studies on acid
deposition have been carried out. As a result of these studies, national governments
have intervened to control acid deposition problems. Such efforts are discussed at the
end of this chapter. First, the basic science of acid deposition is discussed.
10.2. CAUSES OF ACIDITY
In Chapter 5, pH was defined as
pH log
10
[H
] (10.3)
where [H
] is the molarity (moles per liter) of H
in a solution containing a solvent and
one or more solutes. The pH scale, shown in Fig. 10.3 for a limited range, varies from less
than 0 (lots of H
and very acidic) to greater than 14 (very little H
and very basic or
alkaline). Neutral pH, the pH of distilled water, is 7.0. At this pH, the molarity of H
is
10
7
mol L
1
. A pH of 4 means that the molarity of H
is 10
4
mol L
1
. Thus, water at a
pH of 4 is 1,000 times more acidic (contains 1,000 times more H
ions) than is water at a
pH of 7.
The molarity of H
is related to that of OH
by the equilibrium relationship,
H
2
O(aq)
E
H
OH
Liquid Hydrogen Hydroxide (10.4)
water ion ion
ACID DEPOSITION 257
Figure 10.2. Robert Angus Smith (1817–1884).
