Jacobson M.Z. Atmospheric Pollution
Подождите немного. Документ загружается.

288 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
his invention to the American Chemical Society in April 1930 by inhaling CFC-12,
then blowing it over a candle flame, extinguishing the flame. The invention was not
disclosed previously because the Frigidaire department of General Motors needed time
to file patents on a family of compounds related to the invention (Bhatti, 1999).
In 1931, CFC-12 was produced by the DuPont chemical manufacturer under the trade
name Freon, a name chose by Midgley and his assistants. Its first use was in small ice
cream cabinets. In 1934, it was used in refrigerators and whole-room coolers. Soon after,
it was used in household and automotive air conditioning systems. In 1932, CFC-11
[CFCI
3
(g), trichlorfluoromethane], was first produced. Its first use was in large air condi-
tioning units. CFCs became airborne only when coolants leaked or were drained.
In 1943, Goodhue and Sulli
van of the U.S. Department of Agriculture developed a
method to use CFC-11 and -12 as a propellant in spray cans. CFCs flowed out of a spray
can’s nozzle, carrying with them a mist containing other ingredients. Spray cans were
used to propel hair sprays, paints, deodorants, disinfectants, polishes, and insecticides.
CFC-11 and 12 have also been used as blowing agents in foam production. Foam
is used in insulation, disposable cups and cartons, and fire extinguishers. CFCs are
released to the air during foam-production, itself. CFCs in the air spaces of foam are
usually confined and not an important source of atmospheric CFCs.
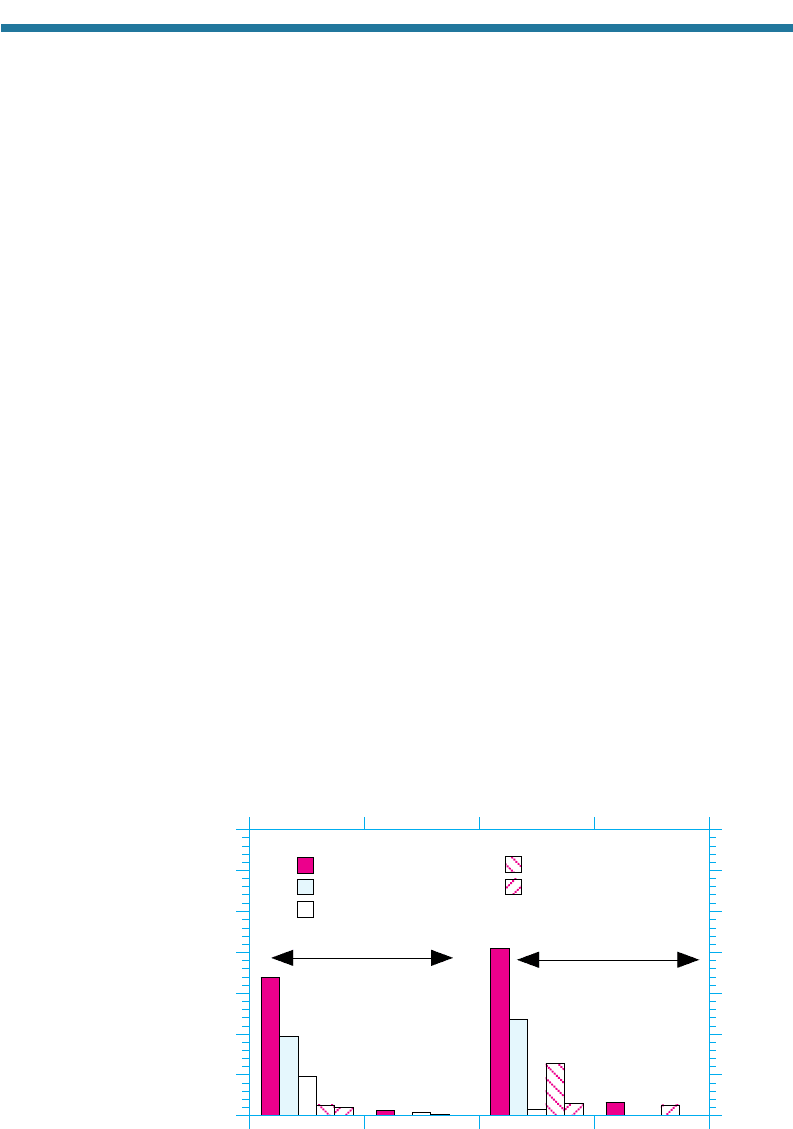
Figure 11.11 shows the reported sales of CFC-11 and -12 in 1976 and 1998. The fig-
ure shows that in 1976, almost 58 percent of CFC-11 and -12 were sold for use as
propellants in spray cans. Secondary use for CFC-11 was as a blowing agent and for
CFC-12 was as a refrigerant. Regulation of CFCs (Section 11.10) resulted in large
decreases in the use of CFC-11 and -12 between 1976 and 1998 and increases in replace-
ment compounds. By 1998, total sales of CFCs were about 6 percent of those in 1976.
Several other CFCs were developed, some of which are listed in Table 11.2. CFC-113,
for example, was first produced in 1934 and used in air conditioning units. It has since
been used primarily as a solvent in the microelectronics industry and in the dry-cleaning
industry. It has also been used as a spray-can propellant and a blowing agent in foam
production.
0
100
200
300
400
500
600
700
1976 1998 1976 1998
Total
Propellant
Blowing agent
Refrigerant
Other
CFC sales (1,000 metric tons/yr)
Year
CFC-12CFC-11
57.4%
28.5%
7.9%
57.6%
6.2%
11.0%
25.9%
5.5%
57.7%
3.7%
31.1%
7.5%
8.9%
3.6%
80.8%
6.7%
Figure 11.11. Reported sales of CFC-11 and -12 in 1976 and 1998. Percentages are of the
total for the year.
Source: AFEAS (2001).

11.5.1.2. Other Chlorine Compounds
Chlorofluorocarbons are a subset of chlorocarbons, which are compounds con-
taining carbon and chlorine. Hydrochlorofluorocarbons (HCFCs) are another subset
of chlorocarbons. HCFCs are similar to CFCs except that HCFCs have at least one
hydrogen atom. The hydrogen atom allows HCFCs to be broken down in the tropo-
sphere by reaction with OH(g). OH(g) does not readily break down CFCs. Because
HCFCs break down more readily than do CFCs, a smaller percentage of emitted
HCFCs than CFCs reaches the stratosphere. Nevertheless, because HCFCs contain
chlorine and some HCFCs reach the stratosphere, HCFCs are still a danger to stratos-
pheric ozone. HCFC-22, first produced in 1943, is the most abundant HCFC in the air
today. HCFC-22 has been used as a refrigerant, spray-can propellant, and blowing
agent in foam production.
Other chlorocarbons include carbon tetrachloride [CCl
4
(g)], methyl chloroform
[CH
3
CCl
3
(g)], and methyl chloride [CH
3
Cl(g)]. Carbon tetrachloride is used as an
intermediate in the production of CFCs and HCFCs and as a solv
ent and grain fumi-
gant. Methyl chloroform is used as a degreasing agent, a dry-cleaning solvent and an
industrial solvent. Methyl chloride is produced synthetically only in small quantities
and used in the production of silicones and tetramethyl lead intermediates (Singh,
1995). Most methyl chloride in the air is produced biogenically in the oceans.
Another chlorine-containing gas in the troposphere is hydrochloric acid
[HCl(g)]. HCl(g) has larger natural than anthropogenic sources. Natural sources
include evaporation of chloride from sea-spray and volcanic emissions. Although
some anthropogenic emissions of HCl(g) are from waste incineration, about 98 per-
cent are from coal combustion (Saxena et al. 1993).
11.5.1.3. Bromine Compounds
Although chlorine-containing compounds are more abundant than are bromine-
containing compounds, the latter compounds are more efficient, molecule for molecule, at
destroying ozone. The primary source of stratospheric bromine is methyl bromide
[CH
3
Br(g)], which is produced biogenically in the oceans and emitted as a soil fumigant.
Other sources of bromine are a group of synthetically produced compounds termed
Halons, which are used in fire extinguishers and as fumigants. The most common Halons
are H-1301 [CF
3
Br(g)], H-1211 [CF
2
ClBr(g)], and H-2402 [CF
2
BrCF
2
Br(g)]. Methyl
bromide and Halons are bromocarbons because they contain both bromine and carbon.
11.5.1.4. Fluorine Compounds
Compounds that contain hydrogen, fluorine, and carbon but not chlorine or
bromine are hydrofluorocarbons (HFCs). HFCs were produced in abundance only
recently as a replacement for CFCs and HCFCs. Because the fluorine in HFCs has lit-
tle effect on ozone, production of HFCs may increase in the future. Unfortunately,
because they absorb thermal-IR radiation, HFCs will enhance global warming if their
use increases. The most abundantly emitted HFC to date has been HFC-134a
[CH
2
FCF
3
(g)]. Related to HFCs are perfluorcarbons (PFCs), such as perfluoroethane
[C
2
F
6
(g)], and sulfur hexafluoride [SF
6
(g)].
11.5.2. Lifetimes and Mixing Ratios of Chlorinated Compounds
Once emitted, CFCs take about one year to mix up to the tropopause. Because they are
chemically unreactive and cannot be broken down by solar wavelengths that reach the
GLOBAL STRATOSPHERIC OZONE REDUCTION 289
troposphere, CFCs are not removed chemically from the troposphere. Instead, they
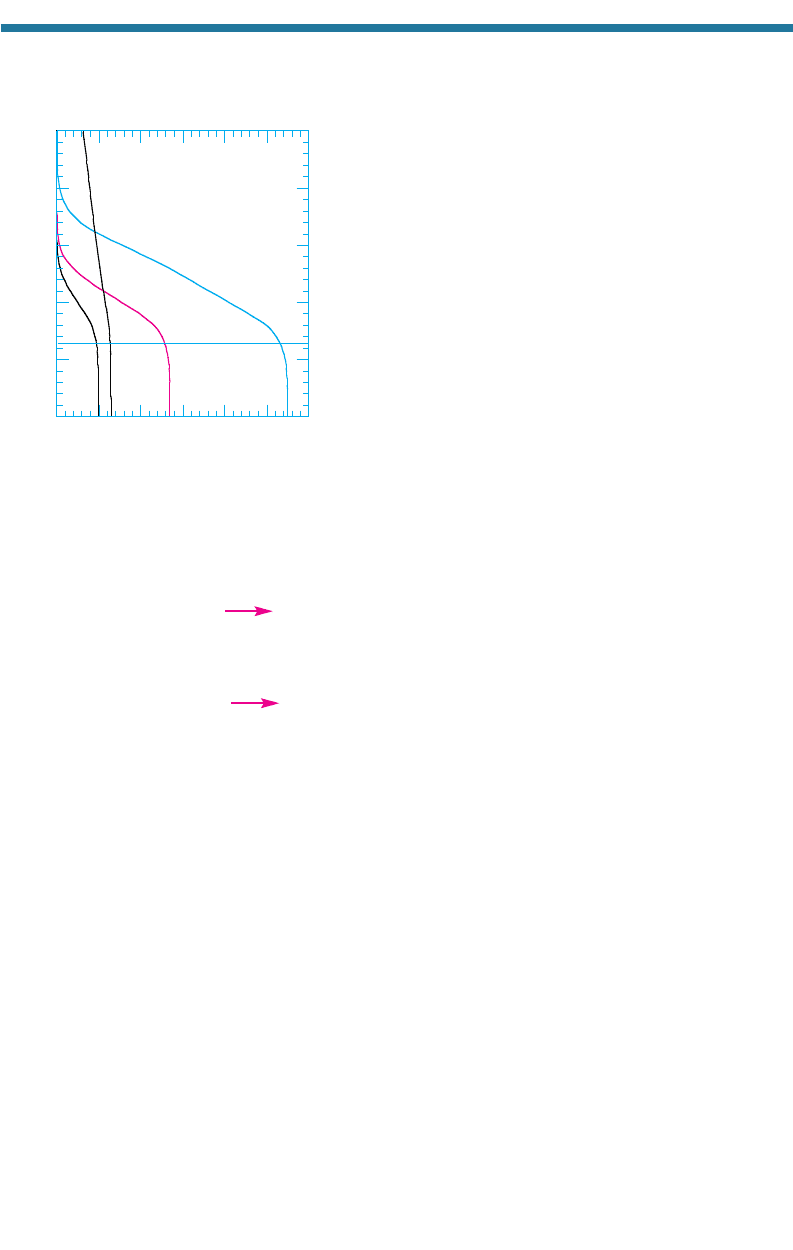
become well mixed in the troposphere and slowly penetrate to the stratosphere. Today, the
tropospheric mixing ratios of CFC-11 and CFC-12, the
two most abundant CFCs, are about 270 and 550 pptv,
respectively (Table 11.2 and Fig. 11.12).
11.5.2.1. Lifetimes of CFCs
Because the stratosphere is one large temperature
inversion, vertical transport of ozone through it is slow.
About 10 Mt of chlorine in the form of CFCs reside in
the troposphere, and the transfer rate of CFC-chlorine
from the troposphere to the middle stratosphere is about
0.1 Mt per year. In this simplified scenario, the average
time required for the transfer of a CFC molecule from
the troposphere to the middle stratosphere is about 100
years.
CFCs are broken down in the stratosphere only
when they are exposed to far-UV radiation (wave-
lengths of 0.01 to 0.25 m), and this exposure occurs at
an altitude of 12 to 20 km and higher
. At such altitudes,
far-UV wavelengths photolyze CFC-11 and CFC-12 by
CFCl
3
(g) h C
•
FCl
2
(g) C
•
l(g) 250 nm
CFC-11 Dichlorofluoro- Atomic (11.21)
methyl radical chlorine
CF
2
Cl
2
(g) h C
•
F
2
Cl(g) C
•
l(g) 230 nm
CFC-12 Dichlorofluoro- Atomic (11.22)
methyl radical chlorine
At 25 km, e-folding lifetimes of CFC-11 and CFC-12 against photolysis under maximum-
sunlight conditions are on the order of 23 and 251 days, respectively. Average lifetimes
are on the order of two to three times these values.
In sum, the limiting factor in CFC decomposition in the stratosphere is not trans-
ported from the surface to the tropopause or photochemical breakdown in the
stratosphere, but transported from the tropopause to the middle stratosphere. Table
11.2 indicates that the overall lifetimes of CFC-11 and CFC-12 between release at the
surface and destruction in the middle stratosphere are about 55 and 116 years, respec-
tively. The lifetime of CFC-12 is longer than that of CFC-11, partly because the
former compound must climb to a higher altitude in the stratosphere before breaking
apart than must the latter. Because of their long overall lifetimes, some CFCs emitted
in the 1930s through 1950s are still present in the stratosphere. Those emitted today
are likely to remain in the air until the second half of the twenty-first century.
11.5.2.2. Lifetimes of Non-CFCs
Lifetimes of non-CFC chlorinated compounds are often shorter than are those of
CFCs. The lifetimes of CCl
4
(g), HCFC-22(g), CH
3
CCl
3
(g), CH
3
Cl(g), and HCl(g)
between emission and chemical destruction are about 35, 12, 5, 1.3, and less than 0.1
year, respectively. Non-CFCs generally have shorter lifetimes than do CFCs because
290 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
0 100 200 300 400 500 600
0
10
20
30
40
50
Altitude (km)
CFC-11
HCFC-22
CFC-12
CCl
4
(g)
Mixing ratio (pptv)
Tropopause
Figure 11.12. Variation of CFC-11, CFC-12,
HCFC-22, and CCl
4
(g) with altitude at 30N
latitude. Smoothed and scaled from
Jackman et al. (1996) to present-day near-
surface mixing ratios.
they react faster with OH(g) than do CFCs and are often more water soluble than are
CFCs. The benefit of a shorter lifetime for a chlorine-containing compounds is that, if
breakdown occurs in the troposphere, the chlorine released can be converted to
HCl(g), which is highly soluble and can be removed readily by rainout. Because the
stratosphere does not contain clouds, except for ice-containing clouds that form sea-
sonally over the poles, HCl(g) cannot be removed from the stratosphere by rainout.
Some non-CFCs, such as HCFC-22, photolyze slower than do CFCs, so once HCFC-
22 reaches the middle stratosphere, its concentration builds up there to a greater extent
than do concentrations of several CFCs, as seen in Fig. 11.12.
Of non-CFC chlorine compounds, CH
3
Cl(g), and HCl(g) have the largest natural
sources. The tropospheric e-folding chemical lifetime of CH
3
Cl(g) against reaction by
OH(g) is about 1.5 years; that of HCl(g) against reaction by OH(g) is about 15 to 30
days. HCl(g) is also soluble in w
ater and is absorbed by clouds. Volcanos,
which emit
water vapor and hydrochloric acid, produce clouds and rain that remove HCl(g), pre-
venting most of it from reaching the stratosphere (Lazrus et al., 1979; Pinto et al.,
1989; Tabazadeh and Turco, 1993). The facts that the two major natural sources of
chlorine, CH
3
Cl(g) and HCl(g), have short chemical lifetimes against destruction by
OH(g) and that HCl(g) is soluble in water, whereas CFCs have long chemical lifetimes
and are insoluble, support the contention that CFCs and not naturally emitted chlorine
compounds are responsible for most ozone destruction in the stratosphere.
11.5.2.3. Emissions of Chlorine Compounds to the Stratosphere
Table 11.3 summarizes the relative emissions of anthropogenic and natural chlorine-
containing compounds into the stratosphere in 1994. About 82 percent of chlorine
entering the stratosphere originated from anthropogenic sources. Of the remainder, about
15 percent was methyl chloride, emitted almost exclusively by biogenic sources in the
oceans, and 3 percent was hydrochloric acid, emitted by volcanos, evaporated from sea
spray, and otherwise produced naturally. The relatively large anthropogenic versus natural
GLOBAL STRATOSPHERIC OZONE REDUCTION 291
Table 11.3. Relative Emissions of Selected Chlorine
Compounds into the Stratosphere
CFC-12 CF
2
Cl
2
(g) 28
CFC-11 CFCl
3
(g) 23
Carbon tetrachloride CCl
4
(g) 12
Methyl chloroform CH
3
CCl
3
(g) 10
CFC-113 CFCl
2
CF
2
Cl(g) 6
HCFC-22 CF
2
ClH(g) 3
Contribution to
Trade Name or Stratospheric
Chemical Name Chemical Formula Emissions (Percent)
Source: WMO (1995).
Methyl chloride CH
3
Cl(g) 15
Hydrochloric acid HCl(g) 3
Total 100
Anthropogenic Sources
Natural Sources
source of chlorine into the stratosphere supports the contention that stratospheric ozone
reductions result primarily from anthropogenic chlorine, not natural chlorine.
11.5.3. Catalytic Ozone Destruction by Chlorine
Once released from their parent compounds in the stratosphere, chlorine atoms from
CFCs and non-CFCs react along one of several pathways. Chlorine reacts in a catalytic
ozone destruction cycle,
At midlatitudes, the chain length of this cycle increases from about 10 in the lower
stratosphere to about 1,000 in the middle and upper stratosphere (Lary, 1997).
The primary removal mechanisms of active chlorine [Cl(g)ClO(g)] from the
catalytic cycle are reactions that produce chlorine reservoirs, HCl(g) and chlorine
nitrate [ClONO
2
(g)]. Chlorine reservoirs are called such because they temporarily
store active chlorine, preventing it from destroying ozone. Conversion of Cl(g) to
HCl(g) occurs by
(11.26)
Conversion of ClO(g) to ClONO
2
(g) occurs by
ClO
•
(g) N
•
O
2
(g)
M
ClONO
2
(g)
Chlorine Nitrogen Chlorine (11.27)
monoxide dioxide nitrate
CH
4
(g)
Methane
HO
2
(g)
Hydroperoxy
radical
O
2
(g)
Molecular
oxygen
H
2
(g)
Molecular
hydrogen
H
2
O
2
(g)
Hydrogen
peroxide
Cl(g) +
Atomic
chlorine
HCl(g) +
CH
3
(g)
Methyl
radical
H(g)
Atomic
hydrogen
HO
2
(g)
Hydroperoxy
radical
Hydrochloric
acid
292 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
C
•
l(g) O
3
(g) ClO
•
(g) O
2
(g)
Atomic Ozone Chlorine Molecular
chlorine monoxide oxygen
(11.23)
C
•
lO(g) •O
•
(g) C
•
l(g) O
2
(g)
Chlorine Ground- Atomic Molecular
monoxide state atomic chlorine oxygen
oxygen
(11.24)
•O
•
(g) O
3
(g) 2O
2
(g)
Ground- Ozone Molecular
(11.25)
state atomic oxygen
oxygen
(net process)
At any time, about 1 percent of the non-CFC chlorine in the stratosphere is in the form
of active chlorine. Most of the rest is in the form of a chlorine reservoir. Because CFCs
release their chlorine by photolysis in the middle and upper stratosphere, it follows
that HCl(g) mixing ratios should also peak in the middle and upper stratosphere.
Indeed, observations confirm this supposition.
The HCl(g) reservoir leaks back to atomic chlorine by photolysis, reaction with
OH(g), and reaction with O(g), all of which are slow processes. The e-folding lifetime
of HCl(g) against photolysis, for example, is about 1.5 years at 25 km. HCl(g) also dif-
fuses back to the troposphere, where it can be absorbed by clouds. The ClONO
2
(g)
reservoir leaks back to atomic chlorine by photolysis with an e-folding lifetime of
about 4.5 hours at 25 km.
11.6. EFFECTS OF BROMINE ON GLOBAL OZONE REDUCTION
Like chlorine, bromine affects stratospheric ozone. The primary source of stratospher-
ic bromine is methyl bromide [CH
3
Br(g)], which is produced biogenically in the
oceans and emitted as a soil fumigant. Other sources of bromine are Halons, defined in
Section 11.5.1. The tropospheric mixing ratios of the most common Halons,
CF
2
ClBr(g) (H-1211) and CF
3
Br(g) (H-1301), are both about 2 pptv, less than 1 per-
cent of the mixing ratios of CFC-11 and -12. Nevertheless, the efficiency of ozone
destruction by the bromine catalytic cycle is greater than is that by the chlorine cat-
alytic cycle.
Methyl bromide and Halons photolyze in the stratosphere to produce atomic
bromine. Photolysis of methyl bromide occurs above 20 km by
CH
3
Br(g) h C
•
H
3
(g) B
•
r(g) 260 nm
Methyl Methyl Atomic (11.28)
bromide radical bromine
The e-folding lifetime of CH
3
Br(g) against loss by this reaction is about 10 days at
25 km.
Once atomic bromine is in the stratosphere, it reacts in another catalytic ozone
destruction cycle,
GLOBAL STRATOSPHERIC OZONE REDUCTION 293
B
•
r(g) O
3
(g) BrO
•
(g) O
2
(g)
Atomic Ozone Bromine Molecular (11.29)
bromine monoxide oxygen
BrO
•
(g) •O
•
(g) B
•
r(g) O
2
(g)
Bromine Ground- Atomic Molecular
monoxide state atomic bromine oxygen
oxygen
(11.30)
•O
•
(g) O
3
(g) 2O
2
(g)
Ground- Ozone Molecular
state atomic oxygen
oxygen
(net process)
(11.31)
The chain length of this cycle increases from about 100 at 20 km to about 10
4
at 40 to
50 km (Lary, 1997). The chain length of the bromine catalytic cycle is longer than is
that of the chlorine catalytic cycle because Br(g) is removed more slowly from the
bromine cycle by reaction with CH
4
(g) and H
2
(g) than Cl(g) is removed from the chlo-
rine cycle by reaction with the same chemicals.
When atomic bromine is removed from its catalytic cycle, it forms hydrobromic
acid [HBr(g)] by
(11.32)
When BrO(g) is removed, it forms bromine nitrate [BrONO
2
(g)] by
BrO
•
(g) N
•
O
2
(g)
M
BrONO
2
(g)
Bromine Nitrogen Bromine (11.33)
monoxide
dioxide nitrate
The HBr(g) reservoir leaks slowly back to atomic bromine by reaction with
OH(g). The BrONO
2
(g) reservoir quickly leaks back to atomic bromine by photol-
ysis. The e-folding lifetime of BrONO
2
(g) against photolysis is about 10 minutes
at 25 km.
11.7. REGENERATION RATES OF STRATOSPHERIC OZONE
The presence of chlorine and bromine has decreased levels of ozone in the stratosphere.
If chlorine and bromine could be removed easily from the stratosphere, the stratospheric
ozone layer could regenerate quickly. The problem is that the overall lifetimes of cer-
tain chlorocarbons and bromocarbons are on the order of 50 to 100 years; thus, the
natural removal rate of chlorine- and bromine-containing compounds from the strato-
sphere is slow.
Suppose that all ozone in the stratosphere were destroyed, all ozone-destroying
compounds were removed, but all oxygen remained. How long would the ozone layer
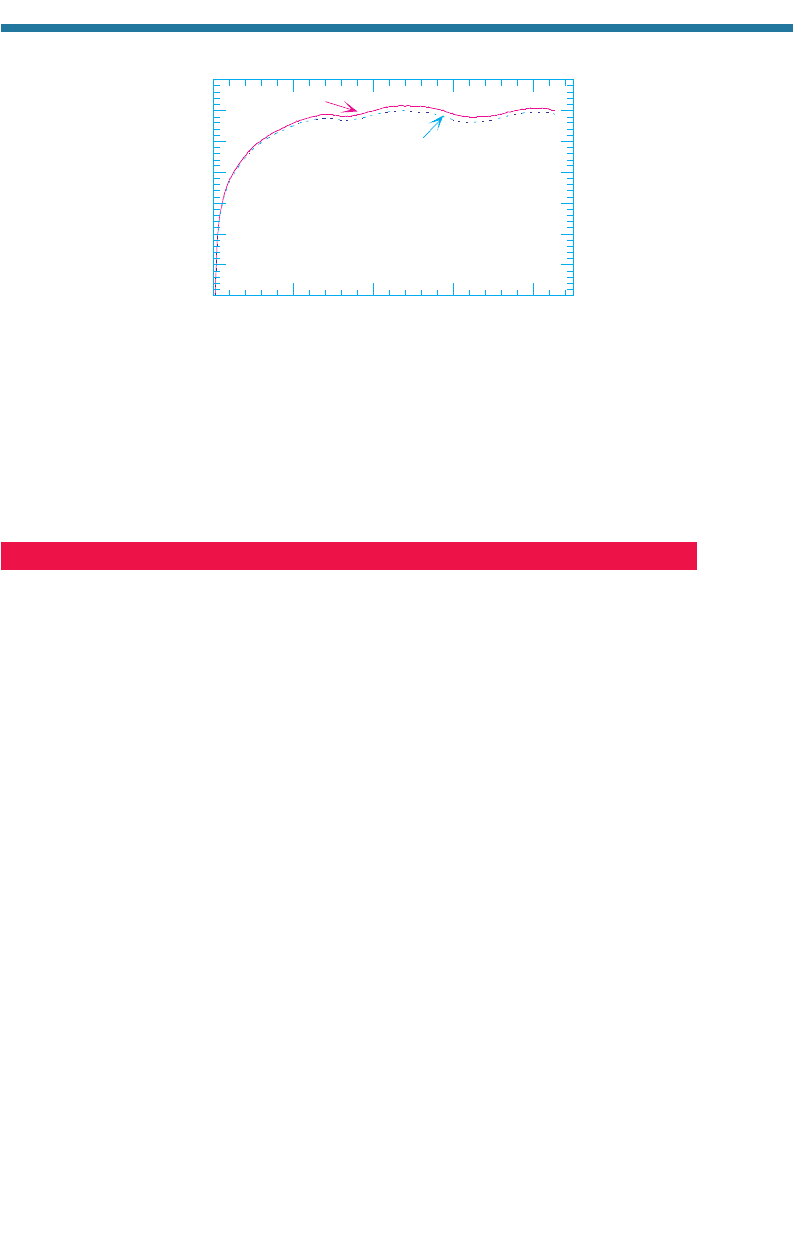
take to regenerate? An estimate can be obtained from Fig. 11.13. This figure shows
results from two computer simulations of the global atmosphere in which all ozone in
the present-day atmosphere was initially removed, but oxygen was not. In the first sim-
ulation, ozone regeneration was simulated in the absence of chlorine and bromine. In
the second, ozone regeneration was simulated in the presence of 1989 concentrations
of chlorine, but in the absence of bromine. In both simulations, the globally averaged
column abundance of ozone regenerated to relatively steady values in less than a year.
Regeneration during the simulation in which chlorine was initially present was about 2
to 3 percent less than that during the no-chlorine case, consistent with the estimated
global reduction in ozone of 2 to 3 percent between the 1970s and 1989 due to chlorine-
containing compounds.
Atomic
bromine
Br(g) +
HBr(g) +
Hydrobromic
acid
HO
2
(g)
Hydroperoxy
radical
H
2
O
2
(g)
Hydrogen
peroxide
HO
2
(g)
Hydroperoxy
radical
O
2
(g)
Molecular
oxygen
294 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
11.8. ANTARCTIC OZONE DEPLETION
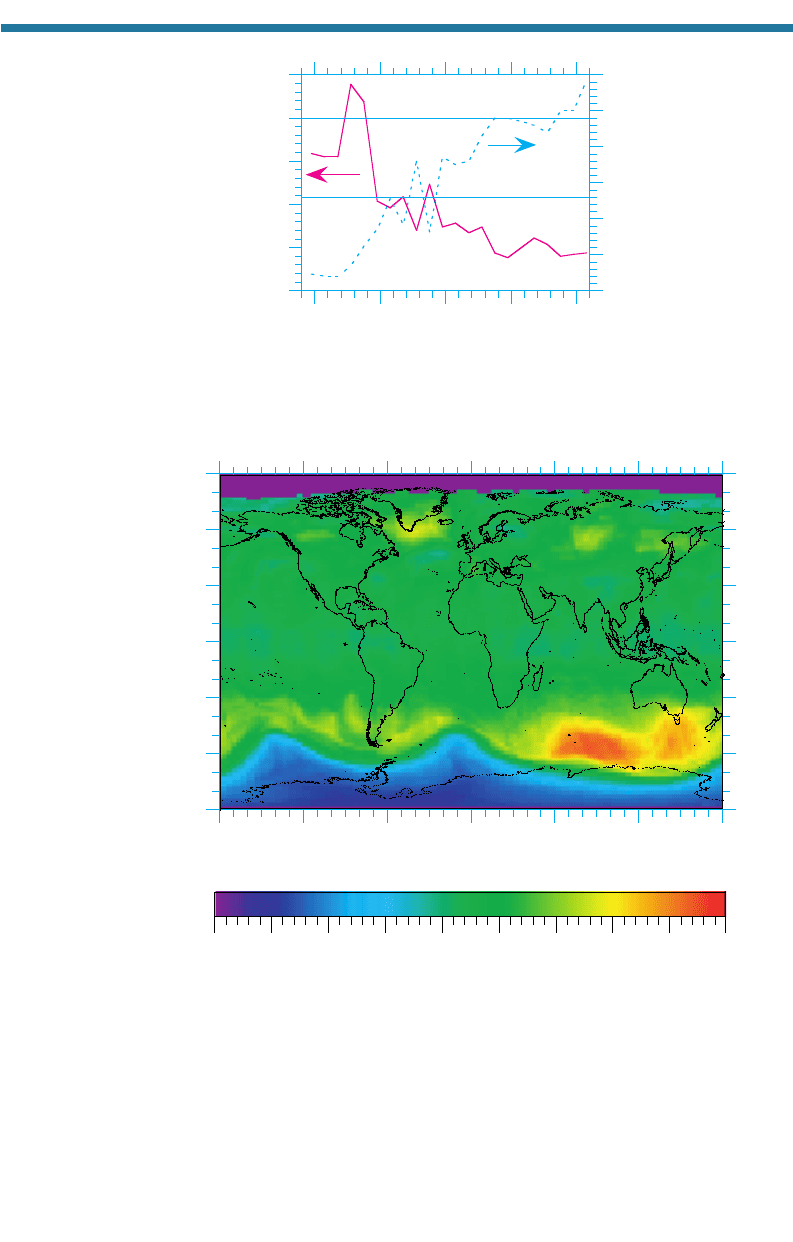
Every September through November since 1980, the minimum ozone column abun-
dance over the Antarctic decreases below its yearly average. Figure 11.14 shows that
in 2000, the lowest measured column abundance over the Antarctic was about 94 DU
(occurring on September 29,
2000), which was 68 percent less than 293.4 DU, the
globally (90S to 90N) and year-2000 averaged ozone column abundance. Between
1981 and 2000, the area over which ozone depletes (the Antarctic ozone hole)
increased, as shown in the figure. The ozone hole area is defined as the area of the
globe over which the ozone column abundance decreases below 220 DUs. The ozone
hole in 2000 covered nearly 30 10
6
km
2
, an area larger than the size of North
America. Most Antarctic ozone depletion occurs between 14 and 18 km in altitude.
Beginning in 1992, springtime ozone decreases were observed up to 24 km and down
to 12 to 14 km.
Figure 11.15 shows the extent of the Antarctic ozone hole on October 1, 2000.
Whereas the minimum on that date was near 94 DU within the polar vortex, an ozone
maximum of 478 DU was observed just outside the vortex. The proximity of the max-
imum to the minimum is one factor that allows the ozone hole to replenish itself from
October to December.
The Antarctic ozone hole now appears every year during the Southern
Hemisphere spring. A smaller Arctic ozone dent (reduction in ozone to 240 to 260
DU) appears during the Northern Hemisphere late winter and spring
(March–May). In 1997, when ozone reductions over the Arctic were the most
severe on record, the minimum ozone value in the dent on April 1 was only 247
DU, appearing at 78.5N, 5.625W. This minimum was not low enough to qualify
as a depletion (less than 220 DU). Nevertheless, the average ozone column abun-
dance 60 to 90N in March 1997 was 22 percent lower than that in March 1979
[Fig. 11.9(a)], indicating that Arctic loss in 1997 was nontrivial. The ozone dent
seemed to disappear in 1999, when the ozone column abundance 60 to 90N in
GLOBAL STRATOSPHERIC OZONE REDUCTION 295
0
50
100
150
200
250
300
350
0 100 200 300 400
Avgerage global ozone column
(DU)
Day and date of simulation
10/1 1/7 4/17 7/26 11/4
No chlorine
With chlorine
Figure 11.13. Change in ozone column abundance, averaged over the globe, during two global
model simulations in which chlorine was present and absent, respectively. In both cases,
ozone was removed initially from the model atmosphere on October 1, 1988. Bromine was
not included in either simulation.
Source: Jacobson (1999a).
March was only 2.2 percent lower than it was in March 1979. The dent reappeared
in March 2000, when the column abundance was 16 percent lower than that in
1979. The hole and dent are caused by set of interlinked factors. One factor linking
global ozone reductions to polar ozone depletion is the presence of chlorine and
bromine in the stratosphere.
296 ATMOSPHERIC POLLUTION: HISTORY, SCIENCE, AND REGULATION
-90
-60
-30
0
30
60
90
-180 -120 -60 600 120 180
Latitude (degrees)
Longitude (degrees)
50 100 150 200 250 300 350 400 450 500
Ozone column abundance (DU)
Figure 11.15. Ozone column abundance (in DU) on October 1, 2000. The figure shows an
ozone hole over the Antarctic (with a minimum value of 94 DU at 86.5S, 64.4W) and an
ozone maximum of 478 DU at 58.5S, 83.125E. No data were available over the North Pole.
Data were obtained from the Total Ozone Mapping Spectrometer (TOMS) satellite and were
made available by NASA Goddard Space Flight Center, Greenbelt, Maryland.
50
100
150
200
250
300
0
5
10
15
20
25
30
1980 1985 1990 1995 2000
Ozone minimum (DU)
Ozone hole area (10
6
km
2
)
Year
Area of North America
Area of Antarctic
continent
Figure 11.14. Minimum ozone column abundances and areal extent of the ozone hole over
the Antarctic region from 1979 to 2000. Data from NASA Goddard Space Flight Center. For
comparison, the area of the Antarctic is about 13 10
6
km
2
and the area of North America
is about 24 10
6
km
2
.

GLOBAL STRATOSPHERIC OZONE REDUCTION 297
Figure 11.16. Polar stratospheric clouds, photographed in spring 2000 in the Arctic. Courtesy
NASA.
11.8.1. Polar Stratospheric Cloud Formation
The ozone hole over the Antarctic appears in part because the
Antarctic winter
(June–September) is very cold. Temperatures are low because much of the polar
region is exposed to 24 hours of darkness each day during the winter, and a wind sys-
tem, the polar vortex, circles the Antarctic. The vortex is a polar front jet-stream wind
system that flows around the Antarctic continent, trapping cold air within the polar
region and preventing an influx of warm air from outside this region.
Because temperatures are low in the Antarctic stratosphere, optically thin clouds,
called polar stratospheric clouds (PSCs) form (Fig. 11.16). These clouds have few
particles per unit volume of air in comparison with tropospheric clouds. Two major
types of clouds form. When temperatures drop to below about 195 K, nitric acid and
water vapor grow on small sulfuric acid–water aerosol particles (Toon et al. 1986).
Initially, it was thought that nitric acid and water molecules deposited to the ice
phase in the ratio 1:3. Such ice crystals have the composition HNO
3
• 3H
2
O and
are called nitric acid trihydrate (NAT) crystals. More recently, it was found that
these particles contain a variety of phases. Some contain nitric acid dihydrate
(NAD) (Worsnop et al. 1993), and others contain supercooled liquid water (liquid
water present at temperatures below the freezing point of water), sulfuric acid, and
nitric acid (Tabazadeh et al. 1994). Together, nitrate containing cloud particles that
form at temperatures below about 195 K in the winter polar stratosphere are called
Type I polar stratospheric clouds.
When temperatures drop below the frost point of water, which is about 187 K for
typical polar stratospheric conditions, a second type of cloud forms. These clouds con-
tain pure water ice and are Type II polar stratospheric clouds. Usually, about 90
percent of PSCs are Type I and 10 percent are Type II (Turco et al. 1989). Typical
