Jacobson M.Z. Atmospheric Pollution
Подождите немного. Документ загружается.


200,000 tons of fine-ground limestone to lakes and watercourses each year. Since the
1970s, more than 7,000 of Sweden’s 17,000 anthropogenically acidified lakes have
been limed. Because lime is consumed 2 to 3 years after its application, acidified lakes
need to be relimed regularly. Other countries that have large liming programs include
Norway, Finland, and Canada.
10.6.3. Calcium Carbonate
Some soils that contain the minerals calcite or aragonite [CaCO
3
(s), calcium carbonate]
have a natural ability to neutralize acids. When acid rain falls onto calcite-containing
soils, the H
is removed by
CaCO
3
(s) 2H
E
Ca
2
CO
2
(g) H
2
O(aq)
Calcium Hydrogen Calcium Carbon Liquid (10.17)
carbonate ion ion dioxide gas water
The same result occurs when soil-dust particles that contain CaCO
3
(s) collide with
acidified raindrops. The erosion of farmland and desert borders in many locations has
enhanced the quantity of soil dust in the air, inadvertently increasing the calcium car-
bonate content of rainwater, decreasing rainwater acidity, and increasing rainwater pH
in nearby regions.
Unfortunately, the same process described by Reaction 10.17 that decreases soil
acidity is partly responsible for the erosion of great statues and buildings made of or
containing marble or limestone. Marble and limestone, which both contain calcite,
erode when they become coated with acidified water. Coating can occur in at least two
ways. The first is when acidified raindrops or aerosol particles deposit directly onto a
marble or limestone surface. The second is when a gas dissolves and forms an acid in
dew or rainwater that has recently coated a surface. For example, when SO
2
(g) dis-
solves in water, it oxidizes to sulfuric acid (Section 10.3.2).
When water containing sulfuric acid coats a calcite surface, the hydrogen ion dis-
solves the calcite by Reaction 10.17, and the sulfate ion reacts with the dissociated
calcium to form the mineral gypsum by
Ca
2
SO
4
2
2H
2
O(aq)
E
CaSO
4
2H
2
O(s)
Calcium Sulfate Liquid Calcium sulfate (10.18)
ion ion water dihydrate (gypsum)
The net result is the formation of a clear-to-white gypsum crust over the marble or
limestone. Bombardment by rain over time removes some of the brittle gypsum
crust. Because the crust now contains part of the statue or building material (the cal-
cium), its removal creates tiny crevices, or pits, causing erosion (Davidson et al.,
1999). Because the gypsum crust and the crevices roughen the surface of a statue or
building, other pollutants, such as soot, more readily bond to the surface, darkening
it, as illustrated in Fig. 10.8. The figure shows photographs of the Cathedral of
Learning, at the University of Pittsburgh, taken in 1930, soon after the start of its
construction, and in 1934. During a four-year period, sulfate from coal smoke emit-
ted by steel mills and locomotives (Section 4.1.6.3) roughened the limestone exterior
of the building, and soot from the same smoke bonded with the roughened exterior,
darkening it.
268 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION

Gypsum forms not only on buildings, but also in soils and aerosol particles.
When rainwater containing the sulfate ion falls on soil containing calcite, the
calcite dissociates by Reaction 10.17. When the soil dries, the calcium ion reacts
with the sulfate ion by Reaction 10.18, producing gypsum. The same process
occurs when soil-dust particles containing calcite collide with acidified raindrops.
Deposition of rain containing sulfuric acid over soils containing calcite and deposi-
tion of particles already containing gypsum have, over time, produced worldwide
deposits of gypsum soil.
10.6.4. Sodium Chloride
Some acidic soils near nonpolluted coastal areas are naturally neutralized by
cations originating from sea spray (e.g., Na
,Ca
2
,Mg
2
,K
) that have deposited
onto soils over the millennia. The pH of natural sea
water ranges from 7.8 to 8.3,
and that of uncontaminated large sea-spray drops is similar, indicating that little
H
exists in such drops. The deposition of sea-spray drops to coastal soils,
and the
subsequent desiccation of these drops produces the mineral halite [NaCl(s),
sodium chloride or common salt]. When sulfate-containing water enters NaCl(s)-
containing soils, NaCl(s) dissolves and dissociates, H
combines with the chloride
ion [Cl
] to form HCl(aq), and HCl(aq) evaporates to the gas phase. The net
process is
NaCl(s) H
E
Na
HCl(g)
Sodium Hydrogen Sodium Hydrochloric (10.19)
chloride ion ion acid
which reduces H
, increases pH, and reduces the acidity of soil water.
ACID DEPOSITION 269
(a) (b)
Figure 10.8. Soiling of the limestone exterior of the Cathedral of Learning at the University of
Pittsburgh between (a) 1930 and (b) 1934 (Davidson et al., 1999). The building was con-
structed between 1929 and 1937. Sulfate and soot from coal smoke caused erosion and
darkening of the building after only 4 years. Photo courtesy of the University Archives,
University of Pittsburgh.
10.6.5. Ammonia
Ammonia gas [NH
3
(g)] is considered an anthropogenic pollutant, but it also neutral-
izes raindrop and soil-water acidity. Sources of ammonia gas were discussed in
Section 5.3.2.3. Once in the air, ammonia gas dissolves in water. The dissolved gas
then reacts with the hydrogen ion to form the ammonium ion by
NH
3
(aq) H
E
NH
4
Dissolved Hydrogen Ammonium (10.20)
ammonia ion ion
The reduction in H
that results from this reaction increases pH, reducing acidity. In
many cases, the pH of rainwater containing the ammonium ion exceeds 6. Aerosol
particles and raindrops containing the ammonium ion deposit to soils and lakes, pro-
viding these surfaces with a neutralizing agent. Soils downwind of high ammonia gas
emissions tend to ha
ve a better neutralizing capacity against acid deposition than do
soils far from ammonia sources if all other conditions are the same.
10.7. RECENT REGULATORY CONTROL OF ACID DEPOSITION
The first major effort to control acid deposition was the U.K. Alkali Act of 1863,
which mandated large reductions of hydrochloric acid gas emissions by soda-ash
manufacturers. In more recent years, the U.S. Clean Air Act Amendments of 1970
led to lower emissions of acid-deposition precursors, namely SO
2
(g) and NO
2
(g). In
1977, the U.S. initiated the National Atmospheric Deposition Program (N
ADP),
whose agenda was to monitor trends of acidity in precipitation. In 1980, the U.S.
Congress passed the Acid Precipitation Act, which funded a program, the National
Acid Precipitation Assessment Program (NAPAP). Under the program, the network
of monitoring stations under NADP was enlarged to produce a National Trends
Network (NTN). NAPAP reported trends observed at the monitoring sites. The U.S.
Clean Air Act Amendments of 1990 mandated a 10 million ton reduction in sulfur
dioxide [SO
2
(g)] emissions from 1980 levels and a 2 million ton reduction in nitro-
gen oxide [NO
x
(g)] emissions from 1980 levels by 2010. To implement these
reductions, the U.S. EPA set up an emission trading system, whereby emitters could
trade among themselv
es for limited rights to release SO
2
(g). Power plants were also
required to install emission monitoring systems. In January 2000, the U.S. EPA
issued a new rule requiring U.S. refiners to cut the sulfur content of gasoline to one-
tenth its value by 2006.
Meanwhile, several studies in the 1970s concluded that winds were transporting
acid-deposition precursors over long distances, often over political boundaries. Such
studies culminated in the 1979 Geneva Convention on Long-Range Transboundary Air
Pollution. The convention was signed by 34 governments and the European
Community, and was the first agreement to deal with an international air pollution
problem. As part of a 1985 amendment to the agreement (the Sulfur Protocol), mem-
ber countries were required to reduce their emissions or transboundary fluxes of sulfur
by 30 percent below 1980 values by 1993. Another amendment in 1988 (the Nitrogen
Oxide Protocol) required countries to reduce their emissions or transboundary fluxes
of nitrogen oxide to their 1987 levels by December 1994. Because the first Sulfur
270 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION

Protocol did not address forest loss in central Europe sufficiently, a second Sulfur
Protocol was signed in 1994 that will result in a 60 percent reduction in sulfur emis-
sions below 1980 values by 2010.
10.7.1. Methods of Controlling Emissions
Several mechanisms are available to control emissions of acid deposition precursors.
These include the mandatory use of low-sulfur coal instead of high-sulfur coal and the
use of emission-control technologies. The amount of SO
2
(g) emitted during coal com-
bustion depends on the sulfur content of the coal. In the United States, about 39
percent of coal is mined in the Appalachian Mountains and the rest is mined west of
the Mississippi River, with Wyoming producing 31 percent of all U.S. coal, the largest
percentage of any state (EIA, 2000). Coal from the Appalachian Mountains has a high
sulfur content. The cost of transporting Appalachian coal to power plants, most of
which are in the midwest and eastern United States, is lower than is the cost of trans-
porting low-sulfur coal from
Wyoming or other western states to these plants. As such,
coal burners prefer to use high-sulfur coal. As a result of the CAAA90 requirement to
reduce SO
2
(g) emissions, the reliance on western U.S. coal is expected to increase.
Use of low-sulfur coal is one mechanism to reduce emission of SO
2
(g) during coal
burning. Another is to remove a certain fraction of sulfur from high-sulfur coal before
burning it. A technology available for reducing SO
2
(g) emission from a stack is the
scrubber, first developed by William Gossage to reduce HCl(g) emission. A modern-
day scrubber works by dissolving SO
2
(g) into small water drops sprayed into an
exhaust stream, then removing the drops on a collecting surface, such as a bed or a
wetted surface.
10.7.2. Effects of Regulation
The U.S. EPA Office of Air and Radiation estimates that sulfate concentrations in rain-
fall were 10 to 25 percent less in 1995 and 1996 than what they would have been if
controls mandated through the Clean Air Act Amendments of 1990 had not been imple-
mented. The largest reductions in sulfate concentrations occurred along the Ohio River
Valley and states downwind. Nitrate concentrations during this period did not improve.
Reductions of sulfur dioxide emissions in Canada have reduced the acidity of some
Canadian lakes and forests. For example, in the late 1960s,
the Sudbury, Ontario, nickel-
smelting stack (Section 6.6.2.4) was the largest individual source of SO
2
(g) in North
America, emitting 5000 tons of SO
2
(g) per day, devastating nearby lakes and forests.
Today, its emissions are below 500 tons of SO
2
(g) per day, and nearby lakes and forests
have partially regenerated. Reductions in the acidity of lakes in Quebec, Atlantic
Canada, and other areas of Ontario have been less dramatic (Environment Canada,
2000b). Many lakes in Sweden have been restored, but many more are still damaged by
acid deposition. Acid deposition problems in eastern Europe and Asia are still severe.
10.8. SUMMARY
In this chapter, the history, science, effects, and control of acid deposition were dis-
cussed. Acidity is determined by pH, which ranges from less than 0 (very acidic) to
more than 14 (very basic). The pH of distilled water is 7, of natural rainwater is 5.0 to
ACID DEPOSITION 271

5.6, and of acid rain or fog is less than 5.0. Acid deposition occurs when sulfuric,
nitric, or hydrochloric acid is emitted into or forms chemically in the air and is subse-
quently deposited as a gas or liquid to the ground, where it harms microorganisms,
fish, forests, agriculture, and structures. In high concentrations in the air, acids can also
harm humans. Severe acid deposition problems arose from increased coal combustion
in the U.K. during the Industrial Revolution and from the growth of the alkali industry
in France and the United Kingdom in the 1800s. Today, sulfuric acid is usually the
most abundant acid in rainwater. Sulfuric acid is produced by gas- and aqueous-phase
oxidation of sulfur dioxide. The latter process is most efficient when cloud drops are
present. In polluted coastal air, nitric acid fog is often a problem. A method of amelio-
rating the effects of acid deposition on lakes is to add a neutralizing agent; such as
slaked lime. In the United States, Canada, and western Europe, government interven-
tion in the form of regulations limiting the emissions of acid-deposition precursors has
resulted in reductions in the acidity of rainwater. Acid deposition problems in eastern
Europe and Asia are still severe.
10.9. PROBLEMS
10.1. Identify all the atmospheric acids produced by Leblanc’s soda ash process.
10.2. In terms of acid deposition precursors, what were the advantages of the Solvay
versus the Leblanc soda ash process?
10.3. Although Leblanc’s process produced HCl(g), which caused widespread acid
deposition problems in the early 1800s, HCl(g) was no longer the most danger-
ous by-product of this process in the late 1800s. Why?
10.4. Describe the two important conversion pathways for S(IV) to S(VI). Which
pathway is more important when aerosol particles are present? Why?
10.5. What are the most important aqueous-phase oxidants of S(IV)?
10.6. Why are nitric acid and hydrochloric acid deposition less of a problem in most
parts of the world than is sulfuric acid deposition?
10.7. How do neutralizing agents reduce the acidity of a lake?
10.8. Suppose rainwater containing the sulfate ion enters a soil containing magne-
sium carbonate [MgCO
3
(s)]. Would the magnesium carbonate act as a
neutralizing agent or enhance the acidity of the water? Show the pertinent
chemical process.
10.9. Identify three products that you use or activities that you do that result in the
emission of acids into atmosphere.
10.10. Identify three ways that acids or acid precursors can be controlled through
legislative action.
272 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION

GLOBAL
STRATOSPHERIC OZONE
REDUCTION
11

T
he stratospheric ozone layer began to form soon after the onset of oxygen-
producing photosynthesis, about 2.3 billion years ago (b.y.a.). It probably
did not develop fully until at least 400 million years ago (m.y.a.), when
green plants evolved and molecular oxygen mixing ratios began to approach their
present levels. Absorption of ultraviolet (UV) radiation by ozone is responsible for
the temperature inversion that defines the present day stratosphere. This absorption is
critical for preventing UV radiation from reaching the surface of the Earth, where it
can harm life. The anthropogenic emission of long-lived chlorine- and bromine-con-
taining compounds into the air since the 1930s and the slow transfer of these
compounds to the stratosphere has caused a nontrivial reduction in the global stratos-
pheric ozone layer since the 1970s. In addition, during September, October, and
November each year since the early 1980s, up to 70 percent of the ozone layer has
been destroyed over the Antarctic. Lesser reductions ha
ve occurred over the Arctic in
March, April, and May each year. Recent international cooperation has helped reduce
emissions and slow further ozone loss. In this chapter, the natural stratospheric ozone
layer, global ozone reduction, and Antarctic/Arctic ozone destruction and regenera-
tion are discussed.
11.1. STRUCTURE OF THE PRESENT-DAY OZONE LAYER
About 90 percent of all ozone molecules in the atmosphere reside in the stratosphere;
most of the remaining molecules reside in the troposphere. Whereas ozone molecules
near the surface harm humans,
animals, plants, trees, and structures, the same ozone
molecules, whether in the stratosphere or in polluted air, shield the Earth from harmful
UV radiation.
A measure of the quantity of ozone in the air is the ozone column abundance,
which is the sum of all ozone molecules above a square centimeter of surface between
the ground and the top of the atmosphere. When this number is divided by 2.7 10
16
,
the result is the column abundance in Dobson units (DUs). Thus, 1 DU is equivalent
to 2.7 10
16
molecules of ozone per square centimeter of surface. The Dobson unit is
named after Gordon M. B. Dobson (1889–1976), a researcher at Oxford University
who, in the 1920s, built the first instrument, now called a Dobson meter, to measure
total ozone column abundance from the ground. In 2000, the globally averaged col-
umn abundance of ozone from 90S to 90°N was 293.4 DU. This column abundance
contains the same number of molecules as a column of air 2.93-mm high at 1 atm of
pressure and 273 K (near-surface conditions). Figure 11.1 illustrates ozone column
abundance.
Figure 11.2 shows a plot of the variation in the ozone column abundance with lati-
tude (zonally averaged – averaged over all longitudes) and month for the year 2000.
The following features can be seen in the figure:
• A year-around equatorial ozone minimum due to upward motion of ozone-poor air
from the troposphere that displaces ozone-rich air horizontally to higher latitudes.
The column abundance over the equator is typically 250 to 290 DUs all year.
• A Northern Hemisphere (NH) spring (March–May) maximum, ranging from 350
to 460 DU, near the North Pole. The maximum is due to the northward transport of
stratospheric ozone from the equator. As ozone converges at the pole, it descends,
increasing the ozone column abundance. The maximum column abundance at a
274
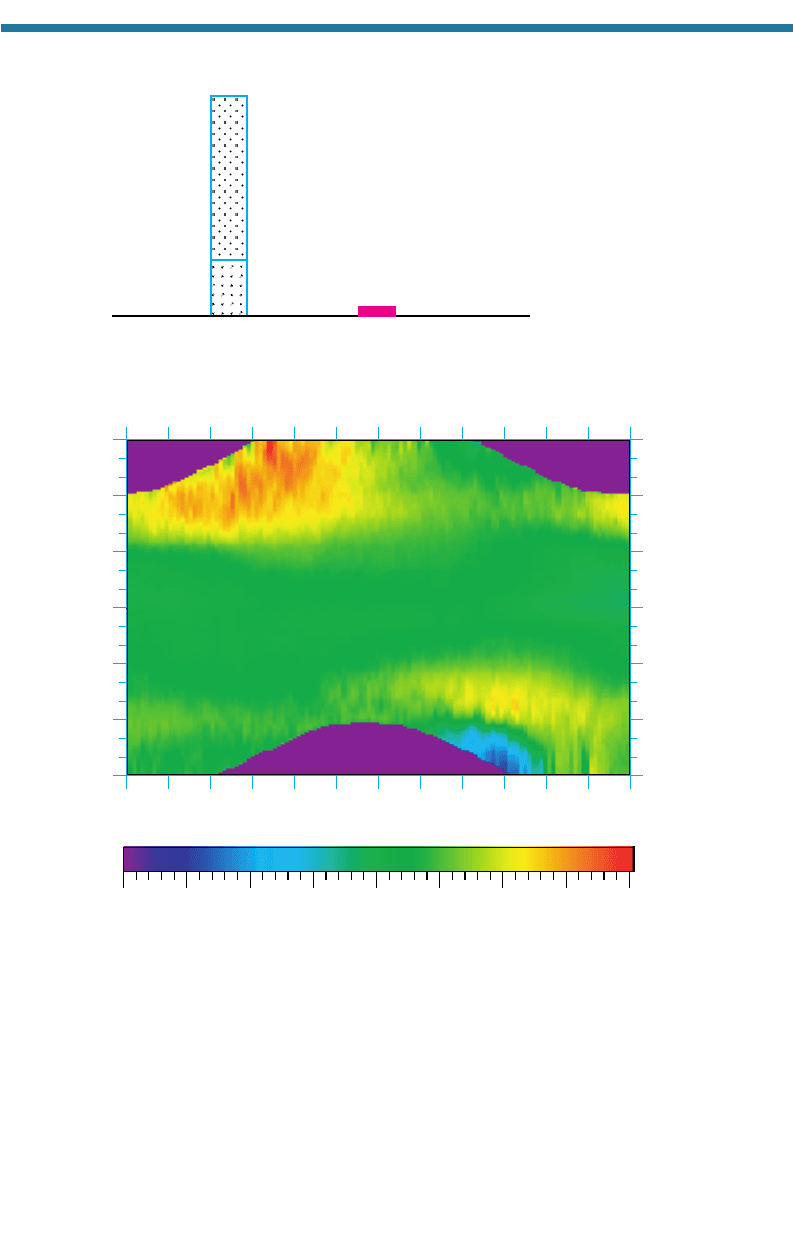
specific location (not zonally averaged) in 2000 was 573 DU on January 28 at
64.5N, 178.125W.
• A Southern Hemisphere (SH) spring (September–November) subpolar (60 to 65S)
maximum, ranging from 350 to 420 DU. The maximum is due to the southward
transport of ozone from the equator. As the ozone moves south, much of it is forced
to descend in front of the polar vortex, a polar front jet-stream wind system that
travels around the Antarctic continent in the upper troposphere and stratosphere.
GLOBAL STRATOSPHERIC OZONE REDUCTION 275
Surface
Top of the atmosphere
293-Dobson Unit column of ozone
= 293 × 2.7 × 10
16
molecules cm
-2
= 2.93-mm column of air at 273 K and 1 atm
2.93-mm high
column of air
Stratosphere
and above
Troposphere
Figure 11.1. Example of globally averaged column abundance of ozone. The number of ozone
molecules per unit area of surface in a 293-DU column of ozone is equivalent to the number
of air molecules in a 2.93-mm high column near the surface. (The figure is not to scale.)
-90
-60
-30
0
30
60
90
Latitude (degrees)
Month
Jan
50 100 150 200 250 300 350 400 450
Ozone column abundance
(
DU
)
Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec
Figure 11.2. Variation of zonally averaged ozone column abundance with latitude and month
during 2000. Blank regions near the poles indicate locations where data were not available.
Data for the figure were obtained from the satellite-based Total Ozone Mapping Spectrometer
(TOMS) and made available by NASA Goddard Space Flight Center, Greenbelt, Maryland.
• A SH spring minimum of less than 150 DU over the South Pole due to chemical
reactions of chlorine and bromine radicals with ozone. This minimum is called
the Antarctic ozone hole. The minimum column abundance at a specific loca-
tion (not zonally averaged) in 2000 was 94 DU on September 29 at 85.5S,
64.375W.
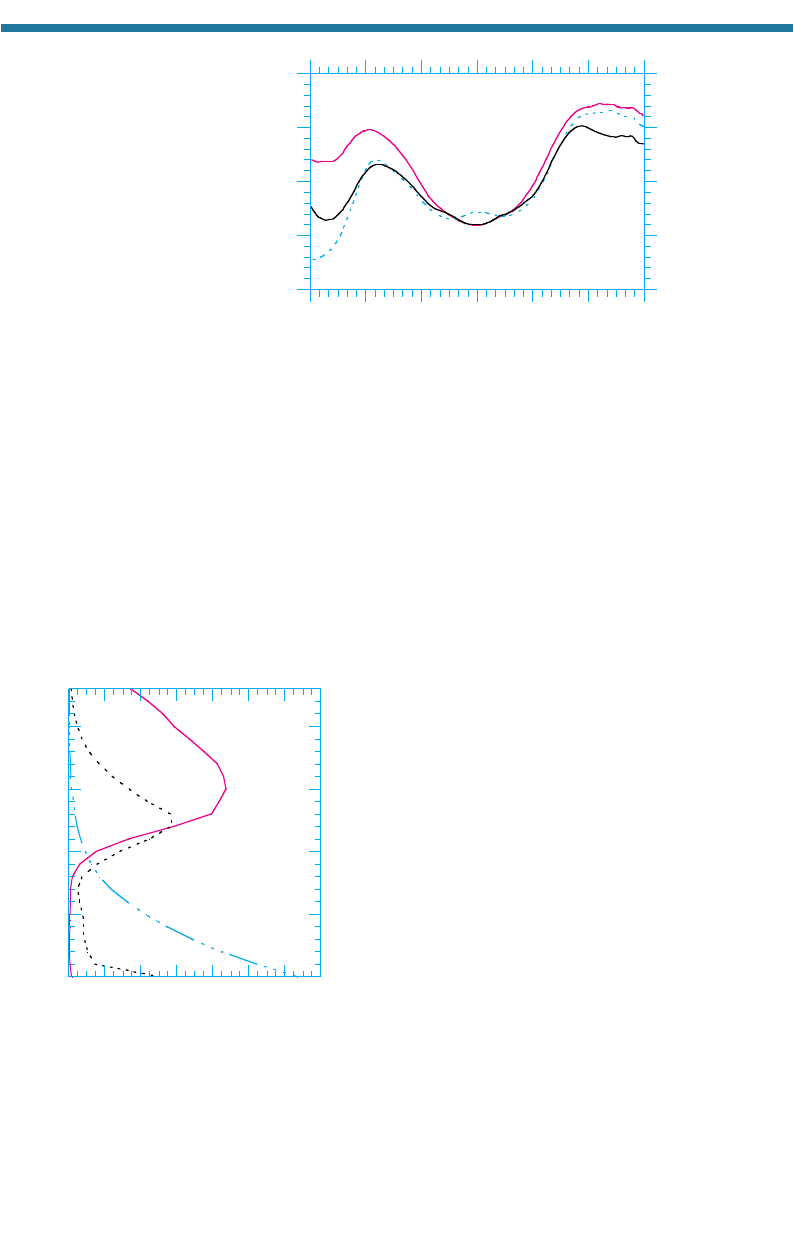
Figure 11.3 shows the variation with latitude of the yearly and zonally a
veraged
ozone column abundance in 1979, 1999, and 2000. The figure shows that the ozone
layer was thin near the equator in all three years.
From 15S to 15N, the ozone column abundance
actually increased in 1999 in comparison with 1979.
Such increases, relative to 1979 ozone, occurred
during about one-third of the years between 1979
and 2000.
In 1979 and earlier, the yearly and zonally aver-
aged ozone column abundance over 60 to 90S was
greater than over the equator. Since then, the season-
al Antarctic ozone hole (Section 11.4) has caused the
ozone column over 60 to 90S to decline, even in the
yearly average. Although the average column abun-
dance over 60 to 90S was slightly higher in 2000
than in 1999, the area of the ozone hole was larger in
2000 than in 1999.
Over 60 to 90N, the ozone column abundance
is always greater than over the equator. In most years
from 1979 to 2000, the column abundance over 60
to 90N was lower than it was in 1979. One excep-
tion was in 1999, when the column abundance was
nearly the same as in 1979. In 2000, another reduction occurred. When a reduction
occurs, it is called an Arctic ozone dent (Section 11.4).
Figure 11.4 shows a typical variation of ozone mixing ratio, ozone number
concentration, and total air number concentration with altitude. The ozone number
276 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
200
250
300
350
400
-90 -60 -30 0 30 60 90
Ozone (DU)
Latitude (degrees)
Zonal and yearly average
1999
1979
2000
Figure 11.3. Variation of year
ly and zonally averaged ozone column abundance with latitude in
1979, 1999, and 2000. Data were obtained from the satellite-based Total Ozone Mapping
Spectrometer (TOMS) and made available by NASA Goddard Space Flight Center, Greenbelt,
Maryland.
02468101214
0
10
20
30
40
Altitude (km)
O
3
(ppmv)
O
3
(molecules cm
-3
10
-12
)
Air (molecules cm
-3
5 3 10
-19
)
Figure 11.4. Example vertical variation in
ozone mixing ratio, ozone number concentra-
tion, and air number concentration with
altitude. The ozone mixing ratio at the surface
is 0.20 ppmv, the level of a Stage 1 smog
alert in the United States.
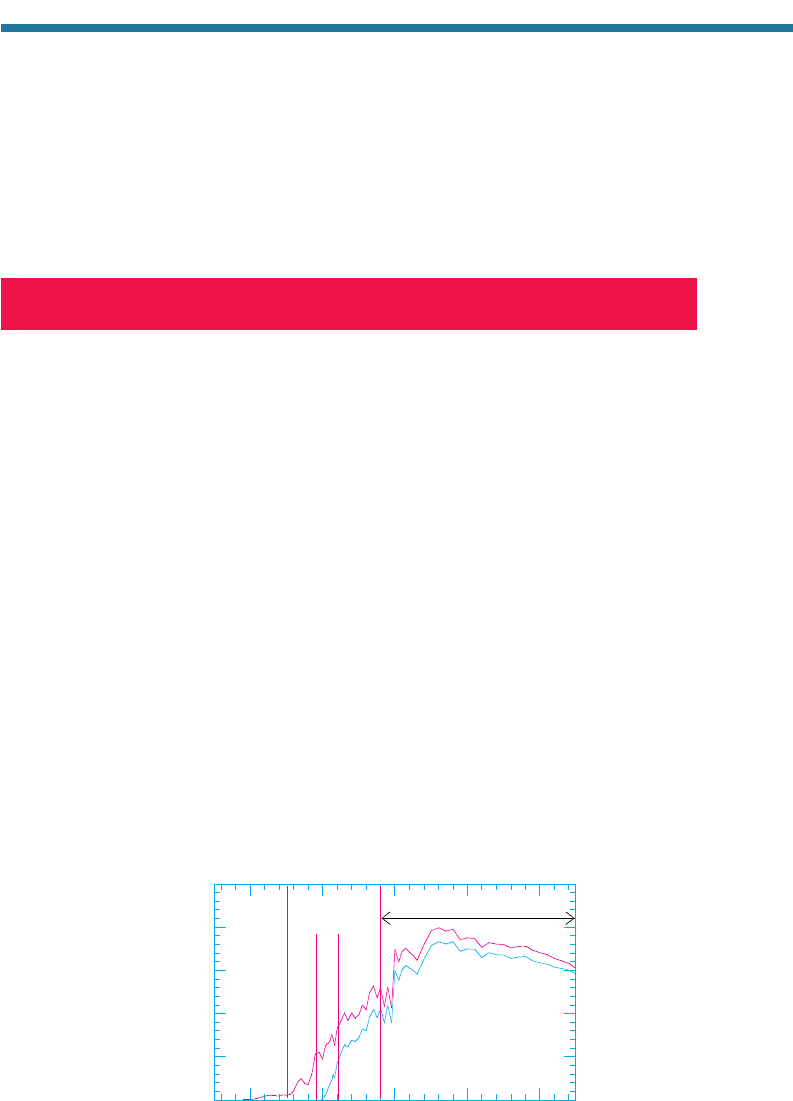
concentration (molecules of ozone per cubic centimeter of air) in the stratosphere
generally peaks at 25 to 32 km altitude. The ozone mixing ratio (number concentration
of ozone divided by that of dry air) peaks at a higher altitude than does the ozone
number concentration. The peak ozone number concentration in the stratosphere is
close to that in polluted urban air. The peak ozone mixing ratio in the stratosphere
(near 10 ppmv) is much higher than is that in polluted urban air (0.2 to 0.35 ppmv) or
free-tropospheric air (0.02 to 0.04 ppmv).
11.2. RELATIONSHIP BETWEEN THE OZONE LAYER
AND UV RADIATION
The ozone layer prevents damaging UV wavelengths from reaching the ground. As
shown in Fig. 2.5, the UV portion of the solar spectrum is divided into far- and near-UV
wavelengths. Near-UV wavelengths are further divided into UV-A, UV-B, and UV-C
wavelengths. Gases, particularly ozone and oxygen, and aerosol particles absorb most
solar UV radiation before it reaches the Earth’s surface. Decreases in stratospheric ozone
increase the transmission of UV to the surface. Enhancements in UV at the surface dam-
age life. In this subsection, processes affecting UV radiation are summarized.
Figure 11.5 shows the intensity of downward UV and visible radiation at the top
of the atmosphere (TOA) and at the ground. The figure shows that, of the incident
solar radiation at the TOA, only wavelengths longer than 0.29 m penetrate to the
ground. Thus, the air filters out all far-UV and UV-C wavelengths. Of the UV that
reaches the ground, about 9 percent is UV-B and the rest (91 percent) is UV-A. Of the
total solar radiation reaching the surface, about 5.2 percent is UV-A/UV-B and the rest
(94.8 percent) is visible/near-IR.
Table 11.1 identifies the major absorbing components responsible for reducing
near- and far-UV radiation between the TOA and ground. Molecular nitrogen [N
2
(g)]
absorbs far-UV wavelengths shorter than 0.1 m in the thermosphere and mesosphere,
and molecular oxygen [O
2
(g)] absorbs wavelengths shorter than 0.245 m in the ther-
mosphere, mesosphere, and stratosphere.
GLOBAL STRATOSPHERIC OZONE REDUCTION 277
0
500
1,000
1,500
2,000
2,500
0.2 0.3 0.4 0.5 0.6
Radiation intensity (W m
-2
μm
-1
)
Wavelength (μm)
TOA
Ground
Visible
10°N, 5°W
August 3, 1990
Solar zenith angle 8.2°
Near UV
Far UV
UV-A
UV-B
UV-C
Figure 11.5. Downward solar radiation less than 0.65 m wavelength at the top of the
atmosphere (TOA) and at the ground at a location near the equator in early August. The
solar zenith angle is the angle of the sun relative to a line perpendicular to the Earth’s
surface.
